World Organisation for Animal Health Seminar on the


































































- Slides: 71

World Organisation for Animal Health

Seminar on the Dialogue and Common Activities between the OIE Member Countries of the European Union and the other Member Countries of the OIE Regional Commission for Europe Ankara, Turkey, 21 -22 November 2005

Dr Dewan SIBARTIE OIE, Central Bureau, Head of the Regional Activities Department 21 -22 November 2005 Presentation of Objectives and Structure of the OIE and of the 4 th Strategic Plan 2006 -2010

The official name of the Organisation “World Organisation for Animal Health” adopted by the International Committee on May 2003

Plan Objectives Member Countries Structure International relations Information system International Standards Reference Laboratories – Collaborating Centres Publications and Website 4 th Strategic Plan of the OIE for 2006 -2010

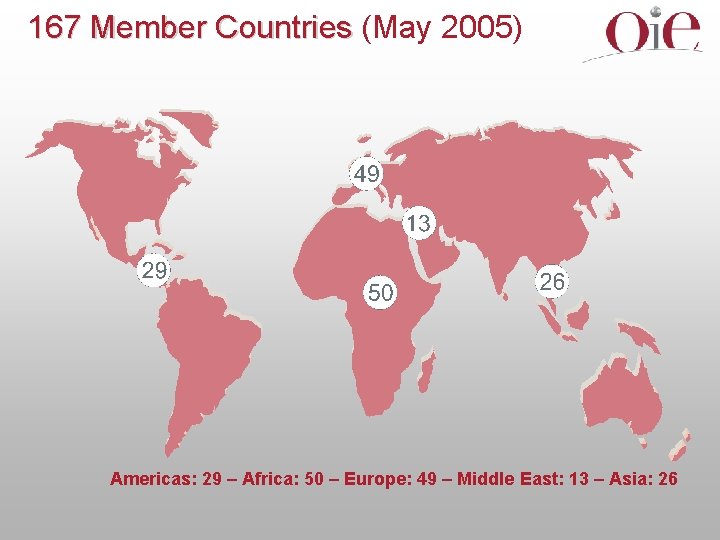
167 Member Countries (May 2005) Americas: 29 – Africa: 50 – Europe: 49 – Middle East: 13 – Asia: 26

OIE Objectives

Objectives 1. To ensure transparency in the global animal disease and zoonosis situation 2. To collect, analyse and disseminate scientific veterinary information 3. To provide expertise and encourage international solidarity in the control of animal diseases 4. Within its mandate under the WTO SPS Agreement, to safeguard world trade by publishing health standards for international trade in animals and animal products 5. To improve the legal framework and resources of national Veterinary Services 6. To provide a better guarantee of the safety of food of animal origin and to promote animal welfare through a science-based

Objectives The historical objectives (1) 1. To ensure transparency in the global animal disease and zoonosis situation

Objectives The historical objectives (2) 2. To collect, analyse and disseminate scientific veterinary information

Objectives New objectives (1) 3. To provide expertise and encourage international solidarity in the control of animal diseases

Objectives New objectives (2) 4. Within its mandate under the WTO SPS Agreement, to safeguard world trade by publishing health standards for international trade in animals and animal products

Objectives New objectives (3) 5. To improve the legal framework and resources of national Veterinary Services

Objectives New mandates (4) 6. To provide a better guarantee of the safety of food of animal origin, To promote animal welfare, through a science-based approach

Contributions Ordinary - 6 categories of countries Volontary - Financing of specific activities (Regional Representations)……. .

