At the end of this unit you should
































- Slides: 49


At the end of this unit you should: 1. Understand the relationship between pressure, force and area; perform simple calculations using this relationship. 2. Be able to explain the relationship between pressure and depth for a liquid. 3. Be able to explain that air has mass and occupies space. 4. Understand that the atmosphere exerts pressure and that atmospheric pressure varies with height. 5. Be able to examine weather charts to observe variations in atmospheric pressure and relate these to weather conditions.

aneroid barometer mercury barometer atmospheric pressure pascal barometer pressure

LIGHTBULB QUESTION The single nail would hurt more because there is a smaller surface area and therefore a greater force is exerted on the nail, whereas your weight is spread across the bed of nails and as such each nail only experiences a small amount of the force.

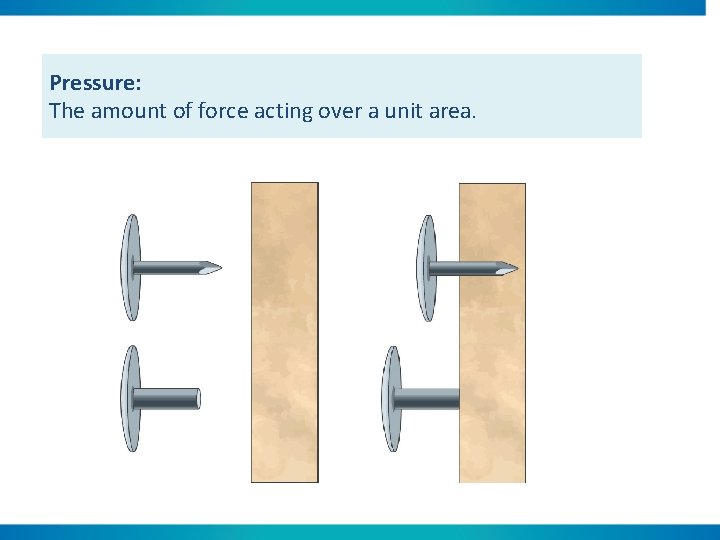
Pressure: The amount of force acting over a unit area.

(a) Try pushing your finger against the desk. Now, using the same amounts of force, push your fist against the desk. What did you notice? Which hurt more? If we want to create big pressures, we need to exert big force on as small an area as possible. Did you notice that if you used the same pressure, it would have hurt your finger more (due to smaller surface area – just like the bed of nails). You were able to apply more force onto the desk using your fist.

(b) Which do you think exerts the greater pressure: (i) An elephant’s foot or high heels? The high heels do, due to the smaller area. However, it does depend on the weight of the person in the heels and the weight and size of the foot of the elephant, but in general the high heels will exert the greater pressure.

(b) Which do you think exerts the greater pressure: (ii) A thumbtack exerting 5 N or the same thumbtack exerting 4 N? The thumbtack exerting 5 N, as it is a larger force over the same area.

(a) What is pressure? Pressure is the force per unit area. (b) What is the unit of pressure? Pascals. (c) Name another unit of pressure. Newton/metres 2.

(d) Which exerts the greatest pressure on a road surface: a bicycle tyre or a car tyre? The bicycle tyre does because it has a smaller surface area. However, the weight of the cyclist needs to be taken into account, but this generally holds true.

(e) Calculate the following: (i) The pressure of a 50 N block with an area of 10 m 2. P = 50/10 P = 5 Pa

(e) Calculate the following: (ii) The pressure of a 75 N block with an area of 100 m 2. P = 75/100 P = 0. 75 Pa

