UPDATE ON ANTIPLATELET THERAPY IN THE TREATMENT AND




































- Slides: 69

UPDATE ON ANTIPLATELET THERAPY IN THE TREATMENT AND PREVENTION OF CARDIOVASCULAR DISEASE Charles H Hennekens, MD, Dr. PH Sir Richard Doll Research Professor of Medicine Charles E. Schmidt College of Medicine Florida Atlantic University Clinical Professor of Preventive Medicine Nova Southeastern University Voluntary Professor of Family Medicine and Community Health University of Miami Miller School of Medicine

Disclosure Dr. Hennekens receives investigator initiated research grant support from Bayer to the Charles E. Schmidt College of Medicine at Florida Atlantic University. He serves as an independent scientist in an advisory role to investigators and sponsors, including as Chair or member of Data and Safety Monitoring Boards to Actelion, Amgen, Anthera, Bayer, Bristol-Myers Squibb, Canadian Institutes of Health Research, Dainippon-Sumitomo, National Association for Continuing Education, , Pozen, Pfizer, Pri. Med, United States (US) Food and Drug Administration, U. S. National Institutes of Health, and Up. To. Date. He serves as an independent scientist in an advisory role to legal counsel for Glaxo. Smith. Kline and Stryker. He serves as speaker for the Association for Research in Vision and Ophthalmology, Astra. Zeneca, International Atherosclerosis Society, and Pfizer. He receives royalties for authorship or editorship of three textbooks and as co-inventor on patents held by Brigham and Women’s Hospital concerning inflammatory markers for cardiovascular disease. He has an investment management relationship with The West-Bacon Group within Sun. Trust Investment Services who has sole discretionary investment authority. He owns no common or preferred stock in any pharmaceutical or device industry.

Death is inevitable but premature death is not. Sir Richard Doll


OBJECTIVES Aspirin in the treatment of CVD n Additive benefits of aspirin and statins n Aspirin in the prevention of CVD n Dual antiplatelet therapy in CVD n Newer antiplatelet agents in CVD n

Milestones For Aspirin 5 th century BC Hippocrates 1897 AD Felix Hoffman/Friedrich Bayer 1900 – present Most widely used drug in the world 1971 Sir John Vane

Moses Receiving The Tablets From God

Milestones For Aspirin 5 th century BC Hippocrates 1897 AD Felix Hoffman/Friedrich Bayer 1900 – present Most widely used drug in the world 1971 Sir John Vane


Milestones For Aspirin 5 th century BC Hippocrates 1897 AD Felix Hoffman/Friedrich Bayer 1900 – present Most widely used drug in the world 1971 Sir John Vane


Milestones For Aspirin 5 th century BC Hippocrates 1897 AD Felix Hoffman/Friedrich Bayer 1900 – present Most widely used drug in the world 1971 Sir John Vane

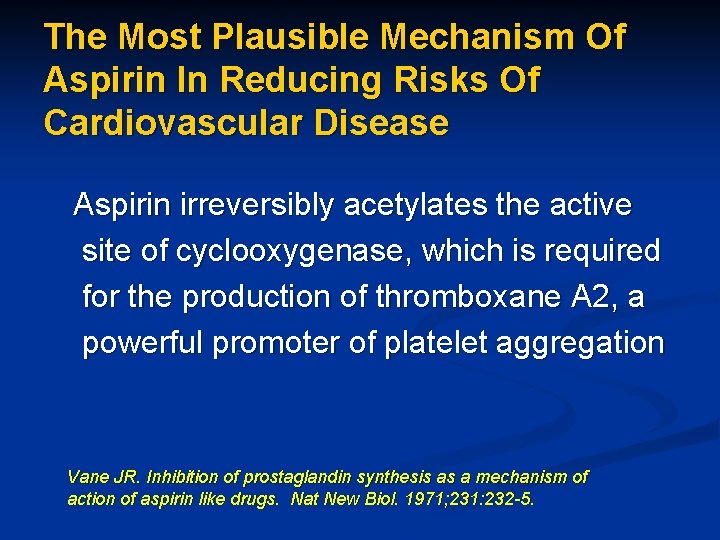
The Most Plausible Mechanism Of Aspirin In Reducing Risks Of Cardiovascular Disease Aspirin irreversibly acetylates the active site of cyclooxygenase, which is required for the production of thromboxane A 2, a powerful promoter of platelet aggregation Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action of aspirin like drugs. Nat New Biol. 1971; 231: 232 -5.

Totality Of Evidence n n Basic research (why) Epidemiology (whether) n descriptive studies s case reports s case series s ecological studies Hennekens. Epidemiology in Medicine. 1987. n analytic studies s observational case-control n cohort s randomized trials n

Observational Epidemiologic Studies n Some but not all case-control & cohort studies indicate that individuals and/or their health care providers who selfselect for aspirin have lower risks of CVD. n For most epidemiologic hypotheses, randomized trials are neither necessary nor desirable n For small to moderate effects, however, the only reliable design strategy is the large randomized trial because the amount of uncontrolled & uncontrollable confounding factors inherent in observational studies can be as large as the effect sizes Hennekens CH, De. Mets D: The need for large scale randomized evidence without undue emphasis on small trials, their meta-analyses or subgroup analyses JAMA 2009

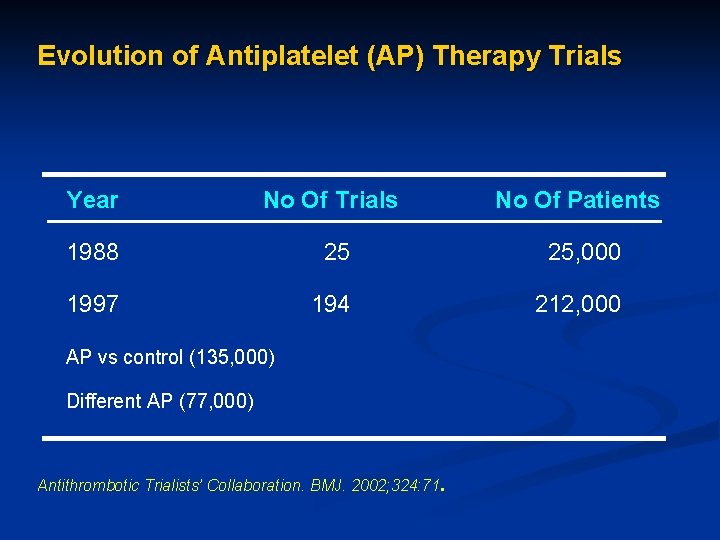
Evolution of Antiplatelet (AP) Therapy Trials Year No Of Trials No Of Patients 1988 25 25, 000 1997 194 212, 000 AP vs control (135, 000) Different AP (77, 000) Antithrombotic Trialists’ Collaboration. BMJ. 2002; 324: 71.

Aspirin in the Treatment of CVD n In a wide range of patients who have survived a prior occlusive event (including MI, occlusive stroke or transient ischemic attack, or other high risk categories including unstable and stable angina, angioplasty, or coronary artery bypass graft), antiplatelet therapy, principally with aspirin, prevents ~25% of serious vascular events, including significant reductions on MI, stroke, and CVD death. n All these patients have 10 -year risks of CHD of 20% or more based on the Framingham risk score recommended by the US NHLBI. Anti. Thrombotic Trialists Collaboration. Lancet, 2002

Benefits of Aspirin on Risk of Stroke n In 158 trials, there were 3, 522 nonfatal and 1, 424 fatal strokes after randomization. n Antiplatelet therapy, principally with aspirin, reduced stroke by about 25%, regardless of whether the patient entered the trial with prior MI, stroke, TIA, or other high -risk conditions. n Antiplatelet therapy, principally with aspirin, increases the absolute risk of hemorrhagic stroke by 3 per 10, 000 treated patients. The upper bound of the 95% confidence interval is less than 1 per 1000 treated patients. Anti. Thrombotic Trialists Collaboration. Lancet, 2002

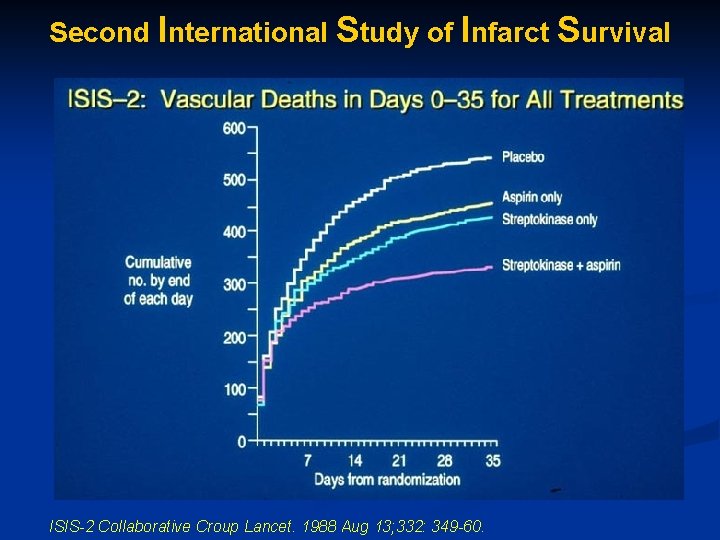
Second International Study of Infarct Survival ISIS-2 Collaborative Croup Lancet. 1988 Aug 13; 332: 349 -60.