OIE Structure

INTERNATIONAL COMMITTEE Administrative Commission Director General Specialist Commissions Regional Commissions Code, Laboratories, Aquatic animals, Scientific Africa, Americas, Europe, Asia. Far East and Oceania, Middle East Central Bureau Collaborating Centres Reference Laboratories Administrative and Financial Department Animal Health Information Department International Trade Department Scientific and Technical Department Regional Activities Department Publications Department Ad hoc Groups Working Groups Regional Representations

The International Committee the highest authority of the OIE comprises all the Delegates meets at least once a year voting by Delegates respects the democratic principle of 'one country, one vote'. elects the members of the governing bodies of the OIE appoints the Director General for a 5 -year mandate

The Delegate He is usually the Chief Veterinary Officer of his country Member of the International Committee (General Session) In permanent contact with the OIE Should inform the OIE of the animal disease situation of his country

The Delegate Ensure that the legislation in force in his country is based on OIE standards and if necessary, on a scientific risk analysis Focal point of the OIE = national specialist focal point (aquatic animal diseases, wildlife, sanitary information systems, veterinary medicinal products

The Administrative Commission (1) Composition: • the President of the International Committee, • the Vice-President, • the Past President, • 6 Delegates, elected for a 3 -year term (with the exception of the former President)

The Administrative Commission (2) President Ø Vice-President Ø Past President Ø Members Ø Ø Auditors Dr Abdoulaye Bouna Niang (Senegal) Dr Barry O’Neil (New Zealand) Dr Romano Marabelli (Italy) Dr Nikola T. Belev (Bulgaria) Dr George Khoury (Syria) Dr Rachid Bouguedour (Algeria) Dr José Molina (Philippines) Dr Carlos A. Correa Messuti (Uruguay) Dr Brian R. Evans (Canada)

The Administrative Commission (3) Role: - represents the Committee during the interval between General Sessions - examines technical and administrative matters and, in particular, the working programme and the proposed budget - to be presented to the International Committee. meets twice a year in Paris

Specialist Commissions Terrestrial Animal Health Standards Commission "Code Commission" Biological Standards Commission - "Laboratories Commission" Scientific Commission for Animal Diseases "Scientific Commission" Aquatic Animal Health Standards Commission "Aquatic Animals Commission"

Régional Commission 5 Regional Commissions - Africa - Americas - Asia, Far East and Oceania - Europe - Middle East Bureau : 1 President 2 Vice-Presidents 1 Secretary General

Central Bureau The Administrative and Financial Department The Animal Health Information Department The Scientific and Technical Department The Regional Activities Department The International Trade Department The Publications Department

Régional Representation 5 Regional Représentations - Africa (Bamako, Mali); sub-representation in SADC - Americas (Buenos Aires, Argentine) - Asia, Far East and Oceania (Tokyo, Japan) - Europe (Sofia, Bulgaria) - Middle East / Moyen Orient (Beyrouth, Lebanon) And A Regional coordination Unit for the Southeast Asia Footand-Mouth Disease Campaign (Bangkok, Thailand)

Working Groups Wildlife Diseases Animal Production Food Safety Animal Welfare

Ad Hoc Groups Set up, when needed, Øwith world renowned scientists Øto prepare decisions of the Specialist Commissions and the International Committee

International Relations

International Relations (1) Institutional cooperation with: WHO World Health Organization FAO Food and Agricultural Organization of the United Nations WTO World Trade Organization CAC Codex Alimentarius Commission IPPC International Plant Protection Convention

International Relations (2) Institutional cooperation with (2): World Bank CABI CAB International ILRI International Livestock Research Institute Regional Organizations: AU-IBAR, PAHO, OIRSA, IICA, CEBEVIRHA, SADC, CPS, European Commission, Andean Community, PVC

International Relations (3) Technical and scientific cooperation with more than 20 regional organisations and international professional associations: Inter alias : IMS IDF FEI IFAH IABs WVA (World Veterinary Association) IFAP (…)

