At the end of this unit you should





















































- Slides: 80


At the end of this unit you should: 1. Be able to produce gases, in particular oxygen, carbon dioxide and hydrogen. 2. Be able to carry out the characteristic tests for oxygen, carbon dioxide and hydrogen gases. 3. Be able to identify some of the properties of carbon dioxide. 4. Be able to explain combustion and the fire triangle. 5. Know how to compare and contrast combustion, respiration and photosynthesis as chemical reactions. 6. Understand that the atmosphere is a mixture of gases in a number of layers.

At the end of this unit you should: 7. Be able to test for the presence of oxygen, carbon dioxide and water vapour in the air. 8. Understand the Law of Conservation of Mass through the production and identification of a gas. 9. Understand the role of carbon dioxide in photosynthesis and extinguishing fire.

activation energy fire triangle aerobic respiration fuel anaerobic respiration Law of Conservation of Mass atmosphere Phlogiston Theory characteristic test photosynthesis combustion respiration downward displacement upward displacement exothermic

LIGHTBULB QUESTION Oxygen is a flammable gas that humans need to live. It is part of the fire triangle and it is required for respiration, which allows energy to be created in the body.

Aerobic Respiration: The release of energy from nutrients in organisms by chemical reaction with oxygen. Anaerobic Respiration: The release of energy from nutrients in organisms without a chemical reaction with oxygen.

Combustion: The release of heat energy from substances by chemical reaction with oxygen. Fuel: A substance that releases its chemical energy as heat energy when reacted with oxygen.

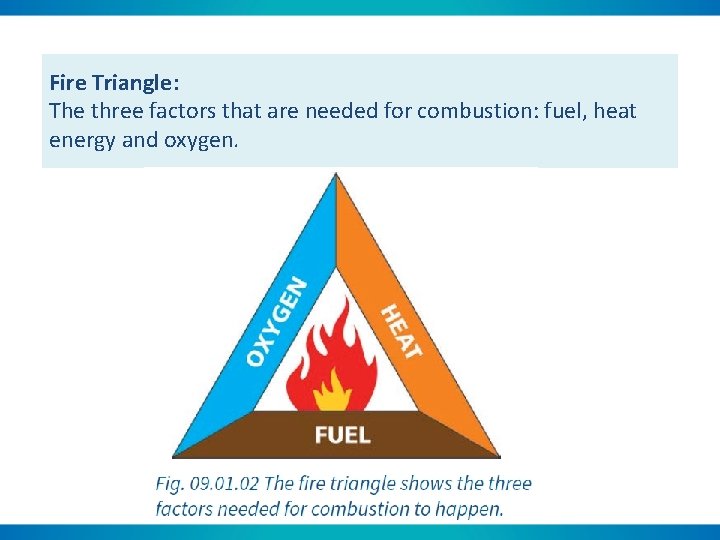
Fire Triangle: The three factors that are needed for combustion: fuel, heat energy and oxygen.

Activation Energy: The minimum amount of energy needed for a reaction to happen. Exothermic: A chemical reaction in which energy is released into the surroundings.

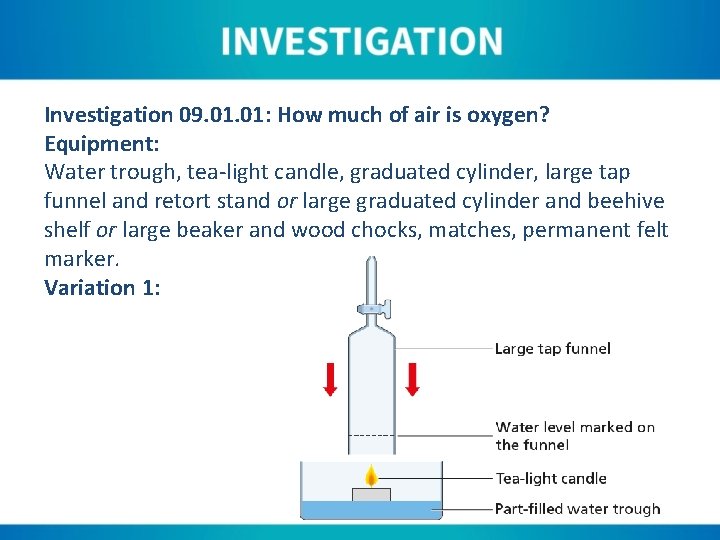
Investigation 09. 01: How much of air is oxygen? Equipment: Water trough, tea-light candle, graduated cylinder, large tap funnel and retort stand or large graduated cylinder and beehive shelf or large beaker and wood chocks, matches, permanent felt marker. Variation 1:

Instructions Variation 1: 1. Fill a water trough to between ⅓ and ½ its volume with tap water. 2. The tap on the funnel should be put in the open position. The tap funnel should then be lowered into the water and the water level marked on its outside with the felt marker. 3. Light a tea-light candle and place it floating in the water trough. 4. The candle should be allowed to burn until a strong consistent flame can be seen (maximum of approximately three minutes). 5. The tap funnel should be lowered over the candle, taking care not to capsize the candle. 6. Once the water levels inside and outside the tap funnel have levelled, the tap can be closed. 7. As the oxygen is consumed by the candle flame, the internal water level will rise significantly and the external level fall slightly.

Instructions Variation 1: 8. Once the candle has been extinguished, the change in the internal water level should be noted by marking the outside of the funnel. 9. The tap funnel can be removed and the tap closed before filling it with water to the first marking. 10. The tap should be opened over a graduated cylinder until the water level drops to the second marking. This volume should be noted. 11. The remainder of the water in the funnel should also be measured and total water in the funnel also recorded. 12. The first (smaller) water volume should be calculated as a percentage of the total water volume.

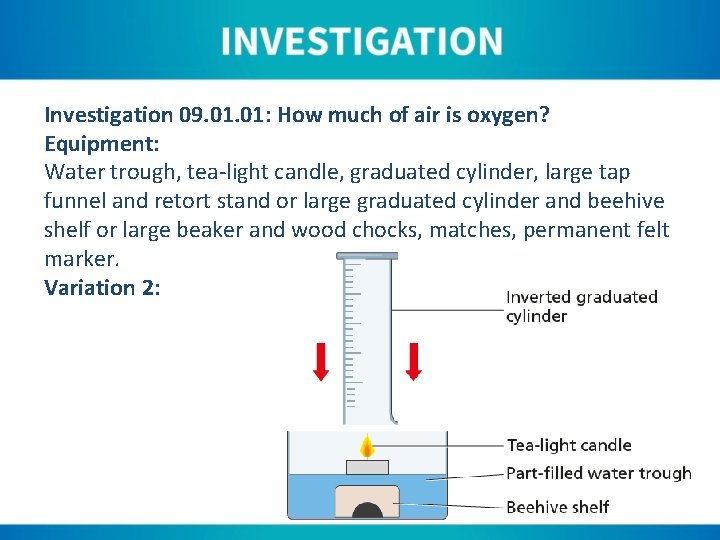
Investigation 09. 01: How much of air is oxygen? Equipment: Water trough, tea-light candle, graduated cylinder, large tap funnel and retort stand or large graduated cylinder and beehive shelf or large beaker and wood chocks, matches, permanent felt marker. Variation 2:

Instructions Variation 2: 1. Fill a water trough to between ⅓ and ½ its volume with tap water. 2. Place a beehive shelf at the bottom of the water trough. 3. Place an inverted large graduated cylinder over the beehive shelf and note the internal water level. 4. Light a tea-light candle and place it floating in the water trough. 5. The candle should be allowed to burn until a strong consistent flame can be seen (maximum of approximately three minutes). 6. The graduated cylinder should be lowered over the candle at a slight angle, taking care not to capsize the candle. The water levels should then equalise.