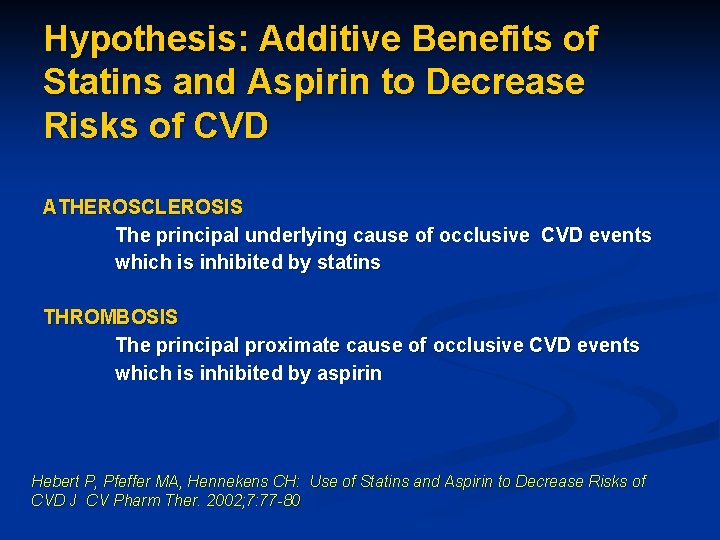
Hypothesis: Additive Benefits of Statins and Aspirin to Decrease Risks of CVD ATHEROSCLEROSIS The principal underlying cause of occlusive CVD events which is inhibited by statins THROMBOSIS The principal proximate cause of occlusive CVD events which is inhibited by aspirin Hebert P, Pfeffer MA, Hennekens CH: Use of Statins and Aspirin to Decrease Risks of CVD J CV Pharm Ther. 2002; 7: 77 -80

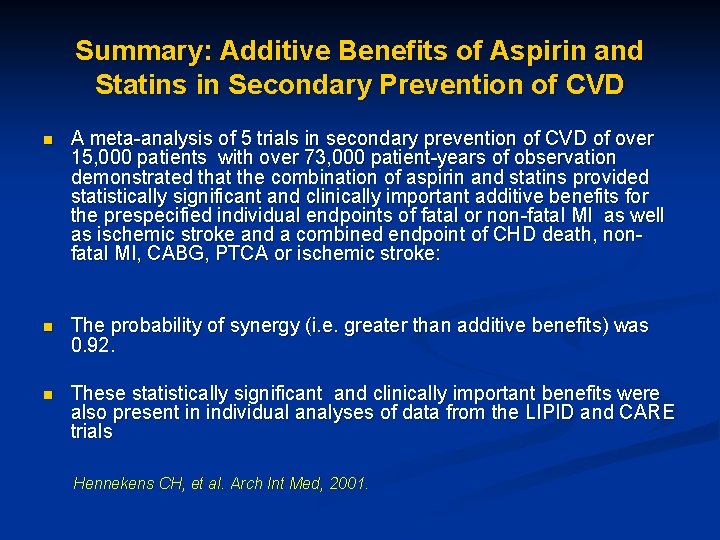
Summary: Additive Benefits of Aspirin and Statins in Secondary Prevention of CVD n A meta-analysis of 5 trials in secondary prevention of CVD of over 15, 000 patients with over 73, 000 patient-years of observation demonstrated that the combination of aspirin and statins provided statistically significant and clinically important additive benefits for the prespecified individual endpoints of fatal or non-fatal MI as well as ischemic stroke and a combined endpoint of CHD death, nonfatal MI, CABG, PTCA or ischemic stroke: n The probability of synergy (i. e. greater than additive benefits) was 0. 92. n These statistically significant and clinically important benefits were also present in individual analyses of data from the LIPID and CARE trials Hennekens CH, et al. Arch Int Med, 2001.

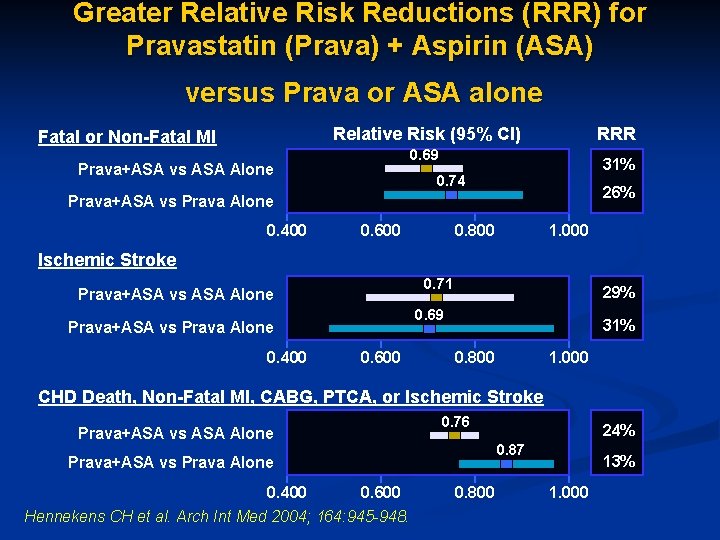
Greater Relative Risk Reductions (RRR) for Pravastatin (Prava) + Aspirin (ASA) versus Prava or ASA alone RRR Relative Risk (95% CI) Fatal or Non-Fatal MI 0. 69 Prava+ASA vs ASA Alone 31% 0. 74 26% Prava+ASA vs Prava Alone 0. 400 0. 600 0. 800 1. 000 Ischemic Stroke 0. 71 Prava+ASA vs ASA Alone 0. 69 Prava+ASA vs Prava Alone 0. 400 29% 0. 600 31% 0. 800 1. 000 CHD Death, Non-Fatal MI, CABG, PTCA, or Ischemic Stroke 0. 76 Prava+ASA vs ASA Alone 0. 87 Prava+ASA vs Prava Alone 0. 400 24% 0. 600 Hennekens CH et al. Arch Int Med 2004; 164: 945 -948. 0. 800 13% 1. 000

Summary: Aspirin in Primary Prevention of CVD n n n In a comprehensive worldwide meta analysis of the 6 randomized trials of primary prevention aspirin produces a statistically significant and clinically important reduction in risk of a first myocardial infarction by about 1/3 but the available data on stroke and cardiovascular death remain inconclusive In these apparently healthy men and women at low risk aspirin is of uncertain net value as the reduction in occlusive events needs to be weighed against any increase in major bleeds. The average 10 year risk of a first CHD event among the apparently healthy men and women in the 6 randomized trials is less than 5%. The chief need is for randomized evidence in apparently healthy individuals whose 10 year risk of a first CHD event is 10 -19%. Until then any decision to use aspirin in primary prevention should be an individual clinical judgement by the healthcare provider. Writing Group (Baigent C, Blackwell L, Buring J, Collins R, Emberson J, Godwin J, Hennekens C, Kearney P, Meade T, Patrono C, Peto R, Roncaglioni R, Zanchetti A). Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data. Lancet. 2009; 373: 1849 -60.