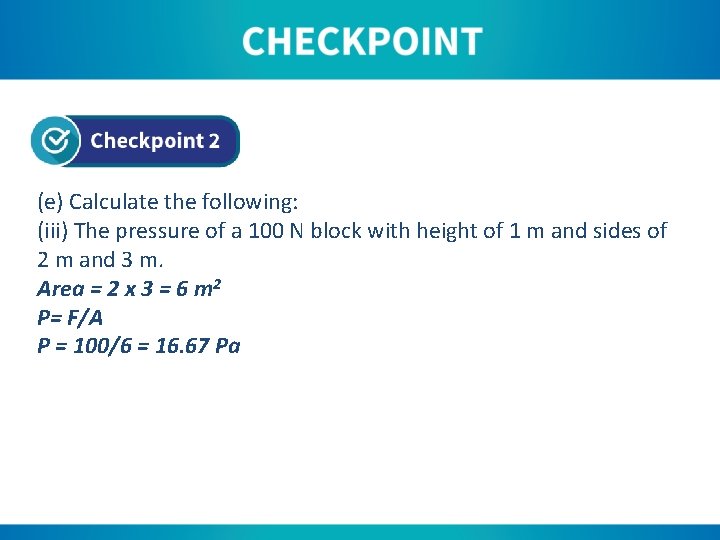
(e) Calculate the following: (iii) The pressure of a 100 N block with height of 1 m and sides of 2 m and 3 m. Area = 2 x 3 = 6 m 2 P= F/A P = 100/6 = 16. 67 Pa

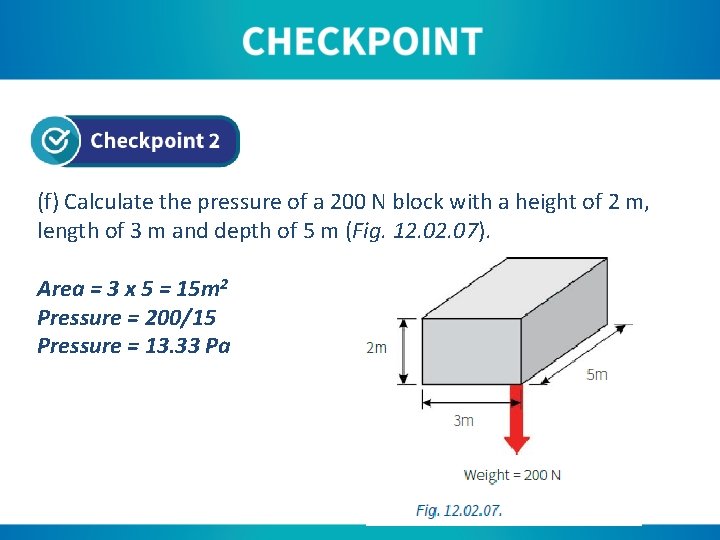
(f) Calculate the pressure of a 200 N block with a height of 2 m, length of 3 m and depth of 5 m (Fig. 12. 07). Area = 3 x 5 = 15 m 2 Pressure = 200/15 Pressure = 13. 33 Pa

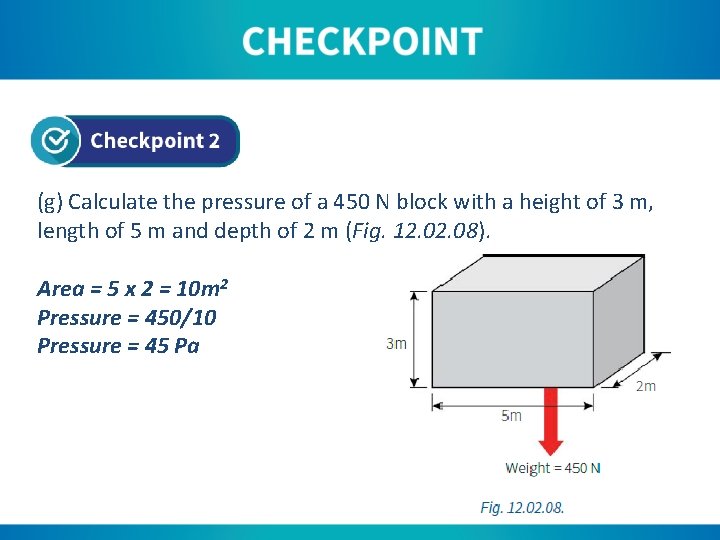
(g) Calculate the pressure of a 450 N block with a height of 3 m, length of 5 m and depth of 2 m (Fig. 12. 08). Area = 5 x 2 = 10 m 2 Pressure = 450/10 Pressure = 45 Pa