Instructions Variation 2: 7. Once the burning candle has consumed the available internal oxygen, the water level will rise. The new internal level should be noted. 8. Using the volume measurements noted from the graduated cylinder, the percentage of oxygen in air can be calculated.

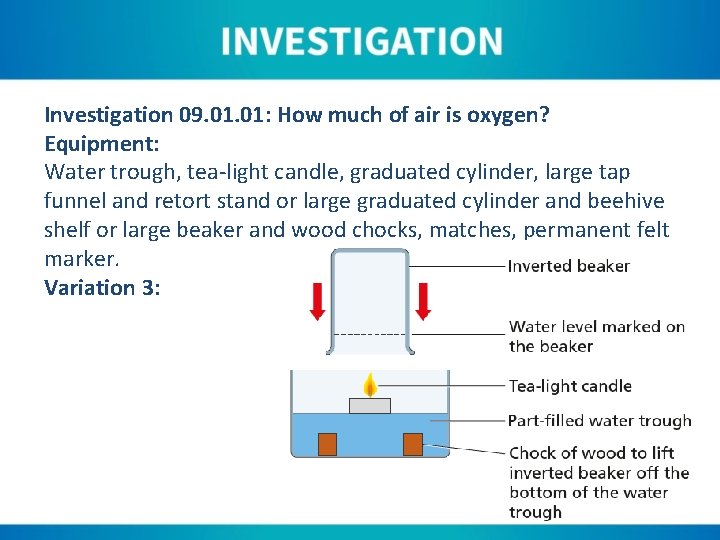
Investigation 09. 01: How much of air is oxygen? Equipment: Water trough, tea-light candle, graduated cylinder, large tap funnel and retort stand or large graduated cylinder and beehive shelf or large beaker and wood chocks, matches, permanent felt marker. Variation 3:

Instructions Variation 3: 1. Fill a water trough to between ⅓ and ½ its volume with tap water. 2. Place two wooden chocks on the base of the water trough so that they can support a large beaker. 3. Place an inverted large beaker cylinder over the beehive shelf and note the internal water level. 4. Light a tea-light candle and place it floating in the water trough. 5. The candle should be allowed to burn until a strong consistent flame can be seen (maximum of approximately three minutes). 6. The large beaker should be lowered over the candle at a slight angle, taking care not to capsize the candle. The water levels should then equalise.

Instructions Variation 3: 7. Once the burning candle has consumed the available internal oxygen, the water level will rise. The new internal level should be marked on the outside of the beaker. 8. The beaker can then be removed and filled with water to the first marking. 9. The beaker should be emptied until the water level drops to the second marking. This volume should be noted. 10. The remainder of the water in the beaker should also be measured and total water in the beaker also recorded. 11. The first (smaller) water volume should be calculated as a percentage of the total water volume.

1. Can another source of flame instead of a candle be used for this investigation? Justify your answer. Candles are made from paraffin wax so are naturally waterproof and will not soak up any water to quench the flame. A candle will also float easily on water. A wide candle could also be cut to a thick disc shape and used instead of a tea-light. A small ‘boat’ of tinfoil could be used to burn some other material, but this may become swamped when covering it.

2. Could the size or shape of the candle, or other pieces of equipment, make a difference to the results of your investigation? No – the investigation is testing the volume of air inside the container that is left after displacement by any solids such as the candle. Regardless of the sizes used, there is a fixed proportion of oxygen available, so size becomes irrelevant.

3. Can you explain why the flame on the candle should be left lit for a few minutes before the upside-down container is placed over it? This allows the wax to melt sufficiently so it can then be burned, creating convection currents that draw in more air until the combustion has reached a self-sustaining temperature.

4. How can you stop water swamping the candle as you place the upside-down container over it? Regardless of the container used, it must be placed slowly over the candle to prevent capsizing and splashing if it rises unevenly within the container.

5. Can you think of a way to measure the amount of oxygen used by the flame? If not using a large graduated cylinder as the container, carefully marking the changing water levels on the outside of the container and measuring the different volumes with a graduated cylinder will work.

6. Suggest reasons why your measurement might not be accurate and how you could improve accuracy in this investigation? Allowing the candle to burn long enough should mean that the flame in the container consumes the oxygen quickly and at a higher temperature, so should combust better than if it was only just lit, as well as reducing the amount of smoke (partly combusted fuel). Practice will increase dexterity and accuracy also.

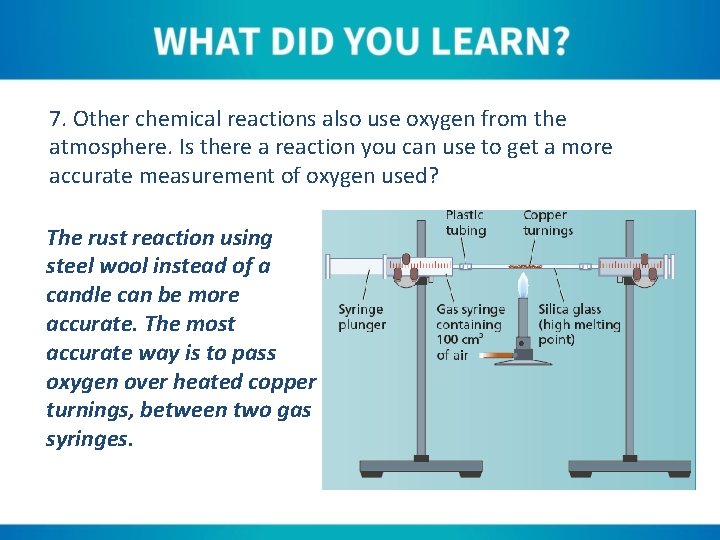
7. Other chemical reactions also use oxygen from the atmosphere. Is there a reaction you can use to get a more accurate measurement of oxygen used? The rust reaction using steel wool instead of a candle can be more accurate. The most accurate way is to pass oxygen over heated copper turnings, between two gas syringes.

8. In your opinion, is the percentage of oxygen in air always the same? Justify your answer. No – oxygen in a room can be used up if there is no ventilation. This and the build-up of exhaled carbon dioxide leads to room becoming ‘stuffy’.

(a) Firefighters use water in most situations. Which factor in the fire triangle does using water remove? Heat. (b) Oil-well firefighters use explosives in the ‘blowout’ technique to extinguish oil-well fires. This is similar to blowing out birthday candles. Which factor in the fire triangle does this remove? Oxygen, because the air moves so fast past the flame it cannot use the oxygen for burning.