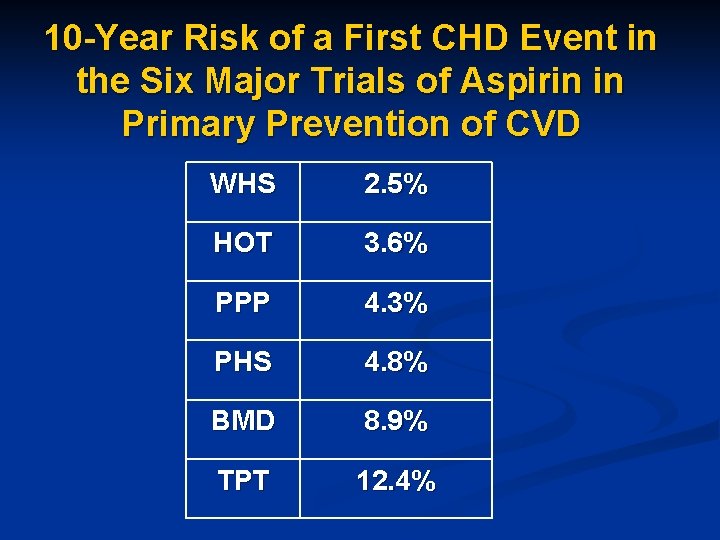
10 -Year Risk of a First CHD Event in the Six Major Trials of Aspirin in Primary Prevention of CVD WHS 2. 5% HOT 3. 6% PPP 4. 3% PHS 4. 8% BMD 8. 9% TPT 12. 4%

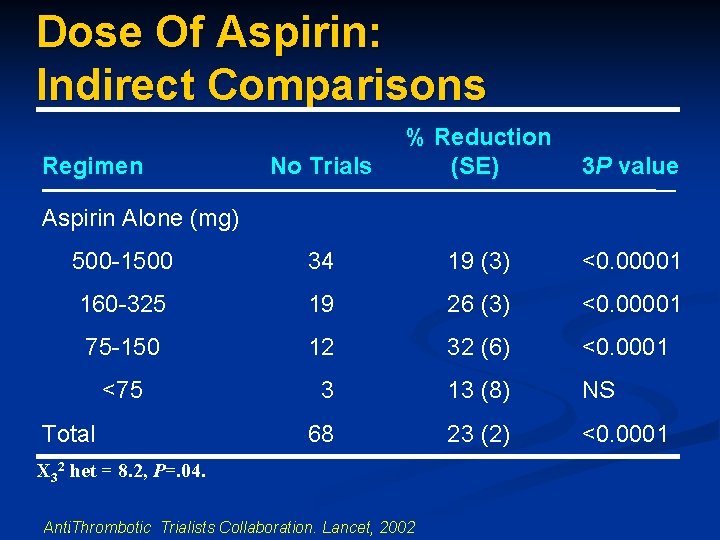
Dose Of Aspirin: Indirect Comparisons Regimen No Trials % Reduction (SE) 3 P value Aspirin Alone (mg) 500 -1500 34 19 (3) <0. 00001 160 -325 19 26 (3) <0. 00001 75 -150 12 32 (6) <0. 0001 <75 3 13 (8) NS 68 23 (2) <0. 0001 Total X 32 het = 8. 2, P=. 04. Anti. Thrombotic Trialists Collaboration. Lancet, 2002.

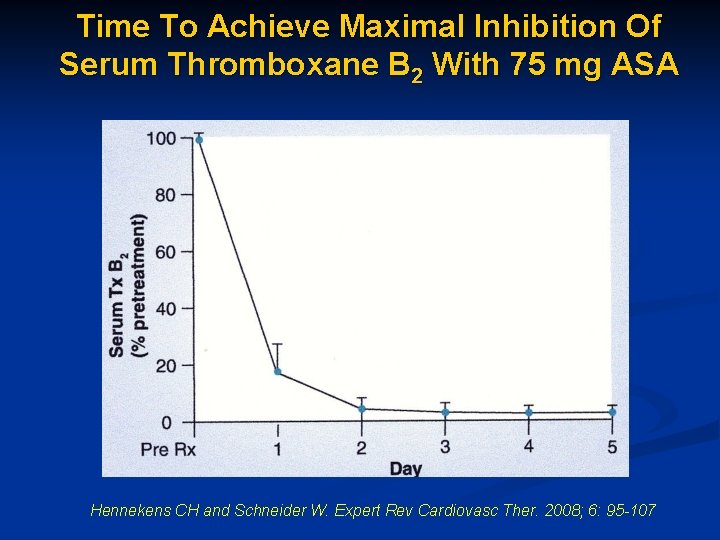
Time To Achieve Maximal Inhibition Of Serum Thromboxane B 2 With 75 mg ASA Hennekens CH and Schneider W. Expert Rev Cardiovasc Ther. 2008; 6: 95 -107

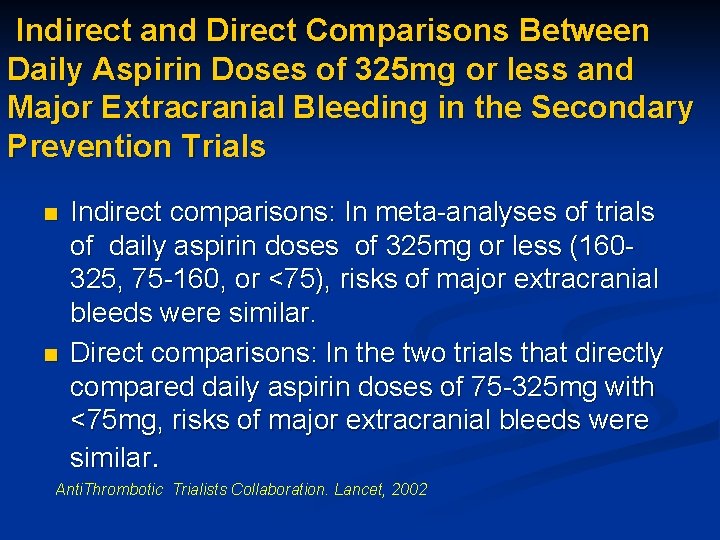
Indirect and Direct Comparisons Between Daily Aspirin Doses of 325 mg or less and Major Extracranial Bleeding in the Secondary Prevention Trials n n Indirect comparisons: In meta-analyses of trials of daily aspirin doses of 325 mg or less (160325, 75 -160, or <75), risks of major extracranial bleeds were similar. Direct comparisons: In the two trials that directly compared daily aspirin doses of 75 -325 mg with <75 mg, risks of major extracranial bleeds were similar. Anti. Thrombotic Trialists Collaboration. Lancet, 2002

Optimal Dosing For Aspirin In CHD Secondary Prevention & Primary Prevention 75 mg – 325 mg Acute CVD Syndrome 162. 5 mg – 325 mg Hennekens CH, Dyken M, Fuster V: Circ. 1997

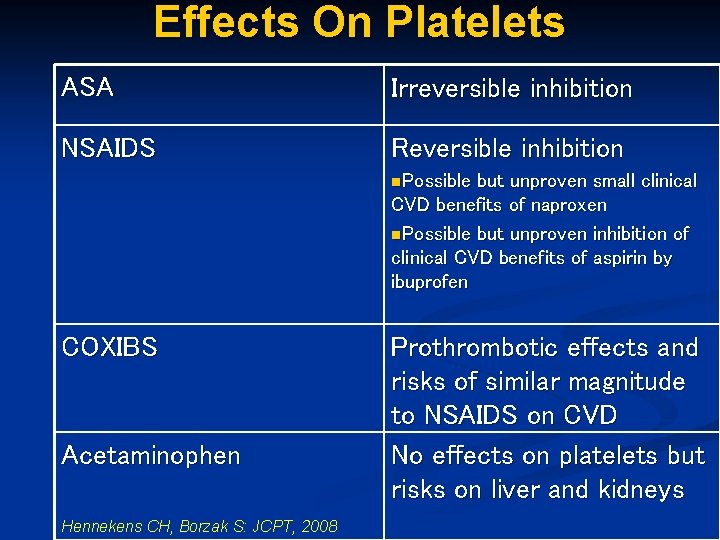
Effects On Platelets ASA Irreversible inhibition NSAIDS Reversible inhibition n. Possible but unproven small clinical CVD benefits of naproxen n. Possible but unproven inhibition of clinical CVD benefits of aspirin by ibuprofen COXIBS Acetaminophen Hennekens CH, Borzak S: JCPT, 2008 Prothrombotic effects and risks of similar magnitude to NSAIDS on CVD No effects on platelets but risks on liver and kidneys