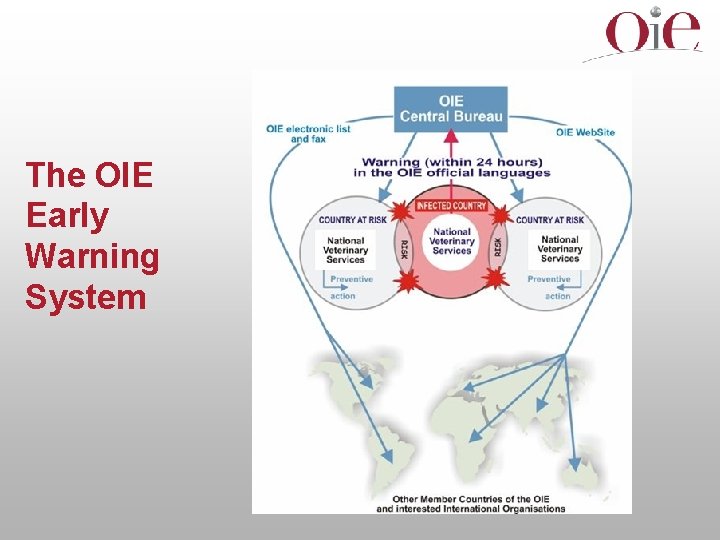
OIE Information System Promote transparency in and knowledge of global animal disease situation

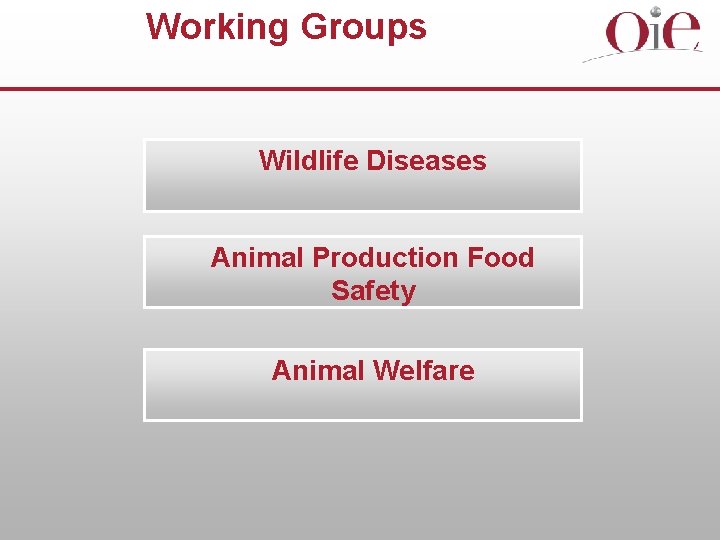
Source of Early Warning Disease reports Reports from Member Countries Reports from worldwide network of OIE Reference Labs. Active search and tracking of unofficial sources, such as scientific publications and Pro. Med, and lay publications, with Member Country verification Improved Member Country surveillance § § Policies Internal and international resources

The OIE Early Warning System

The OIE Global Information System

OIE animal disease notification system Criteria for inclusion in the OIE list: ý International spread ý Significant spread within naive population ý Zoonotic potential ý Emerging diseases (new infection resulting from the evolution of an existing pathogen or parasite resulting in a change of host range, vector, pathogenicity or strain; or the occurrence of a previously unrecognized infection or disease. )

Global Early Warning System (GLEWS) Joint OIE/FAO/WHO initiative Animal disease and zoonoses tracking Emergency response Trends analysis predictions Capacity building of Veterinary Services for surveillance and early warning and response (animal sector) List of priority animal diseases, zoonoses and emerging diseases

OIE International Standards

OIE International Standards Terrestrial Animal Health Code – mammals, birds and bees Aquatic Animal Health Code – fish, molluscs and crustaceans Manual of Diagnostic Tests and Vaccines for Terrestrial Animals Manual of Diagnostic Tests for Aquatic Animals

Why are standards necessary (1) Safety of international trade of animals and animal products Harmonization of legislations and control methods in countries Narrow the gap between rich and poor countries (…)

Why are standards necessary (2) Surveillance and control of animal diseases and zoonoses = Intern. Public Good (IPG) IPG implementation is a duty of governments International community, international org. , donors and NGO influence and support Public – Private sector contracts

How fast are they changing and in response to what pressure? Pressure by exporting countries to increase trade Pressure by importing countries to protect themselves (e. g. Avian influenza) Ethics and public health protection