(c) Which factor is the most difficult to remove from a fire? Explain your answer. Fuel. It isn’t always possible to separate fuel from the heat or oxygen if it is one mass, e. g. a large log or a pool of liquid or gas.

Characteristic Test: A chemical test that identifies a specific substance and no other. Gas Test Characteristic Result Oxygen Place a glowing wooden splint into gas Splint relights Hydrogen Place a burning wooden splint into gas Burns with a pop Carbon dioxide Pass through limewater Turns limewater milky-white

You are given three gas jars labelled X, Y and Z. You are told that a different gas has been placed in each gas jar. The three gases are oxygen, carbon dioxide and nitrogen. You have enough limewater to test all three gases, but only two wooden splints, so you can only test two gases with these. How would you carry out an investigation to identify the three gases? Test two gas jars with glowing splints. If one relights, oxygen has been identified. If neither relights the untested gas jar is oxygen. The two gas jars can then be tested with limewater to distinguish between carbon dioxide and nitrogen. The one that does not turn limewater milky is nitrogen.

Law of Conservation of Mass: Matter can be neither created nor destroyed but rather converted from one form to another.

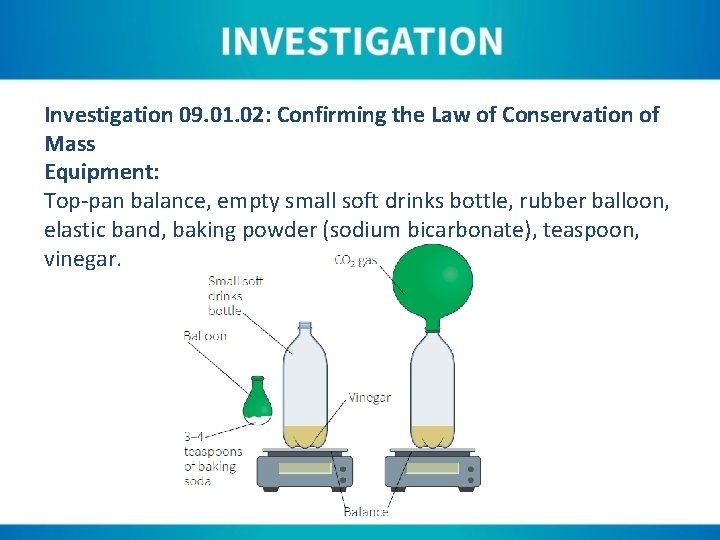
Investigation 09. 01. 02: Confirming the Law of Conservation of Mass Equipment: Top-pan balance, empty small soft drinks bottle, rubber balloon, elastic band, baking powder (sodium bicarbonate), teaspoon, vinegar.

Instructions: 1. Place 50 cm 3 of vinegar in a clean, dry soft drinks bottle. 2. Stretch the rubber balloon several times and then add two– three teaspoons of vinegar to it. 3. Place the bottle, balloon and elastic band onto the top-pan balance. Note the total mass. 4. Stretch the neck of the balloon over the neck of the bottle, and fix in place with the elastic band. 5. Lift the bottom of the balloon up to tip the baking powder into the bottle. 6. Allow the reaction to continue until no more gas is produced (bubbling stops). 7. Note the total mass.

1. How could you identify this gas? Test with limewater first. If it does not give a positive test, testing with a glowing splint will establish if it is oxygen or not.

2. Is this a good method to demonstrate the Law of Conservation of Mass? Justify your answer. Yes – the equipment is simple and easy to use, and it is possible to measure changes in state of matter without losing any gas.

3. Can you think of other substances that could be used to prove the Law of Conservation of Mass with a different gas? Hydrogen peroxide can be substituted for vinegar, and a small amount of manganese dioxide for the baking powder, to produce oxygen using the same equipment.

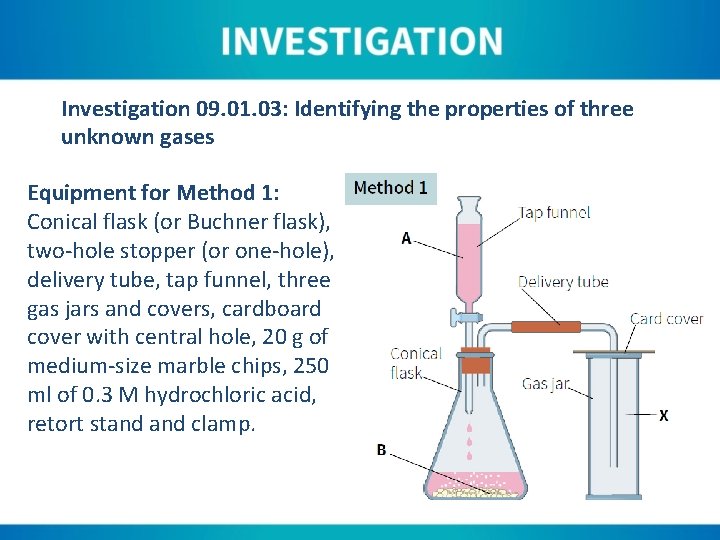
Investigation 09. 01. 03: Identifying the properties of three unknown gases Equipment for Method 1: Conical flask (or Buchner flask), two-hole stopper (or one-hole), delivery tube, tap funnel, three gas jars and covers, cardboard cover with central hole, 20 g of medium-size marble chips, 250 ml of 0. 3 M hydrochloric acid, retort stand clamp.

Instructions for Method 1: 1. Add the marble chips to the flask. 2. Seal the flask with the stopper and make sure the tap of the funnel is closed. 3. Clamp the flask at the neck to prevent toppling. 4. Fill the reservoir of the tap funnel. 5. Connect the delivery tube to the flask and pass through the cover of the gas jar. 6. Allow approximately half the volume of the tap funnel to run into the flask, making sure to close the tap after doing this.

Instructions for Method 1: 7. Allow the reaction to continue for ninety seconds before replacing the first gas jar with another. 8. Test the gas by adding a small amount of limewater and shaking. 9. The second gas jar can be tested with limewater. Also test with a lighted splint and a glowing splint.

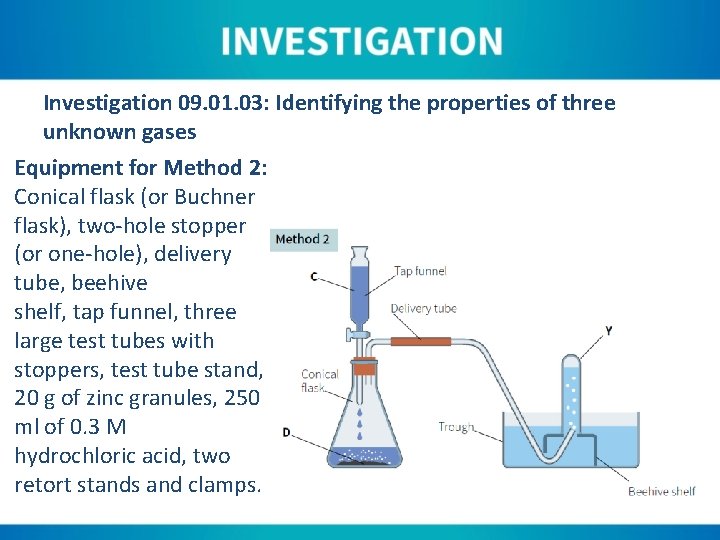
Investigation 09. 01. 03: Identifying the properties of three unknown gases Equipment for Method 2: Conical flask (or Buchner flask), two-hole stopper (or one-hole), delivery tube, beehive shelf, tap funnel, three large test tubes with stoppers, test tube stand, 20 g of zinc granules, 250 ml of 0. 3 M hydrochloric acid, two retort stands and clamps.