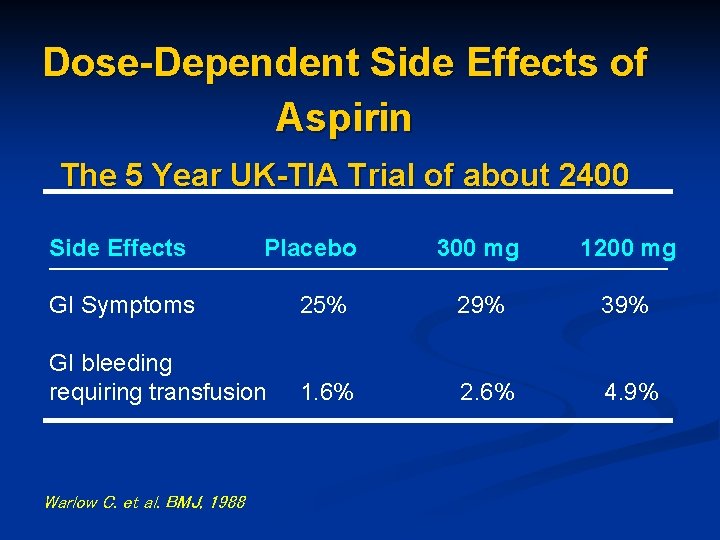
Dose-Dependent Side Effects of Aspirin The 5 Year UK-TIA Trial of about 2400 Side Effects Placebo 300 mg 1200 mg GI Symptoms 25% 29% 39% GI bleeding requiring transfusion 1. 6% 2. 6% 4. 9% Warlow C. et al. BMJ, 1988

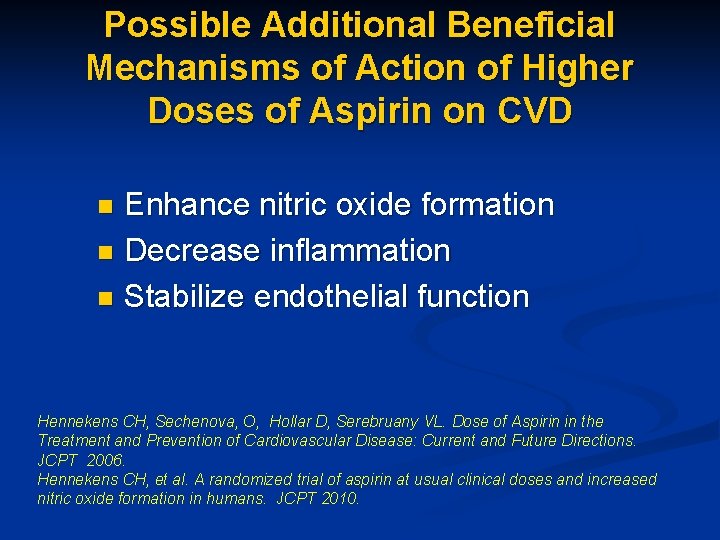
Possible Additional Beneficial Mechanisms of Action of Higher Doses of Aspirin on CVD Enhance nitric oxide formation n Decrease inflammation n Stabilize endothelial function n Hennekens CH, Sechenova, O, Hollar D, Serebruany VL. Dose of Aspirin in the Treatment and Prevention of Cardiovascular Disease: Current and Future Directions. JCPT 2006. Hennekens CH, et al. A randomized trial of aspirin at usual clinical doses and increased nitric oxide formation in humans. JCPT 2010.

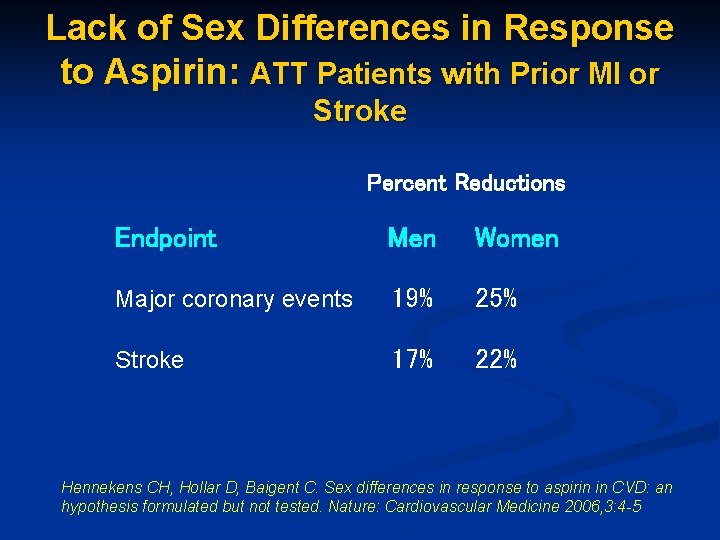
Lack of Sex Differences in Response to Aspirin: ATT Patients with Prior MI or Stroke Percent Reductions Endpoint Men Women Major coronary events 19% 25% Stroke 17% 22% Hennekens CH, Hollar D, Baigent C. Sex differences in response to aspirin in CVD: an hypothesis formulated but not tested. Nature: Cardiovascular Medicine 2006, 3: 4 -5

Dual Antiplatelet Therapy Risks versus Monotherapy n Benefits and risks: Aspirin + Dipyrimadole Aspirin + Clopidogrel Issues with Clopidogrel versus Prasugrel Clopidogrel versus Ticagrelor n

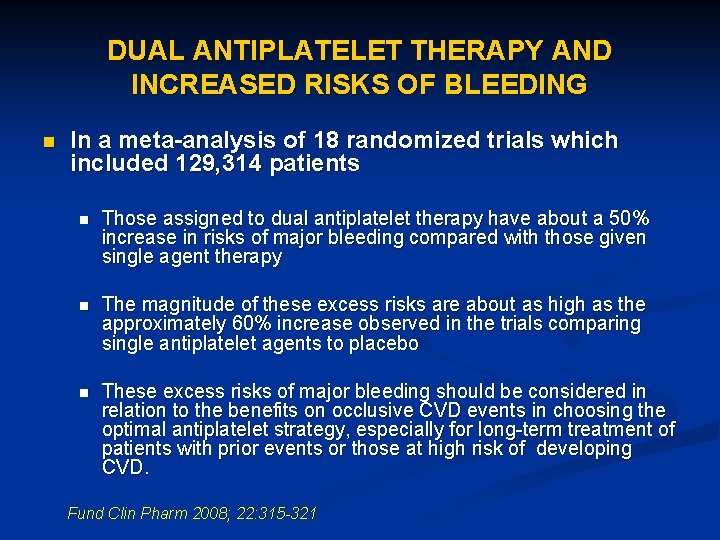
DUAL ANTIPLATELET THERAPY AND INCREASED RISKS OF BLEEDING n In a meta-analysis of 18 randomized trials which included 129, 314 patients n Those assigned to dual antiplatelet therapy have about a 50% increase in risks of major bleeding compared with those given single agent therapy n The magnitude of these excess risks are about as high as the approximately 60% increase observed in the trials comparing single antiplatelet agents to placebo n These excess risks of major bleeding should be considered in relation to the benefits on occlusive CVD events in choosing the optimal antiplatelet strategy, especially for long-term treatment of patients with prior events or those at high risk of developing CVD. Fund Clin Pharm 2008; 22: 315 -321

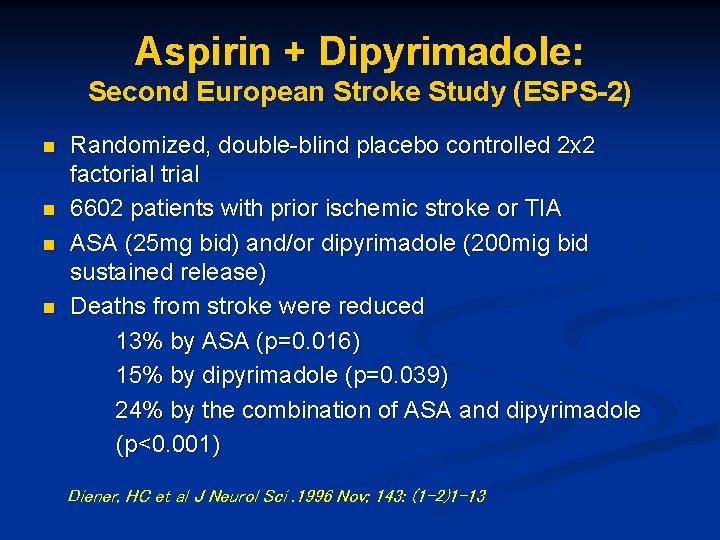
Aspirin + Dipyrimadole: Second European Stroke Study (ESPS-2) n n Randomized, double-blind placebo controlled 2 x 2 factorial trial 6602 patients with prior ischemic stroke or TIA ASA (25 mg bid) and/or dipyrimadole (200 mig bid sustained release) Deaths from stroke were reduced 13% by ASA (p=0. 016) 15% by dipyrimadole (p=0. 039) 24% by the combination of ASA and dipyrimadole (p<0. 001) Diener, HC et al J Neurol Sci. 1996 Nov; 143: (1 -2)1 -13