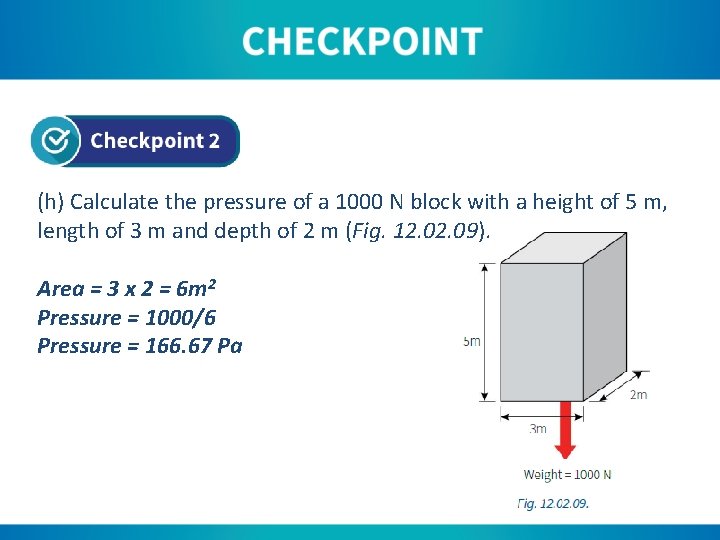
(h) Calculate the pressure of a 1000 N block with a height of 5 m, length of 3 m and depth of 2 m (Fig. 12. 09). Area = 3 x 2 = 6 m 2 Pressure = 1000/6 Pressure = 166. 67 Pa

Investigation 12. 01: Pressure in a liquid Equipment: Empty 2 -litre bottle, water, pin, sink/basin.

Investigation 12. 01: Pressure in a liquid Equipment: Empty 2 -litre bottle, water, pin, sink/basin. Instructions: 1. Fill a 2 -litre bottle with water. 2. Using a compass or other sharp object, puncture three equal holes vertically down the bottle (ensure these holes are punched in a straight line). 3. Observe what happens.

1. What observations did you make during this investigation? The water ‘ran out’ of the bottle much quicker at the bottom of the bottle than at the top.

2. Can you describe a pattern/relationship from your observations? As the depth increases, the pressure in the liquid increases. This is why divers can only go a certain distance under water safely. If they descend too much, they risk blowing their ear drums or collapsing a lung due to the immense pressure of the water above them.

Investigation 12. 02: Horizontal pressure in a liquid Equipment: Empty 2 -litre bottle, cap, water, pin, sink/basin. Instructions: Using a different bottle, or covering up the holes of the first bottle, fill it up again with water, ensuring to put the cap on the top this time. Place the bottle on its side. Uncover the holes or make new ones in exactly the same place as in Investigation 12. 01.

1. What observations did you make this time? The water ‘ran out’ of the bottle at the same rate through each hole.

2. How do your observations compare to those made in Investigation 12. 01? While in Investigation 12. 01 the pressure of the water increased in depth, in this investigation there is no increase in depth and therefore no increase in pressure.

3. Can you describe a pattern/relationship for the pressure horizontally in a liquid? The pressure horizontally across a liquid is at a constant pressure.