Instructions for Method 2: 1. Two-thirds fill the water trough and place a beehive shelf in the centre of the trough. 2. Place the delivery tube so that it runs from the flask and up through the beehive shelf. The tube should only slightly protrude above the top of the beehive shelf. 3. Fill the test tubes with water, by slowly immersing in the water at an angle. This allows all the air to escape. 4. Invert one test tube and place it over the centre of the beehive shelf. Secure by clamping in a retort stand. 5. Allow approximately half the volume of the tap funnel to run into the flask, making sure to close the tap after doing this.

Instructions for Method 2: 6. Allow the reaction to continue for approximately thirty seconds as this allows the air in the system to be expelled. Air will bubble out from under the test tube/beehive shelf as this happens. 7. Stopper the test tube under the water level and substitute with another test tube. 8. Test the filled test tube by removing the stopper and quickly placing a lit splint into the mouth of the tube. Also test with limewater and a glowing splint.

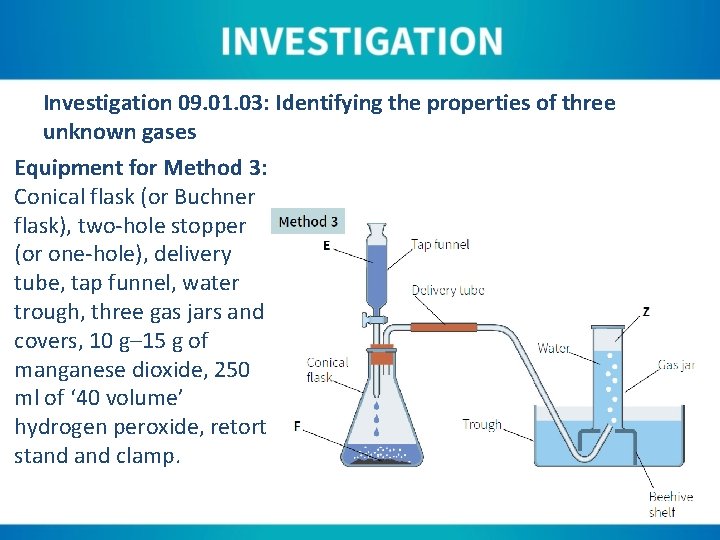
Investigation 09. 01. 03: Identifying the properties of three unknown gases Equipment for Method 3: Conical flask (or Buchner flask), two-hole stopper (or one-hole), delivery tube, tap funnel, water trough, three gas jars and covers, 10 g– 15 g of manganese dioxide, 250 ml of ‘ 40 volume’ hydrogen peroxide, retort stand clamp.

Instructions for Method 3: 1. Two-thirds fill the water trough and place a beehive shelf in the centre of the trough. 2. Place the delivery tube so that it runs from the flask and up through the beehive shelf. The tube should only slightly protrude above the top of the beehive shelf. 3. Fill the gas jars with water by slowly immersing in the water at an angle. This allows all the air to escape. 4. Invert one gas jar and place it over the centre of the beehive shelf. 5. Allow approximately half the volume of the tap funnel to run into the flask, making sure to close the tap after doing this.

Instructions for Method 3: 6. Allow the reaction to continue for approximately ninety seconds as this allows the air in the system to be expelled. Air will bubble out from under the gas jar/beehive shelf as this happens. 7. Cover the gas jar under water so that no gas is lost. 8. Test the gas with a glowing splint. Also test with limewater and a burning splint.

1. How can you prove which gas is heavier than air? Compare the mass of a sealed container of each gas with the mass of a sealed container of air. Alternatively, a rubber balloon could be filled with each gas and then dropped to see which falls through the air.

2. Should you rely on the first sample of each gas that you collect? Explain your answer. No, as it may be contaminated with air from the apparatus. The second sample should also be tested to confirm the initial result.

3. Does solid F get used up? Why do you think this happens? Solid F does not get used up. It is a catalyst and is there to speed up the reaction by reducing the activation energy without getting used up itself.

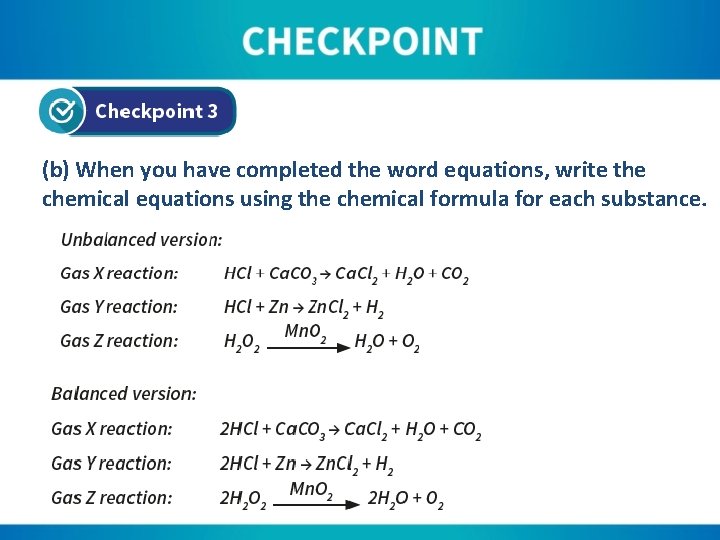
(a) Using the information above and the information given to you by your teacher, write a word equation for each gas production reaction: Gas X reaction: liquid A + solid B → salt + water + gas X Gas X reaction: hydrochloric acid + marble chips → calcium chloride + water + carbon dioxide Gas Y reaction: liquid C + solid D → salt + gas Y Gas Y reaction: hydrochloric acid + zinc → zinc chloride + hydrogen Gas Z reaction: liquid E Solid F water + gas Z manganese dioxide Gas Z reaction: hydrogen peroxide water + oxygen

(b) When you have completed the word equations, write the chemical equations using the chemical formula for each substance.

LIGHTBULB QUESTION Air feels ‘heavy’ because increased humidity makes it more difficult to breathe, requiring more muscle effort, therefore giving the impression that the air is heavier.