Aspirin + Dipyridamole: PROFESS n n n 20, 332 post-ischemic stroke patients within 120 days Randomized to aspirin 25 mg +extended release dipyrimadole 200 mg bid vs clopidogrel 75 mg qd After 2. 5 years there were similar rates of the primary prespecified composite endpoint of stroke, MI or vascular death. NEJM, 2008; 359: 1238 -1251

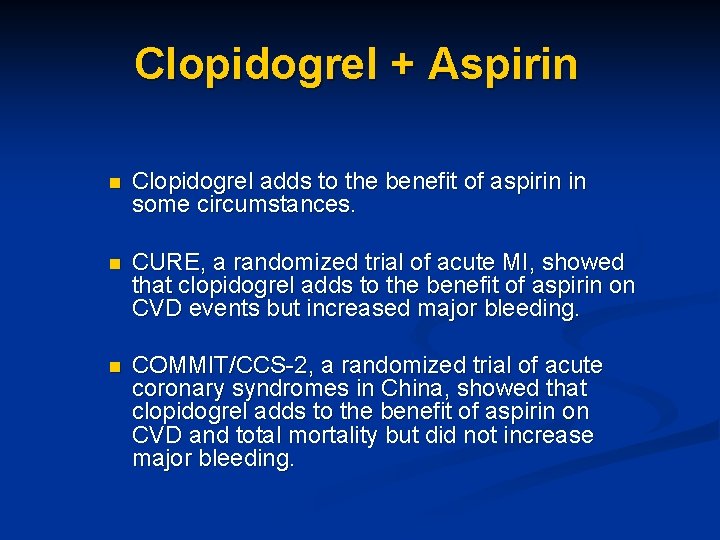
Clopidogrel + Aspirin n Clopidogrel adds to the benefit of aspirin in some circumstances. n CURE, a randomized trial of acute MI, showed that clopidogrel adds to the benefit of aspirin on CVD events but increased major bleeding. n COMMIT/CCS-2, a randomized trial of acute coronary syndromes in China, showed that clopidogrel adds to the benefit of aspirin on CVD and total mortality but did not increase major bleeding.

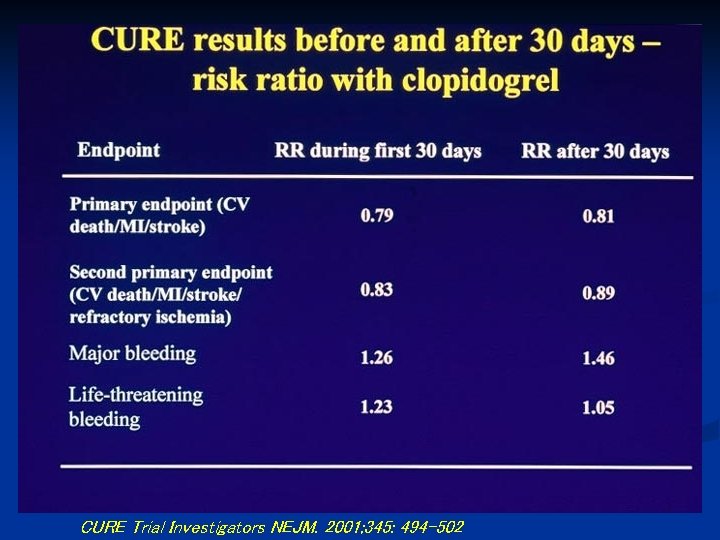
CURE Trial Investigators NEJM. 2001; 345: 494 -502

Second Chinese Cardiac Study: COMMIT n n n Randomized, double-blind, 2 x 2 factorial trial of clopidogrel and metoprolol 45, 852 patients within 24 hours of onset of symptoms of suspected acute myocardial infarction Randomization in clopidogrel arm to daily 75 mg clopidogrel+162 mg aspirin(22, 960) or placebo +160 mg aspirin(22, 891) COMMIT Collaborative Group. Lancet 2005; 366: 1607 -1621.

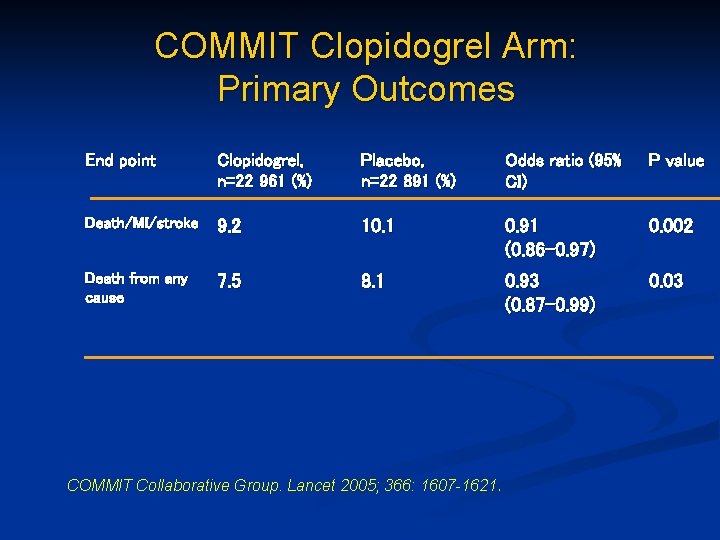
COMMIT Clopidogrel Arm: Primary Outcomes End point Clopidogrel, n=22 961 (%) Placebo, n=22 891 (%) Odds ratio (95% CI) P value Death/MI/stroke 9. 2 10. 1 0. 91 (0. 86 -0. 97) 0. 002 Death from any cause 7. 5 8. 1 0. 93 (0. 87 -0. 99) 0. 03 COMMIT Collaborative Group. Lancet 2005; 366: 1607 -1621.

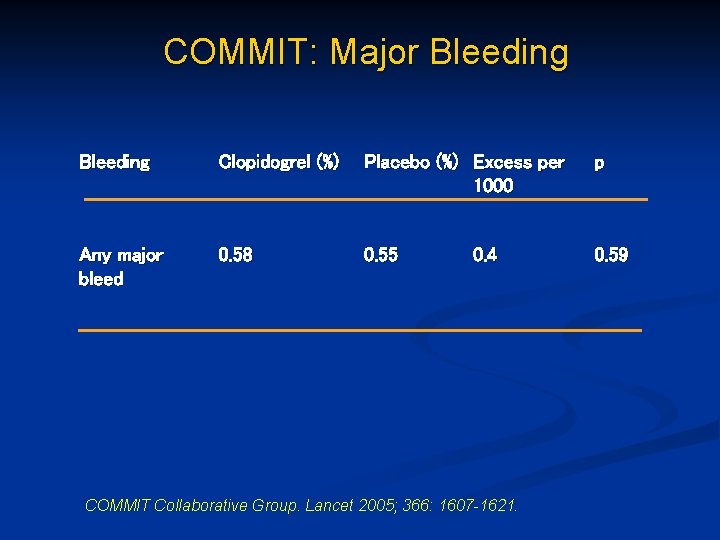
COMMIT: Major Bleeding Clopidogrel (%) Placebo (%) Excess per 1000 p Any major bleed 0. 58 0. 55 0. 59 0. 4 COMMIT Collaborative Group. Lancet 2005; 366: 1607 -1621.