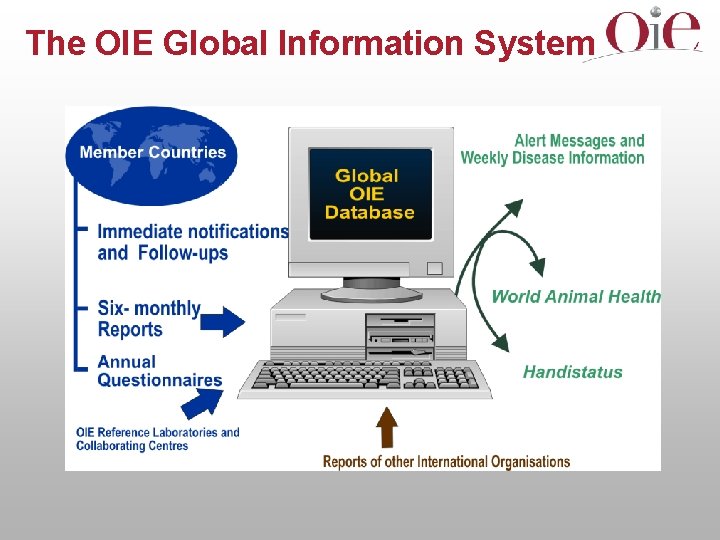
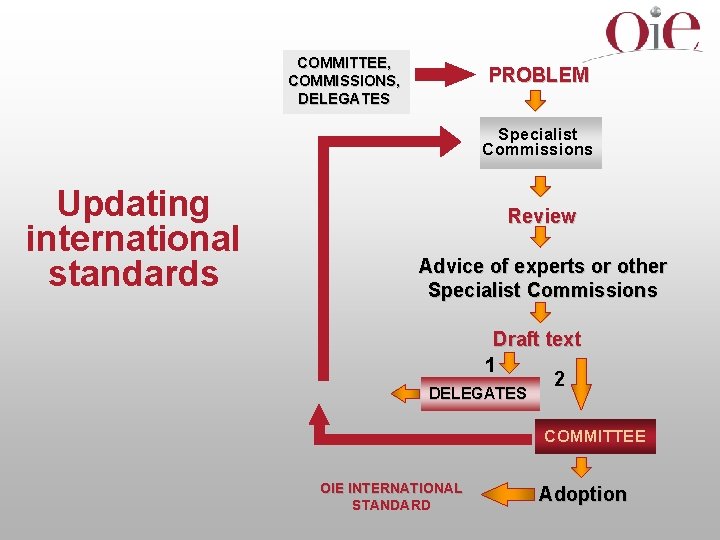
COMMITTEE, COMMISSIONS, DELEGATES PROBLEM Specialist Commissions Updating international standards Review Advice of experts or other Specialist Commissions Draft text 1 2 DELEGATES COMMITTEE OIE INTERNATIONAL STANDARD Adoption

Reference Laboratories Collaborating Centres

Reference Laboratories (1) 155 Reference Laboratories in 30 Countries covering 92 diseases or topics

Reference Laboratories (2) Expert centres for worldwide standardization Store and distribute reference reagents Conduct and validate diagnostic tests Coordinate technical and scientific studies Provide technical and scientific training Organise laboratory proficiency testing

Collaborating Centres (1) 15 Collaborating Centres in 9 Countries

Collaborating Centres (2) Expert centres on horizontal subjects, for the OIE and Member Countries Assist in the elaboration of procedures for the harmonization of international standards Coordinate activities on cooperation Provide technical training Organize and host scientific meetings for the OIE

OIE Publications & Web Site

Publications of the OIE Bulletin months every 3 Scientific and Technical Review months every 4 World Animal Health year every Technical items, Newsletters, Specialised books variabl e

On the OIE Web Site… Early warning Weekly Disease Information International Standards (Codes, Manuals, etc. ) Scientific and Technical Review (contents and abstracts) Scientific and general information on OIE activities Animal diseases and zoonoses Editorials from the Director General