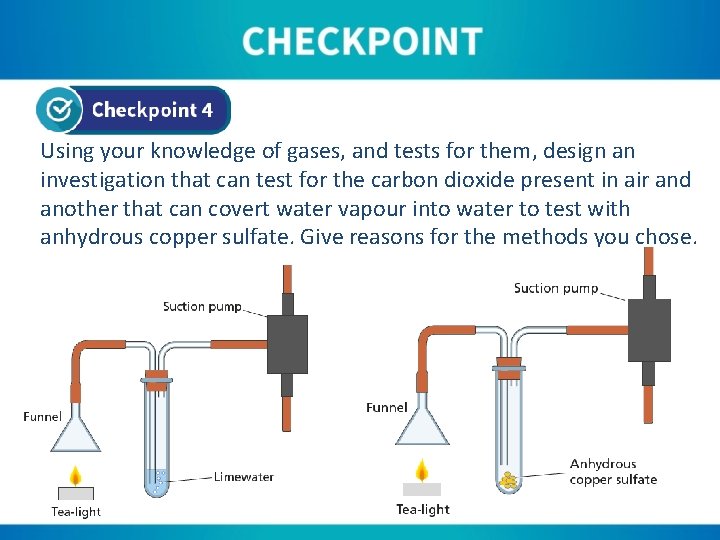
Using your knowledge of gases, and tests for them, design an investigation that can test for the carbon dioxide present in air and another that can covert water vapour into water to test with anhydrous copper sulfate. Give reasons for the methods you chose.

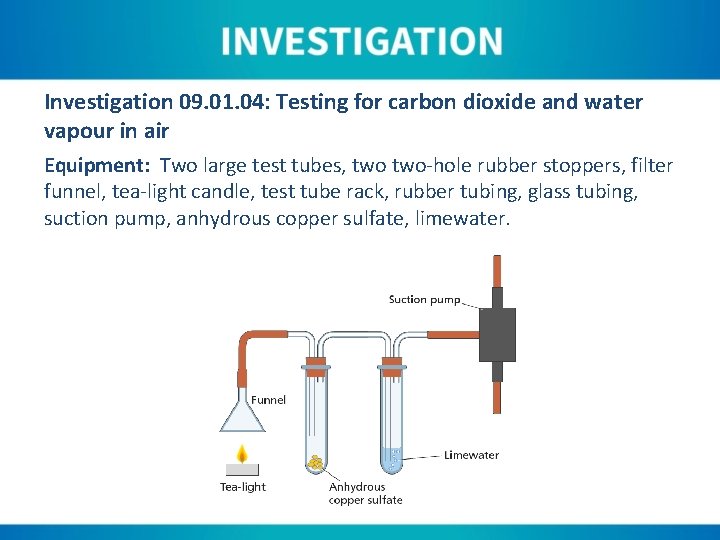
Investigation 09. 01. 04: Testing for carbon dioxide and water vapour in air Equipment: Two large test tubes, two-hole rubber stoppers, filter funnel, tea-light candle, test tube rack, rubber tubing, glass tubing, suction pump, anhydrous copper sulfate, limewater.

Instructions: 1. Light the tea-light candle and allow to burn for several minutes. 2. Set up the apparatus as shown, making sure that the anhydrous copper sulfate is placed in a clean, dry test tube. 3. The test tubes can be placed in a test tube rack (or set up in clamps on a retort stand). 4. Make sure that the suction pump is securely attached to a tap. 5. Turn on the tap fully to ensure that air is sucked through the apparatus. 6. Place the candle under the funnel, and observe any changes that occur.

1. If you repeat the investigation again, will you get the same results? Justify your answer. If the same candle is used and the test reagents are refreshed, the results should be the same, unless contaminated.

2. Can you think of how to combine these two tests into one equipment set-up? If the two test tubes are linked, air could be sucked through.

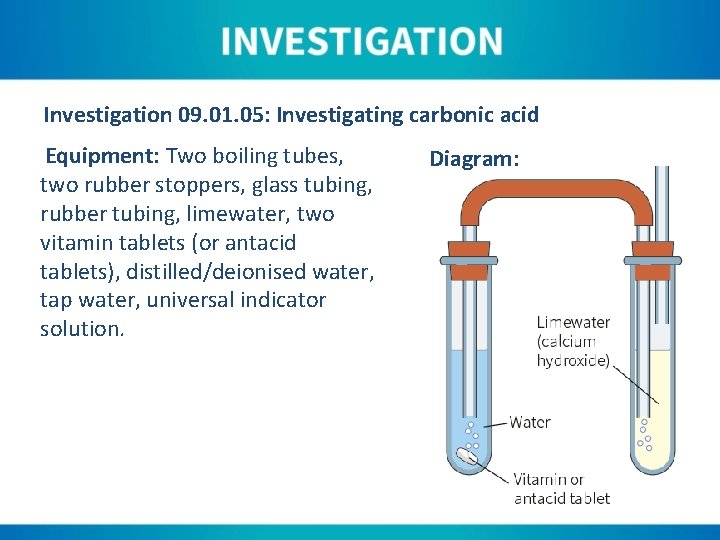
Investigation 09. 01. 05: Investigating carbonic acid Equipment: Two boiling tubes, two rubber stoppers, glass tubing, rubber tubing, limewater, two vitamin tablets (or antacid tablets), distilled/deionised water, tap water, universal indicator solution. Diagram:

Instructions: 1. Half-fill one boiling tube with fresh limewater and place the stopper as shown. 2. Half-fill the other boiling tube with tap water, add one of the tablets and seal with a stopper. 3. Record your observations. 4. Clean and reset the apparatus. 5. Replace the limewater with deionised water, and add three drops of universal indicator solution. Stopper and shake gently to mix thoroughly. 6. Add the tablet to the tap water and seal with a stopper. 7. Record your observations.

1. Is using a vitamin tablet (or antacid tablet) a useful way to produce carbon dioxide gas? Justify your answer. It is useful to produce small amounts for simple testing, but would be impractical and expensive as a large number of tablets would be needed. 2. What does the second part of the experiment tell you about carbon dioxide? Carbon dioxide is acidic when mixed in water.

3. Would using deionised water instead of tap water to dissolve the tablet make a difference to the reaction? It would not make any noticeable difference as soluble tablets all contain powdered ascorbic acid, which is watersoluble. This dissolves the calcium carbonate in the tablets, releasing carbon dioxide (this is the neutralisation reaction of a carbonate base).

4. Can you name another solid and liquid that could be used to produce carbon dioxide? Vinegar and baking powder or hydrochloric acid any calcium carbonate source such as marble, crushed limestone or chalk powder.

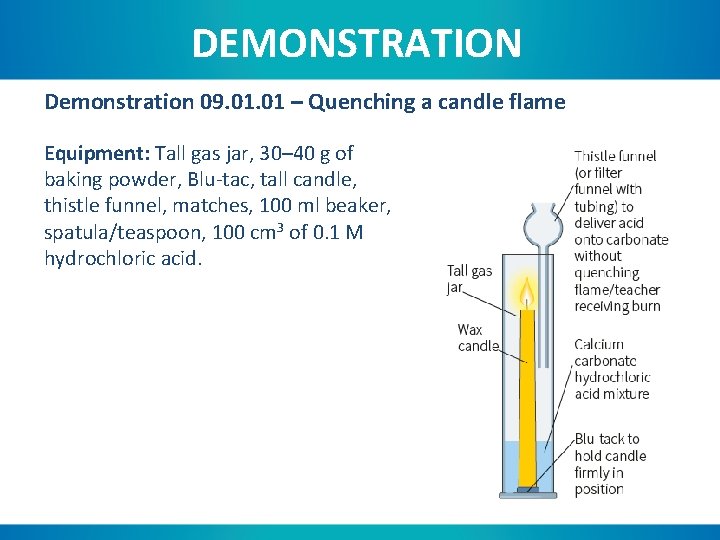
DEMONSTRATION Demonstration 09. 01 – Quenching a candle flame Equipment: Tall gas jar, 30– 40 g of baking powder, Blu-tac, tall candle, thistle funnel, matches, 100 ml beaker, spatula/teaspoon, 100 cm 3 of 0. 1 M hydrochloric acid.