CHARISMA A randomized, double-blind placebo controlled trial of 15, 603 patients (79% ) with established CVD and 21% with multiple risk factors designed to test whether clopidogrel should be continued beyond 1 year in addition to aspirin. n All patients received daily aspirin(75 -162 mg) and were randomized to daily clopidogrel(75 mg) or placebo n Clopidogrel patients had an event rate of 6. 8% and placebo patients had an event rate of 7. 3%. n CHARISMA demonstrated no significant benefit long term when clopidogrel is added to aspirin. n Rates of severe bleeding were similar but clopidogrel patients experienced significantly higher rates of moderate bleeding. n There was possible effect modification by presence or absence of prior events, a post hoc formulated hypothesis not directly tested in this trial. Bhatt DL, et al; N Engl J Med. 2006. 54: 1706 -1717 n

ISIS-2 Investigators, Lancet, 1988

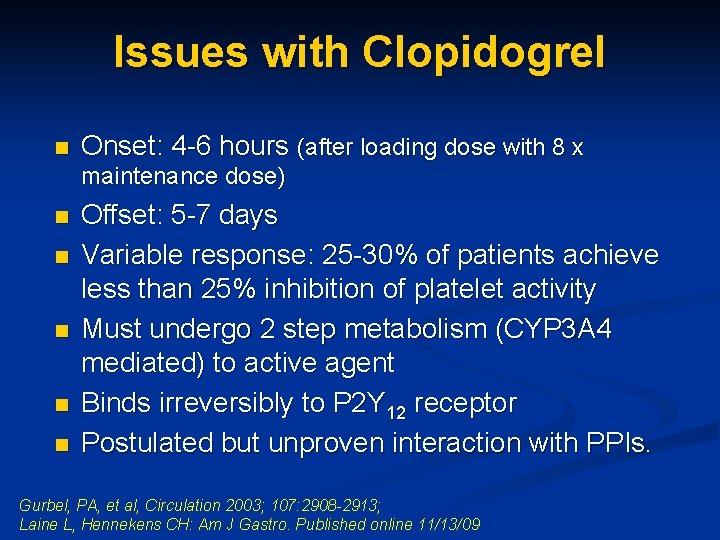
Issues with Clopidogrel n Onset: 4 -6 hours (after loading dose with 8 x maintenance dose) n n n Offset: 5 -7 days Variable response: 25 -30% of patients achieve less than 25% inhibition of platelet activity Must undergo 2 step metabolism (CYP 3 A 4 mediated) to active agent Binds irreversibly to P 2 Y 12 receptor Postulated but unproven interaction with PPIs. Gurbel, PA, et al, Circulation 2003; 107: 2908 -2913; Laine L, Hennekens CH: Am J Gastro. Published online 11/13/09

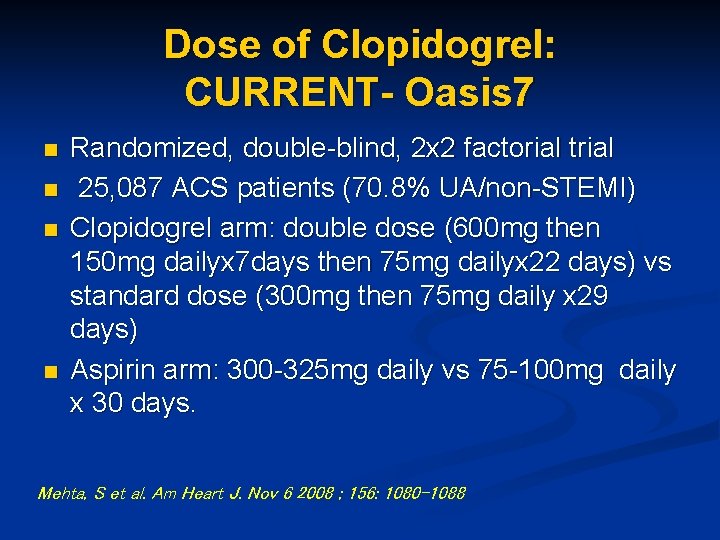
Dose of Clopidogrel: CURRENT- Oasis 7 n n Randomized, double-blind, 2 x 2 factorial trial 25, 087 ACS patients (70. 8% UA/non-STEMI) Clopidogrel arm: double dose (600 mg then 150 mg dailyx 7 days then 75 mg dailyx 22 days) vs standard dose (300 mg then 75 mg daily x 29 days) Aspirin arm: 300 -325 mg daily vs 75 -100 mg daily x 30 days. Mehta, S et al. Am Heart J. Nov 6 2008 ; 156: 1080 -1088

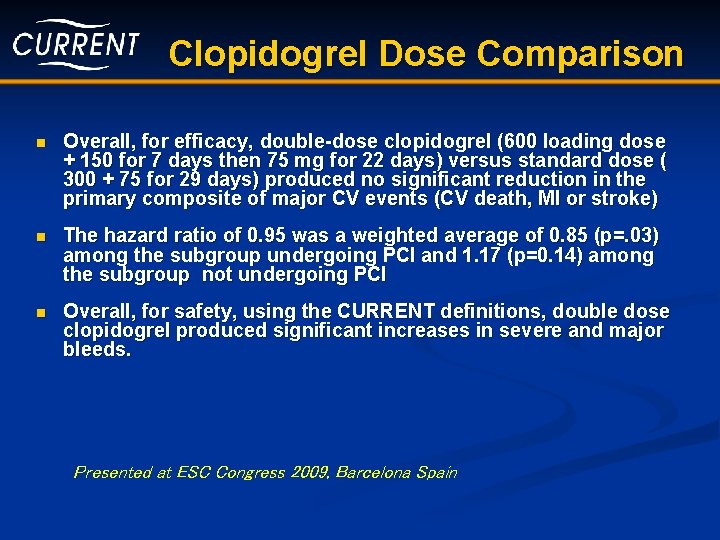
Clopidogrel Dose Comparison n Overall, for efficacy, double-dose clopidogrel (600 loading dose + 150 for 7 days then 75 mg for 22 days) versus standard dose ( 300 + 75 for 29 days) produced no significant reduction in the primary composite of major CV events (CV death, MI or stroke) n The hazard ratio of 0. 95 was a weighted average of 0. 85 (p=. 03) among the subgroup undergoing PCI and 1. 17 (p=0. 14) among the subgroup not undergoing PCI n Overall, for safety, using the CURRENT definitions, double dose clopidogrel produced significant increases in severe and major bleeds. Presented at ESC Congress 2009, Barcelona Spain

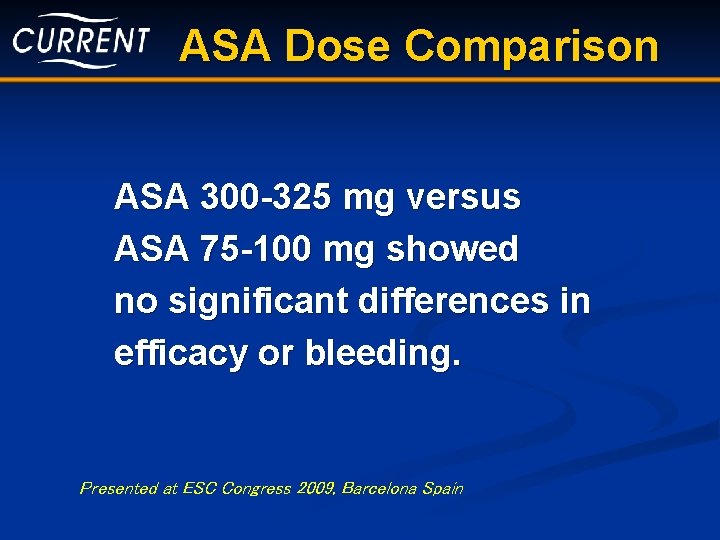
ASA Dose Comparison ASA 300 -325 mg versus ASA 75 -100 mg showed no significant differences in efficacy or bleeding. Presented at ESC Congress 2009, Barcelona Spain