4 th OIE Strategic Plan for 2006 -2010

Procedures adopted Consultations with Regional and Specialist Commissions Interim report discussed during the May 2004 General Session Extraordinary meeting of the OIE Administrative Commission (Montebello, Canada, November 2004) Permanent support from an expert : Dr Alan Randell, former Codex Commission Secretary

Procedures adopted Elaboration of a new project proposed for adoption by the Administrative Commission in February 2005, at the OIE headquarters in Paris Submission of the project to the Member Countries after translation into the OIE working languages (French, English, Spanish) Discussion and adoption by the OIE International Committee in May 2005

Subsequent phases Establishment of a work schedule for the Director General, based on the agreed Plan Director General develops a financial plan taking into account contributions from Member Countries Proposed by Administrative Commission (February 2006), for adoption by International Committee (May 2006)

What does a Strategic Plan mean ? Defines a five year policy having a strong legal basis but based on consensus Provides a framework that allows the Director General to schedule his annual work programmes for 5 years Allows adjustments, if any, after a period of 3 years

2005/2010 Strategic Plan Reasserts the relevance of the goals of the former Strategic Plan and provides for their consolidation Clarifies the OIE’s objectives and major missions Ensures a balance between missions to be achieved and available resources detailed in the work programme of the Director General

The OIE’s global objective The OIE was created in 1924 to prevent animal diseases from spreading all over the world The 4 th Strategic Plan provides a further step and extends the OIE’s global mandate to “the improvement of animal health all over the world”

Main consequences coming from this new mandate To alleviate poverty To improve Public Health by controlling/eradicating zoonoses including food borne diseases To improve the sanitary safety of international trade in animals and animal products To facilitate the access to regional and international markets for all countries

Main consequences arising from this new mandate Promotion of animal welfare through the improvement of animal health and its sustainability by the development of international standards Improvement of National Veterinary Services to adopt and enforce regulations Strengthening of the position of the OIE as a leading international Organisation in the interest of Member Countries

New priorities of the IVth Plan Consolidation of 3 missions from the former Strategic Plan Ø To ensure transparency in the global animal disease situation Ø Elaboration and publication of science based standards, especially within the WTO-SPS Agreement Ø Elaboration and publication of guidelines for the prevention, control and eradication of animal diseases, including zoonoses. Evaluation of the health status of Member countries with respect to

New strategic items Capacity building: training of OIE Delegates and their collaborators including focal points designated to liaise with the OIE on sanitary information system, aquatic animals, wild life, veterinary medicinal products…) By using new mechanisms such as Standards and Trade Development Facility (STDF) Strengthening the OIE’s influence on global, regional and national governance policies regarding animal health and scientific research Strengthening the position of the OIE as an advisor of Member Countries to settle sanitary disputes

Practical consequences Reinforcement of OIE capacities: Ø Necessity to strengthen Regional Representations Ø Defining a financing mechanism by the Member Countries of each region (in addition to the financing programme of the host country) Ø Staff reinforcement, development of internship, support from the private sector (within the framework of existing rules) Ø Necessity to formalise relations between Regional Representatives and elected Bureaus of

Practical consequences Scientific influence: Ø Necessity to reinforce the OIE Network of Collaborating Centers and Reference Laboratories Ø Development of twinning procedures and other specific projects for laboratories support particularly in developing countries Ø More involvement of the OIE in zoonotic diseases

Practical consequences Influence on global governance of animal health Ø Develop further the OIE’s communication department Ø Clarify further the relationship with WHO and FAO, by negotiating detailed Agreements and alliances for operational and specific programmes Ø Pursue lobbying with multi and bi-lateral Organisations in order to persuade them that investing in animal health and Veterinary Services is a major national and global priority

Practical consequences Influence on national policies Ø Convince Governments of the importance of the OIE Delegate Ø Convince Governments that further investment in monitoring and preventing animal diseases represents a low cost insurance compared to high costs involved in combating animal diseases Ø Support Delegates from developing countries to participate in standards-setting process and attending SPS and Codex meetings