DEMONSTRATION Instructions: 1. Use some Blu-tac to fix the candle in place. 2. Add the baking powder around the base of the candle. 3. Light the candle and allow to burn for several minutes to establish a strong flame. 4. Using the thistle funnel, add the hydrochloric acid to the gas jar.

1. If the reaction mixture started producing carbon dioxide almost immediately, why did it take so long for the flame to die out? Carbon dioxide is more dense than air so has to build up within the gas jar. 2. If the reaction was producing oxygen, what would happen, and would it happen faster than if carbon dioxide was being produced? Justify your answer. Oxygen would float to the top of the gas jar and the candle flame would burn more strongly. This could be proven by substituting approximately 10 g of manganese dioxide for the baking powder, and 20 volume hydrogen peroxide for the hydrochloric acid.

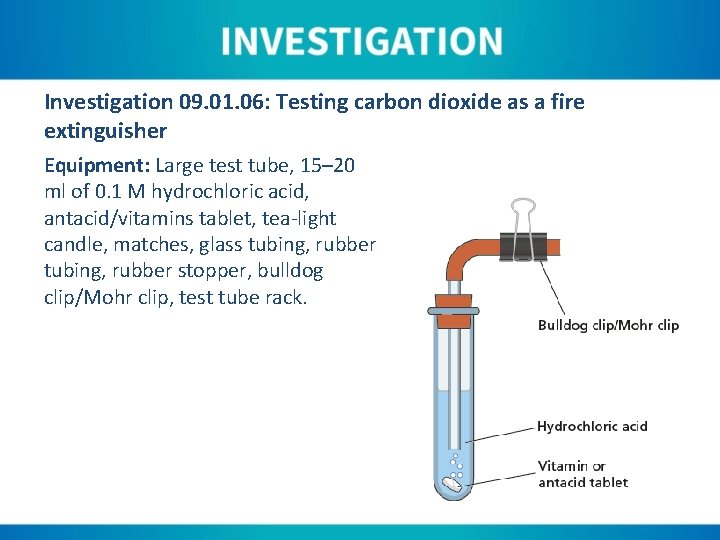
Investigation 09. 01. 06: Testing carbon dioxide as a fire extinguisher Equipment: Large test tube, 15– 20 ml of 0. 1 M hydrochloric acid, antacid/vitamins tablet, tea-light candle, matches, glass tubing, rubber stopper, bulldog clip/Mohr clip, test tube rack.

Instructions: 1. Light the tea-light candle and allow to establish a strong flame. 2. Place the hydrochloric acid in the test tube. 3. Attached the clip to the rubber tubing. 4. Add the antacid to the test tube and stopper immediately. 5. Wait forty-five seconds, then place the end of the rubber tubing near the flame. 6. Remove the clip and observe what happens.

1. Suggest the factors that could affect how well carbon dioxide works as a fire extinguisher. The volume and concentration of acid; the amount of calcium carbonate in the tablet. 2. What steps would you need to take to ensure that this was a fair test? Repeat the procedure with the same amounts and concentrations, and compare across several different types of vitamin or antacid tablets.

3. Would you recommend your design to a fire extinguisher company? Explain your answer. No – it requires acid to be used, which can be a danger itself.

Copy and Complete In this unit I learned that aerobic respiration is the release of energy from food in organisms by chemical reaction with oxygen. When heat energy is released from other chemicals by reacting with oxygen this is called combustion. Oxygen is produced by reacting hydrogen peroxide and manganese dioxide. The black powder used in this reaction is a catalyst so does not get used up. Oxygen causes a glowing splint to relight. One part of the fire triangle is oxygen; the other parts are fuel and heat. Hydrogen burns with a pop when a lighted splint is placed in it.

Copy and Complete This gas is produced when zinc metal is reacted with hydrochloric acid. A lighted splint is extinguished when placed in carbon dioxide gas. Hydrochloric acid and calcium carbonate react to give this gas. Marble, chalk/ limestone and baking powder all contain calcium carbonate. Carbon dioxide can be used for fire extinguishers, stage effects and fizzy drinks. This gas is absorbed by plants during photosynthesis to make food for the plants.

1. Complete the word equations below by writing a suitable ‘fuel’ for each one: Respiration: PASTA + oxygen HEAT carbon dioxide + water + energy Combustion: COAL + oxygen HEAT carbon dioxide + water + energy

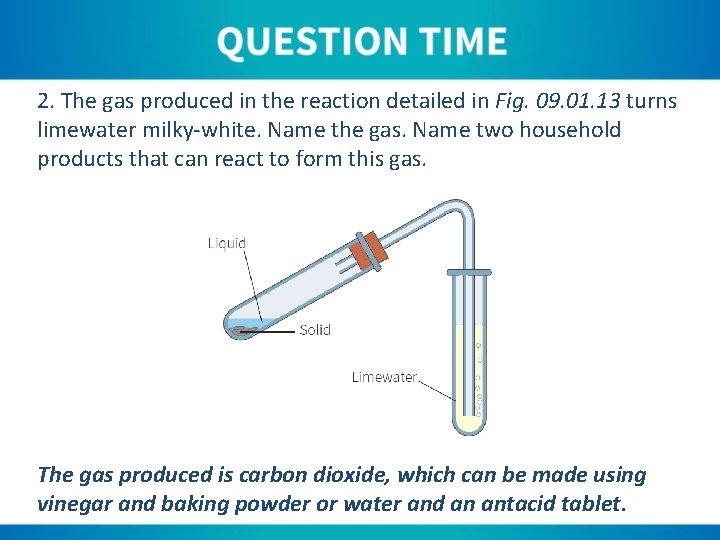
2. The gas produced in the reaction detailed in Fig. 09. 01. 13 turns limewater milky-white. Name the gas. Name two household products that can react to form this gas. The gas produced is carbon dioxide, which can be made using vinegar and baking powder or water and an antacid tablet.