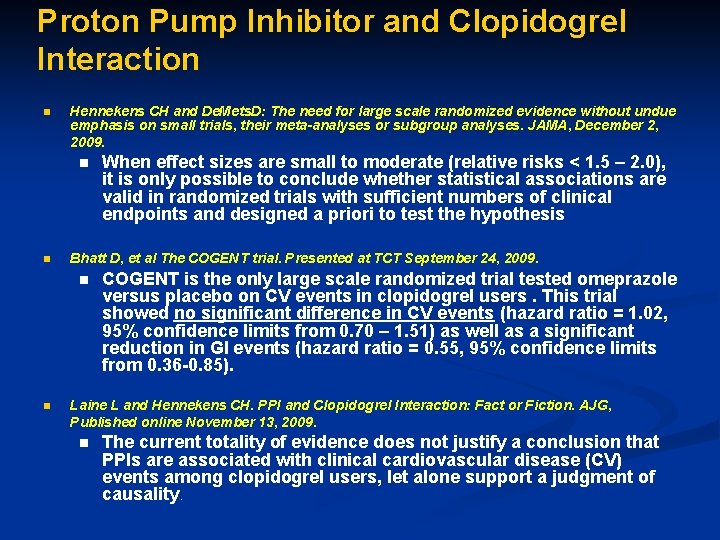
Proton Pump Inhibitor and Clopidogrel Interaction n Hennekens CH and De. Mets. D: The need for large scale randomized evidence without undue emphasis on small trials, their meta-analyses or subgroup analyses. JAMA, December 2, 2009. n n Bhatt D, et al The COGENT trial. Presented at TCT September 24, 2009. n n When effect sizes are small to moderate (relative risks < 1. 5 – 2. 0), it is only possible to conclude whether statistical associations are valid in randomized trials with sufficient numbers of clinical endpoints and designed a priori to test the hypothesis COGENT is the only large scale randomized trial tested omeprazole versus placebo on CV events in clopidogrel users. This trial showed no significant difference in CV events (hazard ratio = 1. 02, 95% confidence limits from 0. 70 – 1. 51) as well as a significant reduction in GI events (hazard ratio = 0. 55, 95% confidence limits from 0. 36 -0. 85). Laine L and Hennekens CH. PPI and Clopidogrel Interaction: Fact or Fiction. AJG, Published online November 13, 2009. n The current totality of evidence does not justify a conclusion that PPIs are associated with clinical cardiovascular disease (CV) events among clopidogrel users, let alone support a judgment of causality.

Proton Pump Inhibitor and Clopidogrel Interaction …According to US FDA November 17, 2009 New data show that when clopidogrel and omeprazole are taken together, the effectiveness of clopidogrel is reduced. Patients at risk for heart attacks or strokes who use clopidogrel to prevent blood clots will not get the full effect of this medicine if they are also taking omeprazole. http: //www. fda. gov/Drugs/Drug. Safety/Postmarket. Drug. Safety. Informationfor. Patien tsand. Providers/Drug. Safety. Informationfor. Heathcare. Professionals/ucm 190787. htm

Proton Pump Inhibitor and Clopidogrel Interaction …According to Laine and Hennekens November 13, 2009 In randomized trials, PPIs seem to decrease recurrent ulcer bleeding in patients who bled on low-dose aspirin and continue aspirin. In addition, randomized, placebo-controlled trials show that both PPIs and histamine-2 receptor antagonists decrease the development of endoscopic ulcers in lowdose aspirin users. Current consensus recommendations do not specifically address clopidogrel monotherapy, but do state that patients taking dual antiplatelet therapy should receive a PPI. Laine L and Hennekens CH. PPI and Clopidogrel Interaction: Fact or Fiction. AJG, Published online November 13, 2009.

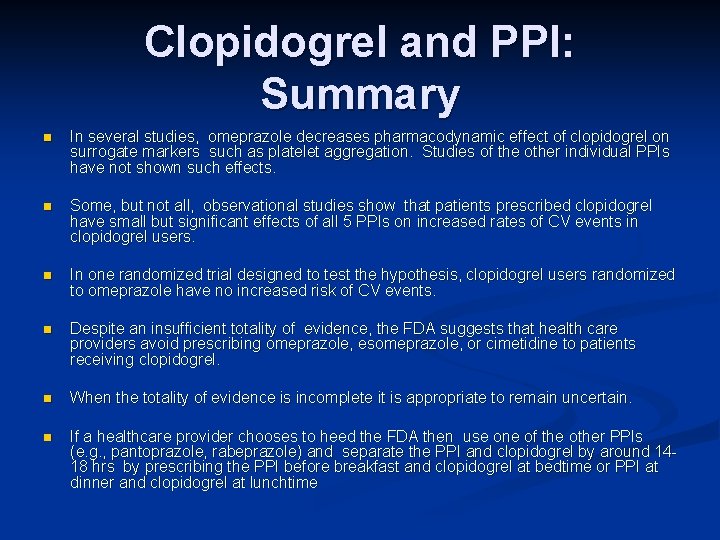
Clopidogrel and PPI: Summary n In several studies, omeprazole decreases pharmacodynamic effect of clopidogrel on surrogate markers such as platelet aggregation. Studies of the other individual PPIs have not shown such effects. n Some, but not all, observational studies show that patients prescribed clopidogrel have small but significant effects of all 5 PPIs on increased rates of CV events in clopidogrel users. n In one randomized trial designed to test the hypothesis, clopidogrel users randomized to omeprazole have no increased risk of CV events. n Despite an insufficient totality of evidence, the FDA suggests that health care providers avoid prescribing omeprazole, esomeprazole, or cimetidine to patients receiving clopidogrel. n When the totality of evidence is incomplete it is appropriate to remain uncertain. n If a healthcare provider chooses to heed the FDA then use one of the other PPIs (e. g. , pantoprazole, rabeprazole) and separate the PPI and clopidogrel by around 1418 hrs by prescribing the PPI before breakfast and clopidogrel at bedtime or PPI at dinner and clopidogrel at lunchtime

New Oral Antiplatelet Drugs Adenosine Diphosphate-Receptor Antagonists Prasugrel n n n Thienopyridine More rapid onset of action than clopidogrel Irreversible inhibitor of the P 2 Y 12 receptor Ticagrelor * n n n Cyclo-pentyl-triazopyrimidine (CPTP) More rapid onset of action than clopidogrel Reversible inhibitor of the P 2 Y 12 receptor * Not approved by FDA

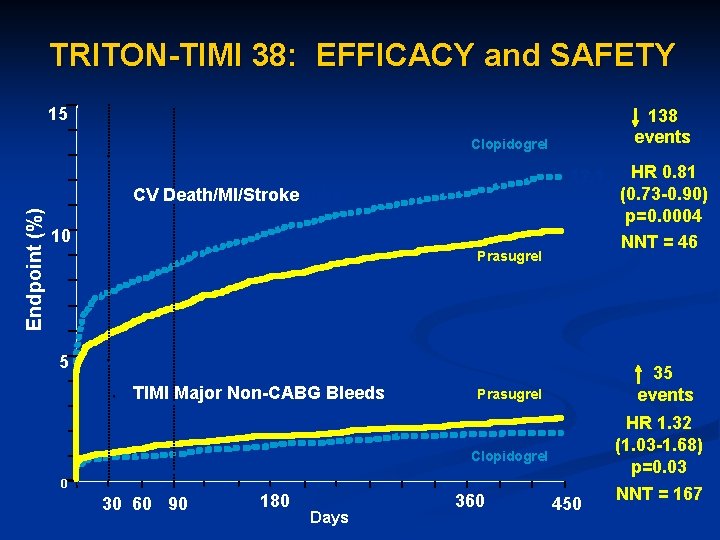
Triton-TIMI 38 13, 608 patients with moderate to high-risk acute coronary syndromes with scheduled PCI n Randomized to prasugrel (60 mg loading dose and a 10 mg daily maintenance dose) or clopidogrel (300 mg loading dose and a 75 mg daily maintenance dose) for 6 -15 months. n Triton –TIMI Investigators. NEJM; 357: 2001 2015

TRITON-TIMI 38: EFFICACY and SAFETY 15 138 events Clopidogrel CV Death/MI/Stroke CV Death / MI / Stroke Endpoint (%) HR 0. 81 (0. 73 -0. 90) p=0. 0004 9. 9 NNT = 46 12. 1 10 Prasugrel 5 TIMI Major Non-CABG Bleeds 35 events Prasugrel Clopidogrel 2. 4 HR 1. 32 1. 8 (1. 03 -1. 68) p=0. 03 0 0 30 60 90 180 Days 270 360 450 NNT = 167

PLATO Ticagrelor vs Clopidogrel in Patients with Acute Coronary Syndromes 18, 624 patients with acute coronary syndromes n Randomization: n Ticagrelor 180 mg loading dose, 90 mg BID n Clopidogrel 300 -600 mg loading dose, 75 mg QD n n All patients received ASA 75 -325 mg Wallentin, L et al NEJM 2009; 361: 1045 -1057