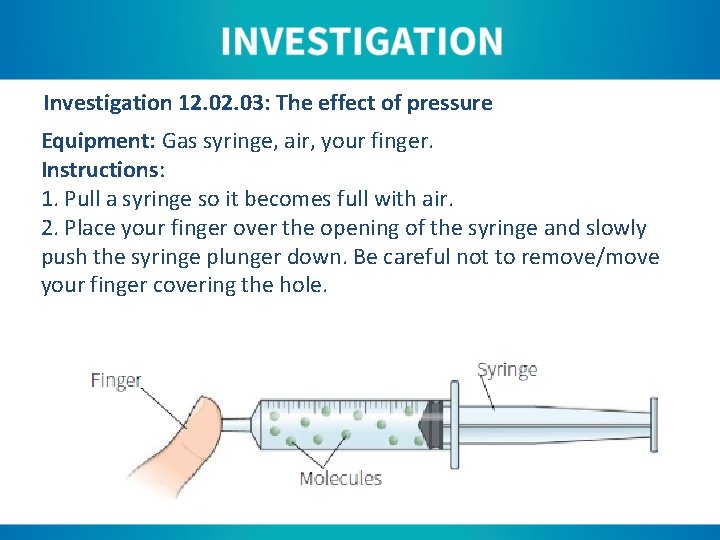
Investigation 12. 03: The effect of pressure Equipment: Gas syringe, air, your finger. Instructions: 1. Pull a syringe so it becomes full with air. 2. Place your finger over the opening of the syringe and slowly push the syringe plunger down. Be careful not to remove/move your finger covering the hole.

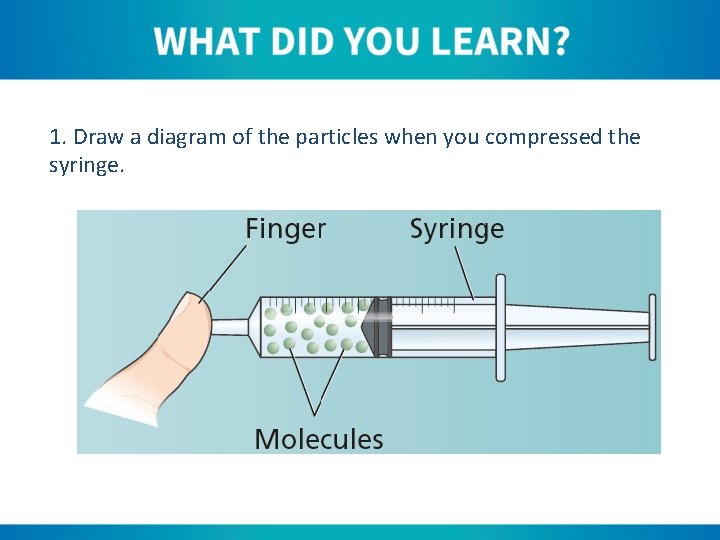
1. Draw a diagram of the particles when you compressed the syringe.

2. Hypothesise what the effect on the temperature is as a result of compressing the air in the syringe. As the pressure increases, the temperature increases. This is because the particles are now closer together and collide more frequently. This increase in collisions means that the energy is increasing inside the syringe and this leads to an increase in temperature.

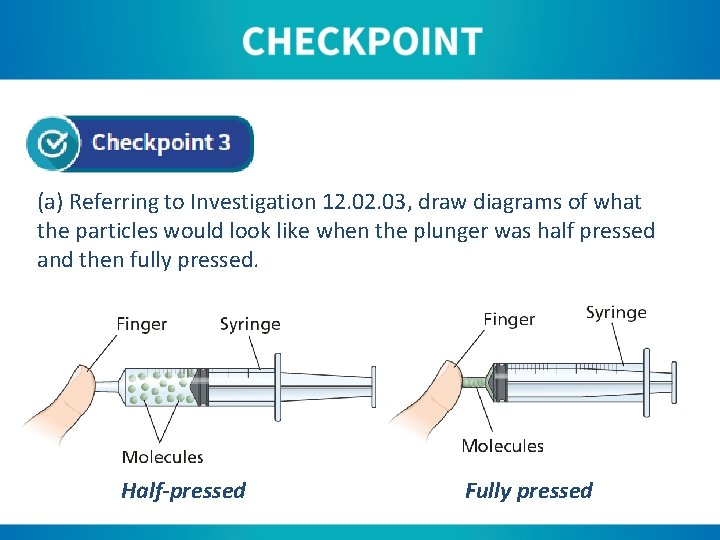
(a) Referring to Investigation 12. 03, draw diagrams of what the particles would look like when the plunger was half pressed and then fully pressed. Half-pressed Fully pressed

(b) Was there a change in temperature? If yes, how do you know? Yes, there should be a change. It may not be detectable by hand but we know because the particles are colliding more often and this leads to an increase in energy.