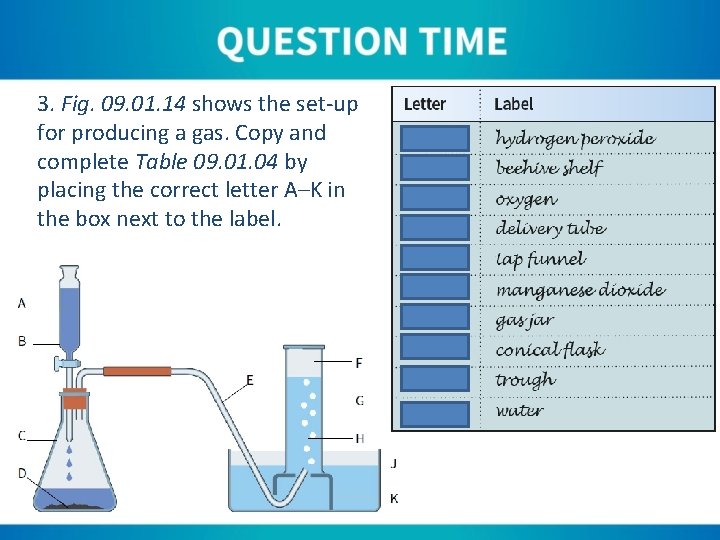
3. Fig. 09. 01. 14 shows the set-up for producing a gas. Copy and complete Table 09. 01. 04 by placing the correct letter A–K in the box next to the label.

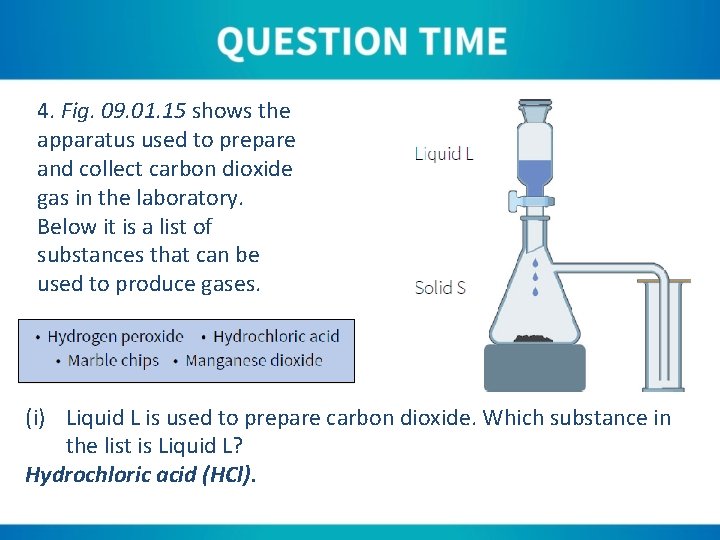
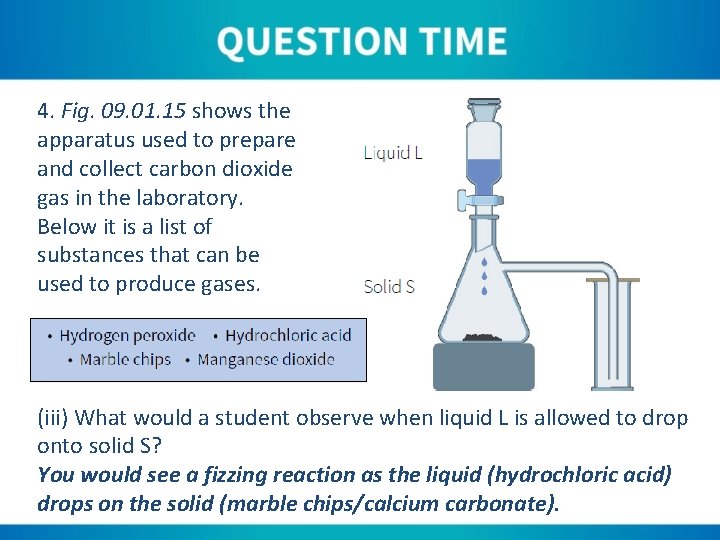
4. Fig. 09. 01. 15 shows the apparatus used to prepare and collect carbon dioxide gas in the laboratory. Below it is a list of substances that can be used to produce gases. (i) Liquid L is used to prepare carbon dioxide. Which substance in the list is Liquid L? Hydrochloric acid (HCl).

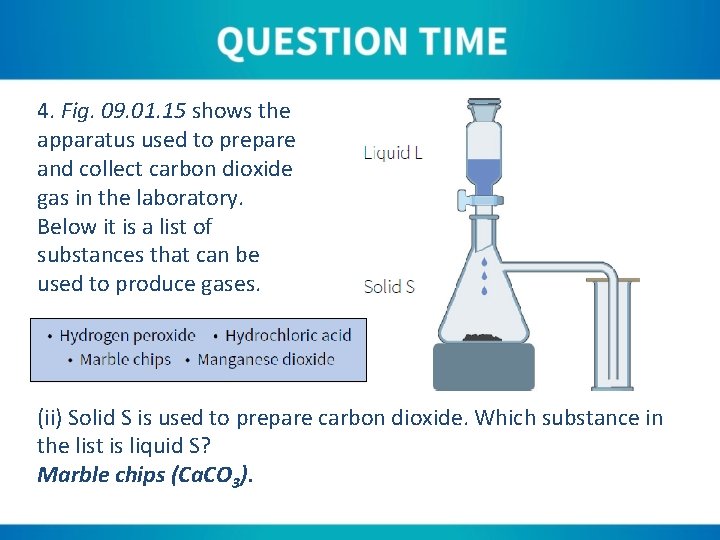
4. Fig. 09. 01. 15 shows the apparatus used to prepare and collect carbon dioxide gas in the laboratory. Below it is a list of substances that can be used to produce gases. (ii) Solid S is used to prepare carbon dioxide. Which substance in the list is liquid S? Marble chips (Ca. CO 3).

4. Fig. 09. 01. 15 shows the apparatus used to prepare and collect carbon dioxide gas in the laboratory. Below it is a list of substances that can be used to produce gases. (iii) What would a student observe when liquid L is allowed to drop onto solid S? You would see a fizzing reaction as the liquid (hydrochloric acid) drops on the solid (marble chips/calcium carbonate).

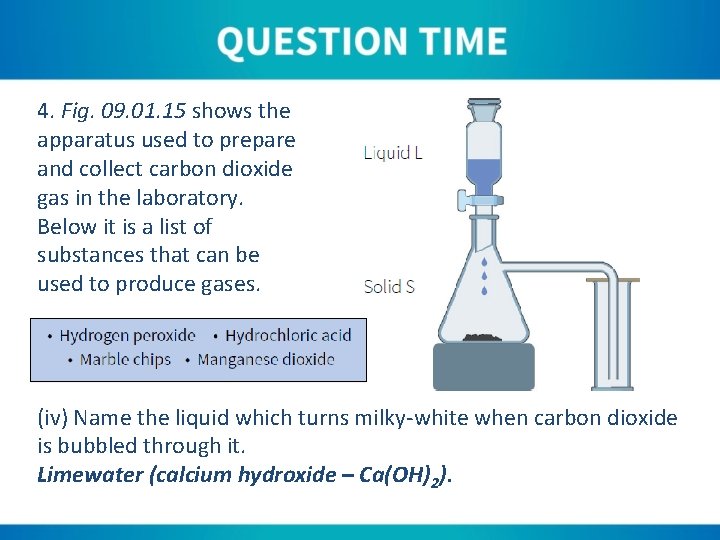
4. Fig. 09. 01. 15 shows the apparatus used to prepare and collect carbon dioxide gas in the laboratory. Below it is a list of substances that can be used to produce gases. (iv) Name the liquid which turns milky-white when carbon dioxide is bubbled through it. Limewater (calcium hydroxide – Ca(OH)2).