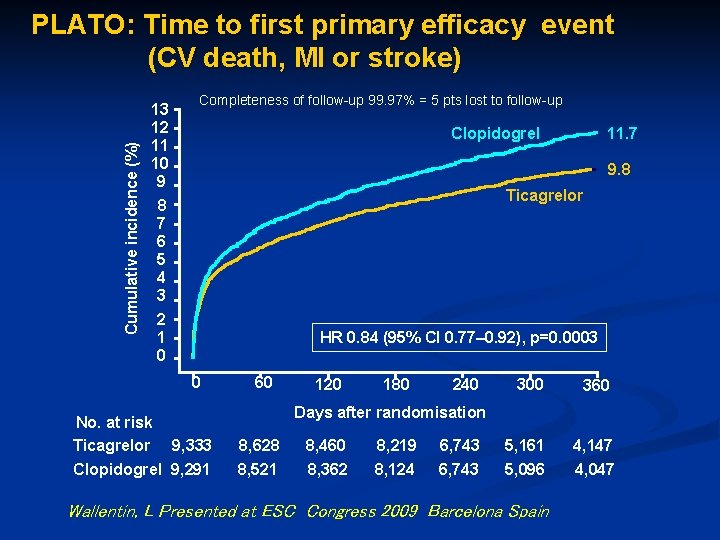
Cumulative incidence (%) PLATO: Time to first primary efficacy event (CV death, MI or stroke) 13 12 11 10 9 8 7 6 5 4 3 2 1 0 Completeness of follow-up 99. 97% = 5 pts lost to follow-up 9. 8 Ticagrelor HR 0. 84 (95% CI 0. 77– 0. 92), p=0. 0003 0 No. at risk Ticagrelor 11. 7 Clopidogrel 9, 333 Clopidogrel 9, 291 60 120 180 240 300 360 Days after randomisation 8, 628 8, 521 8, 460 8, 362 8, 219 8, 124 6, 743 5, 161 5, 096 Wallentin, L Presented at ESC Congress 2009 Barcelona Spain 4, 147 4, 047

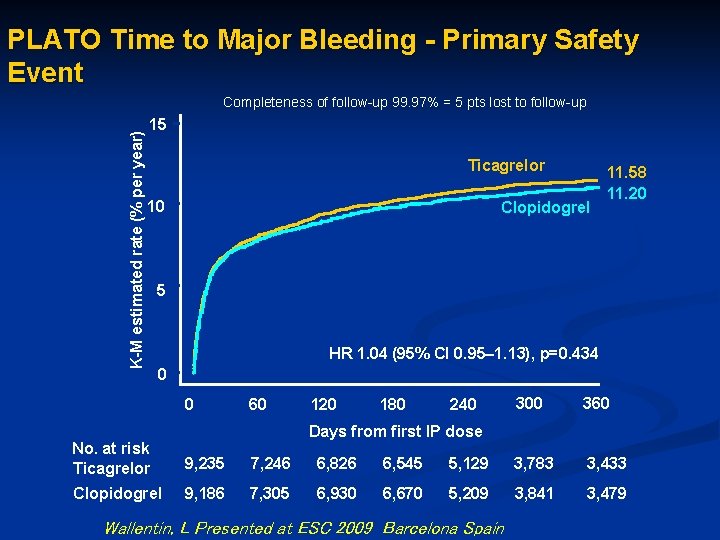
PLATO Time to Major Bleeding - Primary Safety Event K-M estimated rate (% per year) Completeness of follow-up 99. 97% = 5 pts lost to follow-up 15 Ticagrelor 10 Clopidogrel 11. 58 11. 20 5 HR 1. 04 (95% CI 0. 95– 1. 13), p=0. 434 0 0 60 120 180 240 300 360 Days from first IP dose No. at risk Ticagrelor 9, 235 7, 246 6, 826 6, 545 5, 129 3, 783 3, 433 Clopidogrel 9, 186 7, 305 6, 930 6, 670 5, 209 3, 841 3, 479 Wallentin, L Presented at ESC 2009 Barcelona Spain

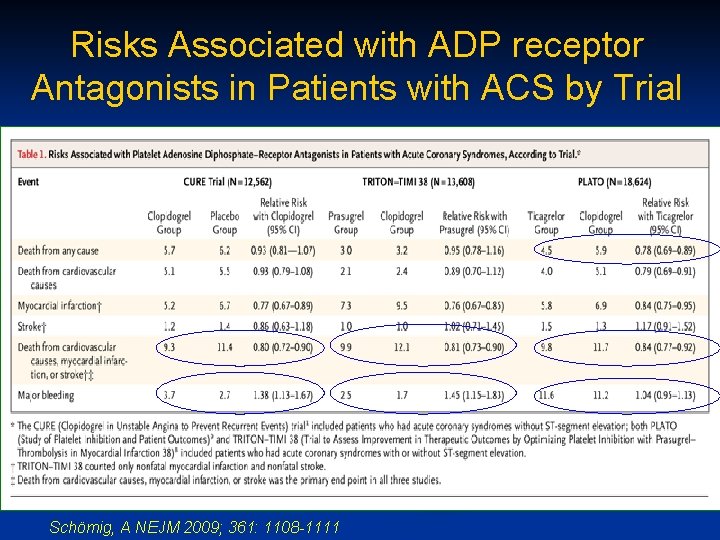
Risks Associated with ADP receptor Antagonists in Patients with ACS by Trial Schӧmig, A NEJM 2009; 361: 1108 -1111

Issues in Clinical Practice Unfortunately, for healthcare providers and their patients, most patients prefer the prescription of pills to the proscription of harmful lifestyles.

Double Cheeseburger, Large Fries, Jumbo Coffee. . Oh And An Aspirin -Gotta Take Care Of The Ticker Y’Know. Aspirin May Reduce Risk Of Heart Attack New Yorker Magazine. 1988.

French Fries 20 years ago 210 calories 2. 4 ounces Today 610 calories How many calories are 6. 9 ounces in these fries? Calorie difference: 400 Calories How to burn* 400 calories: Walk 2 hour 20 minutes *Based on 130 -pound person.

Darwinism and Risk of Cardiovascular Disease

Walking the Dog

Established Risk Factors for CHD Blood cholesterol 10% = 20%-30% in CHD High blood pressure 5 -6 mm Hg = 42% in Stroke = 16% in CHD Cigarette smoking Cessation = 50%-70% in CHD Body weight BMI<25 vs BMI>27 = 35%-55% in CHD Physical activity 20 -minute brisk walk daily = 35%-55% in CHD



“We must all hang together, or assuredly we shall hang separately. ” – Benjamin Franklin July 4, 1776

GOALS OF HEALTH CARE PROVIDERS AND ACADEMIC RESEARCHERS Maximize benefit and minimize risk which is not to be confused with avoidance of risk. Make clinical decisions based on the totality of evidence not dependence on particular subgroups of particular studies. Avoid misstatements of benefit to risk ratios which may increase publicity, academic promotions and grant support in the short run but confuse colleagues and frighten patients and make it more difficult to conduct high quality research ( COX-2 inhibitors and glitazones)

 Guidelines for antiplatelet and fibrinolytic therapy
Guidelines for antiplatelet and fibrinolytic therapy Antiplatelet mechanism of action
Antiplatelet mechanism of action Tavr antiplatelet guidelines
Tavr antiplatelet guidelines Which is an alternative of log based recovery
Which is an alternative of log based recovery Sami2c3
Sami2c3 Both psychoanalysis and humanistic therapy stress
Both psychoanalysis and humanistic therapy stress Bioness integrated therapy system price
Bioness integrated therapy system price Humanistic therapy aims to
Humanistic therapy aims to Basic knowledge of food and beverage
Basic knowledge of food and beverage Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc
V cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số nguyên tố là
Số nguyên tố là Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Sql insert update delete query
Sql insert update delete query Data redundancy and update anomalies
Data redundancy and update anomalies Zechariah
Zechariah Zechariah 2:8-9
Zechariah 2:8-9 University community plan
University community plan Temporary update problem in dbms
Temporary update problem in dbms Sab abdate.com
Sab abdate.com Lnes
Lnes Update data sisdmk
Update data sisdmk Gtcs professional update examples
Gtcs professional update examples Position update formula
Position update formula Position update formula
Position update formula Fiberhome hg6145f firmware
Fiberhome hg6145f firmware Move update compliance
Move update compliance Mdh situation update
Mdh situation update Iqcs master record
Iqcs master record Utils ctl update ctlfile
Utils ctl update ctlfile 811 ticket number
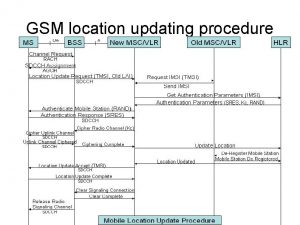
811 ticket number Location update in gsm
Location update in gsm