(c) From your observations, do you think that a relationship exists between pressure and temperature? Yes, a relationship does exist. The pressure is directly proportional to the temperature.

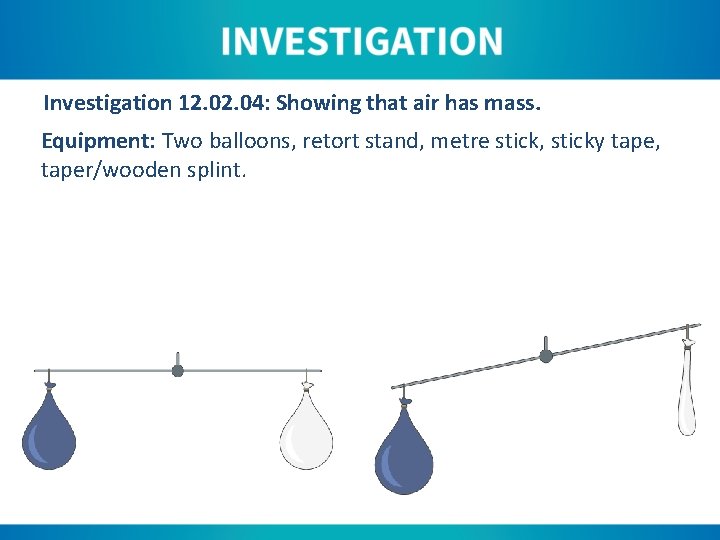
Investigation 12. 04: Showing that air has mass. Equipment: Two balloons, retort stand, metre stick, sticky tape, taper/wooden splint.

Investigation 12. 04: Showing that air has mass. Equipment: Two balloons, retort stand, metre stick, sticky tape, taper/wooden splint. Instructions: 1. Weigh both balloons. 2. Blow up one balloon and weigh it. 3. Balance a metre stick from a retort stand at its centre of gravity. 4. Place a balloon on each side of the metre stick and balance it. 5. Notice that the balloons do not balance on the same point of the metre stick. OR 1. Follow the steps as given except blow up both balloons with different masses of air.

1. How did you ensure this was a fair test? The ruler was hung at its centre of gravity, two similar balloons were used, air was used in one (or both) balloons and the scales was zeroed before any measurements were taken.

2. What precautions did you take to ensure the investigation was carried out safely? Safety googles and full PPE were worn.

3. What possible errors may have occurred during this investigation. Scales not zeroed before measurements taken, or the ruler not balanced.

Atmospheric Pressure: The force per unit area exerted by the weight of the column of air above us.

Investigation 12. 05: Investigating the accuracy of weather forecasting Equipment: Access to a weather channel or news broadcast. Instructions: Take note of the weather forecast tonight for tomorrow’s weather. See if we are to have high or low pressure, then observe the weather tomorrow.

Was the forecast accurate? Be sure to compare the forecast from at least two different sources, i. e. TV, radio, internet, and then comment on the accuracy of each source. You can also do this over a week and comment on the constancy of each source. You should find that the weather reporting is generally quite accurate, and that any changes to the reported weather can be attributed to changes in the Atlantic, which are generally not reported to the public unless there is a major storm or weather front approaching.

Copy and Complete In this unit I learned that pressure is the force per unit area. The unit of pressure is the pascal or the newton/metre 2. Area is a big factor in pressure. If we exert a force over a small area the resulting pressure will be larger than if the same force was applied over a large area. This is why thumbtacks and nails have sharp ends as this increases the pressure. In a liquid, the pressure is greatest at the bottom and smallest at the top. In general we can say the pressure in a liquid increases with depth. I also learned that atmospheric pressure is caused by the weight of the air above us. This means when we have low atmospheric pressure there is less air above us. Low atmospheric pressure gives us bad weather. High atmospheric pressure generally gives us good weather. Normal atmospheric pressure is 101 325 Pa.

1. Define pressure. The force per unit area.

2. What is the unit of pressure? Pascal.

3. A rectangular box of height 6 m and sides of 7 m and 8 m has a weight of 6000 N. Find the pressure it exerts. Area = 7 x 8 = 56 m 2 Pressure = 6000/56 P = 107. 14 Pa

4. Describe an investigation to show that the pressure in a liquid increases with depth. See Investigation 12. 01 Pressure in a liquid on page 363 of your textbook.

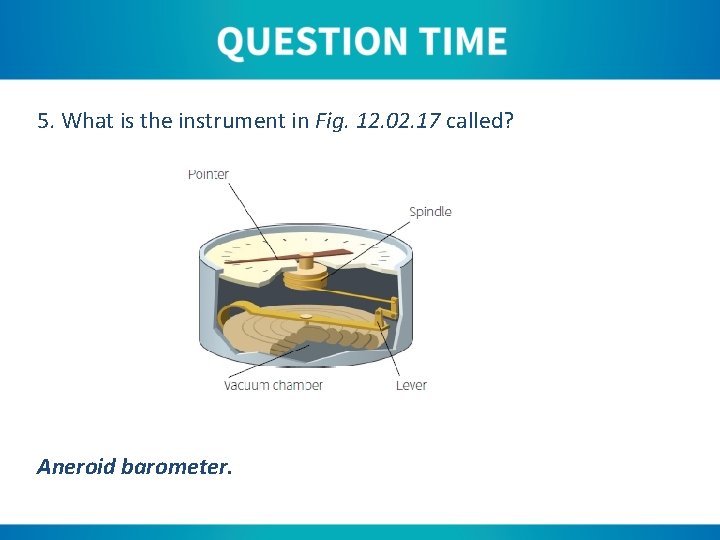
5. What is the instrument in Fig. 12. 02. 17 called? Aneroid barometer.

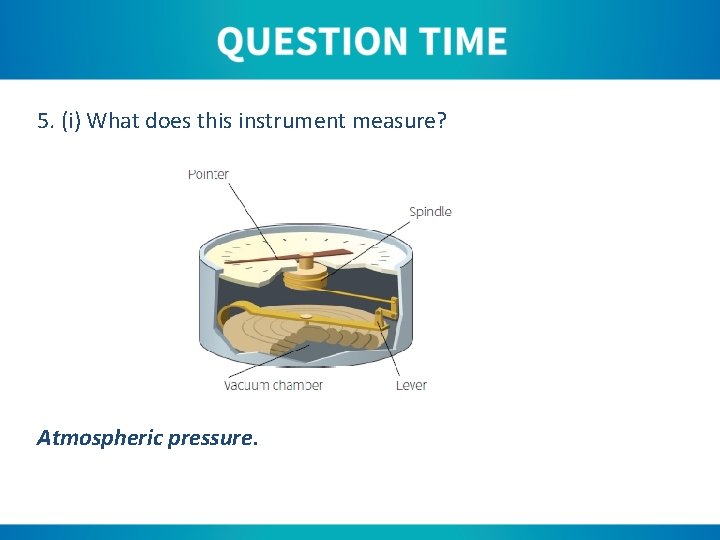
5. (i) What does this instrument measure? Atmospheric pressure.

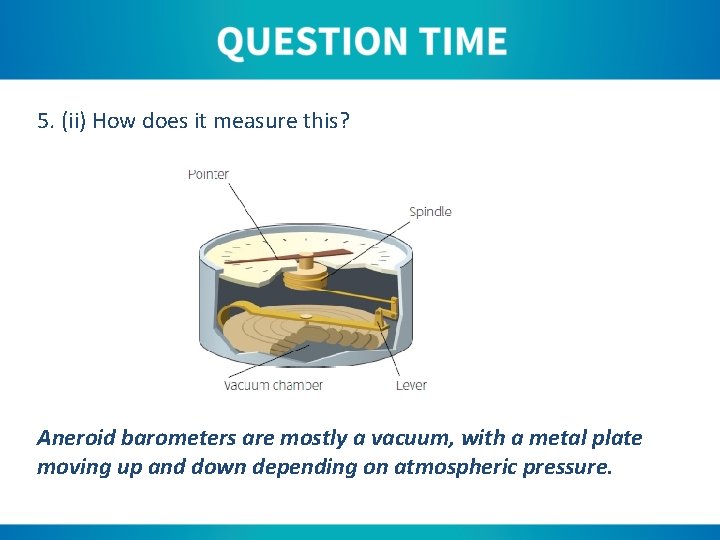
5. (ii) How does it measure this? Aneroid barometers are mostly a vacuum, with a metal plate moving up and down depending on atmospheric pressure.

6. Water boils at 100 o. C at sea level. Do you think water would boil at a higher or lower temperature on Mount Everest? Give reasons to support your answer. Water boils at a lower temperature on the top of Mount Everest because of the lower atmospheric pressure.

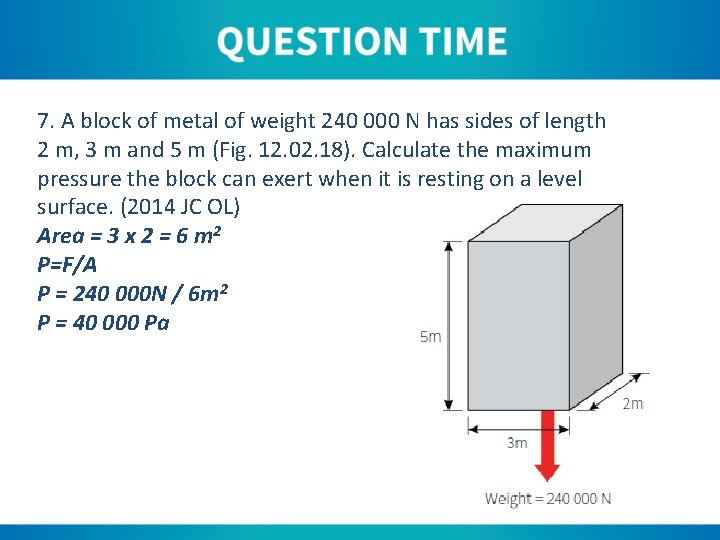
7. A block of metal of weight 240 000 N has sides of length 2 m, 3 m and 5 m (Fig. 12. 02. 18). Calculate the maximum pressure the block can exert when it is resting on a level surface. (2014 JC OL) Area = 3 x 2 = 6 m 2 P=F/A P = 240 000 N / 6 m 2 P = 40 000 Pa

8. Felix Baumgartner set the world record for skydiving when he jumped from an altitude of 39 km above the earth. Did Felix expect the atmospheric pressure to increase or decrease as he fell to earth? What effect would atmospheric pressure have had on his safety during re-entry? Make sure to factor in friction. (Adapted from 2014 JC HL) Felix would expect the atmospheric pressure to increase as he fell towards Earth. As the atmospheric pressure is increasing on the way down, Felix encounters more air. This increases the amount of friction his suit undergoes and might generate heat on his suit or inside his suit. Another issue that might be encountered is the change in pressure. If his pressurised suit wasn’t calibrated to normal atmospheric pressure, then his internal organs might collapse due to the increase in pressure.