Practical consequences As regards finance: Ø The cost (at constant rate currency) of the new priorities and measures of the 4 th Strategic Plan is 25 % higher than the current budget Ø In May 2006, the Director General will propose new financing procedures (also for Regional Representations), including both compulsory and voluntary contributions, to finance the increase in the budget

Conclusion The implementation of the 4 th Strategic Plan through Director General’s programme of work will continue to prove that, since 1924, OIE is of a “Public Good” for the international Community and that the contribution of Member Countries is negligible compared to the services provided in return

World Organisation for Animal Health 12 rue de prony 75017 Paris, France Tel: 33 (0)1 44 15 18 88 – Fax: 33 (0)1 42 67 09 87 Email: oie@oie. int http: //www. oie. int
 World health organisation
World health organisation World health organisation
World health organisation World health organisation bare below the elbow
World health organisation bare below the elbow World health organisation
World health organisation World health organisation
World health organisation World health organisation
World health organisation Terrestrial animals
Terrestrial animals World nature organization (wno)
World nature organization (wno) Objectives of world trade organisation
Objectives of world trade organisation Health promotion world health organization
Health promotion world health organization Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Typiska drag för en novell
Typiska drag för en novell Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Returpilarna
Returpilarna Varför kallas perioden 1918-1939 för mellankrigstiden
Varför kallas perioden 1918-1939 för mellankrigstiden En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Särskild löneskatt för pensionskostnader
Särskild löneskatt för pensionskostnader Vilotidsbok
Vilotidsbok Sura för anatom
Sura för anatom Vad är densitet
Vad är densitet Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Mall debattartikel
Mall debattartikel För och nackdelar med firo
För och nackdelar med firo Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Vätsketryck formel
Vätsketryck formel Publik sektor
Publik sektor Jag har nigit för nymånens skära
Jag har nigit för nymånens skära Presentera för publik crossboss
Presentera för publik crossboss Teckenspråk minoritetsspråk argument
Teckenspråk minoritetsspråk argument Vem räknas som jude
Vem räknas som jude Klassificeringsstruktur för kommunala verksamheter
Klassificeringsstruktur för kommunala verksamheter Mjälthilus
Mjälthilus Claes martinsson
Claes martinsson Centrum för kunskap och säkerhet
Centrum för kunskap och säkerhet Lågenergihus nyproduktion
Lågenergihus nyproduktion Bra mat för unga idrottare
Bra mat för unga idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Hur ser ett referat ut
Hur ser ett referat ut Redogör för vad psykologi är
Redogör för vad psykologi är Stål för stötfångarsystem
Stål för stötfångarsystem Tack för att ni har lyssnat
Tack för att ni har lyssnat Borra hål för knoppar
Borra hål för knoppar Orubbliga rättigheter
Orubbliga rättigheter Stickprovsvariansen
Stickprovsvariansen Tack för att ni har lyssnat
Tack för att ni har lyssnat Steg för steg rita
Steg för steg rita Informationskartläggning
Informationskartläggning Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Toppslätskivling effekt
Toppslätskivling effekt Mästare lärling modell
Mästare lärling modell Egg för emanuel
Egg för emanuel Elektronik för barn
Elektronik för barn Plagg i gamla rom
Plagg i gamla rom Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Kung dog 1611
Kung dog 1611 Humanitr
Humanitr Romarriket tidslinje
Romarriket tidslinje Tack för att ni lyssnade
Tack för att ni lyssnade Multiplikation med uppställning
Multiplikation med uppställning Dikter för barn i skolan
Dikter för barn i skolan Inköpsprocessen steg för steg
Inköpsprocessen steg för steg Rbk mätning
Rbk mätning Etik och ledarskap etisk kod för chefer
Etik och ledarskap etisk kod för chefer Skivepiteldysplasi
Skivepiteldysplasi Myndigheten för delaktighet
Myndigheten för delaktighet Trög för kemist
Trög för kemist