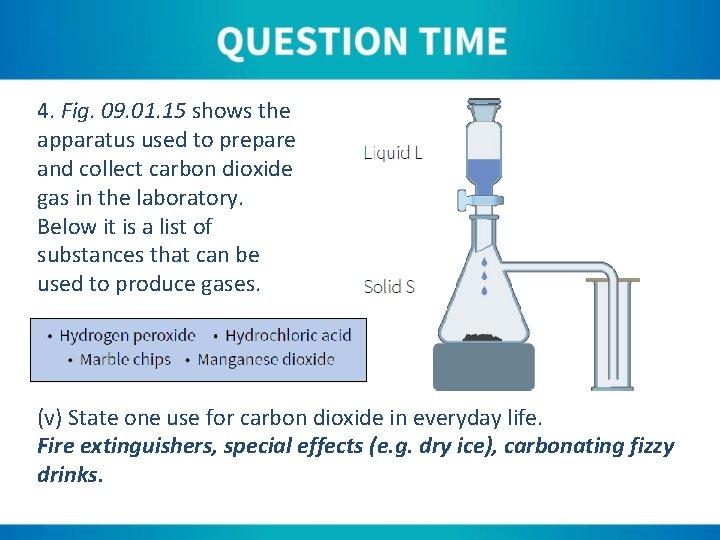
4. Fig. 09. 01. 15 shows the apparatus used to prepare and collect carbon dioxide gas in the laboratory. Below it is a list of substances that can be used to produce gases. (v) State one use for carbon dioxide in everyday life. Fire extinguishers, special effects (e. g. dry ice), carbonating fizzy drinks.

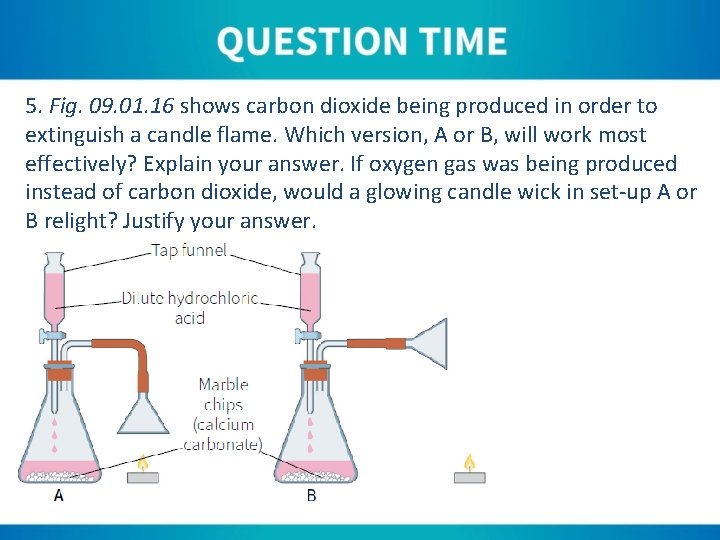
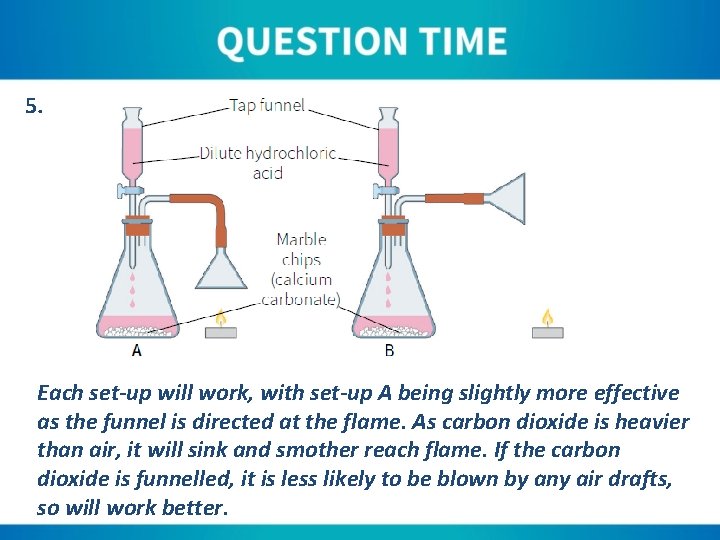
5. Fig. 09. 01. 16 shows carbon dioxide being produced in order to extinguish a candle flame. Which version, A or B, will work most effectively? Explain your answer. If oxygen gas was being produced instead of carbon dioxide, would a glowing candle wick in set-up A or B relight? Justify your answer.

5. Each set-up will work, with set-up A being slightly more effective as the funnel is directed at the flame. As carbon dioxide is heavier than air, it will sink and smother reach flame. If the carbon dioxide is funnelled, it is less likely to be blown by any air drafts, so will work better.
 What are the three stages of passing
What are the three stages of passing Cross traffic is eliminated on controlled access expressway
Cross traffic is eliminated on controlled access expressway Chapter 24 milady review questions
Chapter 24 milady review questions Pressure-volume loop
Pressure-volume loop Stroke volume ejection fraction
Stroke volume ejection fraction Front end of compiler
Front end of compiler Explain front end and back end of compiler in detail
Explain front end and back end of compiler in detail Feride kröpil
Feride kröpil End-to-end wireframe parsing
End-to-end wireframe parsing End to end argument in system design
End to end argument in system design End to end accounting life cycle tasks
End to end accounting life cycle tasks Protect
Protect End to end delay
End to end delay End to end
End to end Comet knowledge graph
Comet knowledge graph Multiple procurement cycles
Multiple procurement cycles Unit 6 review questions
Unit 6 review questions Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa sống lại
Chúa sống lại Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Số nguyên tố là số gì
Số nguyên tố là số gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi You say you love the rain
You say you love the rain Eat meals that are nutritious agree or disagree
Eat meals that are nutritious agree or disagree If you think you can you can poem
If you think you can you can poem Tell me what you eat and i shall tell you what you are
Tell me what you eat and i shall tell you what you are I will always follow you whenever and wherever you go i
I will always follow you whenever and wherever you go i Volleyball score sheet example filled out
Volleyball score sheet example filled out How do you end an email to a teacher
How do you end an email to a teacher The end and thank you images
The end and thank you images End behavior of a graph example
End behavior of a graph example How would you describe the end behavior of the graph
How would you describe the end behavior of the graph How would you describe the end behavior of the graph
How would you describe the end behavior of the graph If you whirl a tin can on the end of a string
If you whirl a tin can on the end of a string What do you do last sunday
What do you do last sunday By the end of this lesson you will be able to
By the end of this lesson you will be able to Bahamian pledge
Bahamian pledge Define career portfolio
Define career portfolio Room service account
Room service account Polyatomic ions list with charges
Polyatomic ions list with charges I think you should be more specific here in step two
I think you should be more specific here in step two Chapter 9 drivers ed
Chapter 9 drivers ed To steer straight forward look
To steer straight forward look Unlike routine claims, persuasive claims:
Unlike routine claims, persuasive claims: