Anticoagulation vs Antiplatelet Therapy after Transcatheter Aortic Valve






- Slides: 27

Anticoagulation vs. Antiplatelet Therapy after Transcatheter Aortic Valve Replacement in Low Risk Patients Toby Rogers, MD, Ph. D Med. Star Heart & Vascular Institute, Washington, DC National Heart Lung and Blood Institute, National Institutes of Health, Bethesda, MD On behalf of the LRT 2. 0 Investigators

Disclosures Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s): Consultant and Proctor: Edwards Lifesciences, Medtronic

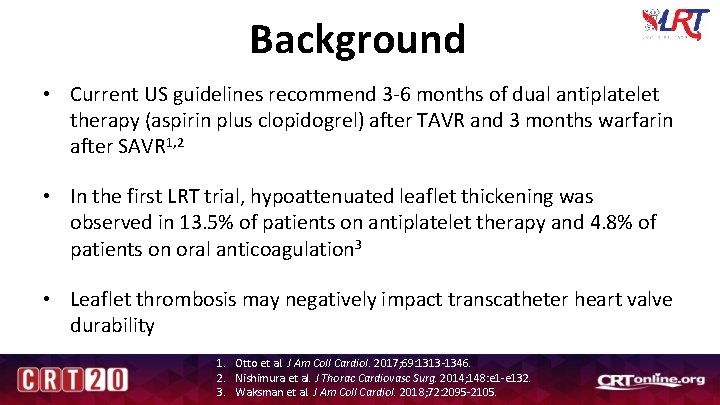
Background • Current US guidelines recommend 3 -6 months of dual antiplatelet therapy (aspirin plus clopidogrel) after TAVR and 3 months warfarin after SAVR 1, 2 • In the first LRT trial, hypoattenuated leaflet thickening was observed in 13. 5% of patients on antiplatelet therapy and 4. 8% of patients on oral anticoagulation 3 • Leaflet thrombosis may negatively impact transcatheter heart valve durability 1. Otto et al. J Am Coll Cardiol. 2017; 69: 1313 -1346. 2. Nishimura et al. J Thorac Cardiovasc Surg. 2014; 148: e 1 -e 132. 3. Waksman et al. J Am Coll Cardiol. 2018; 72: 2095 -2105.

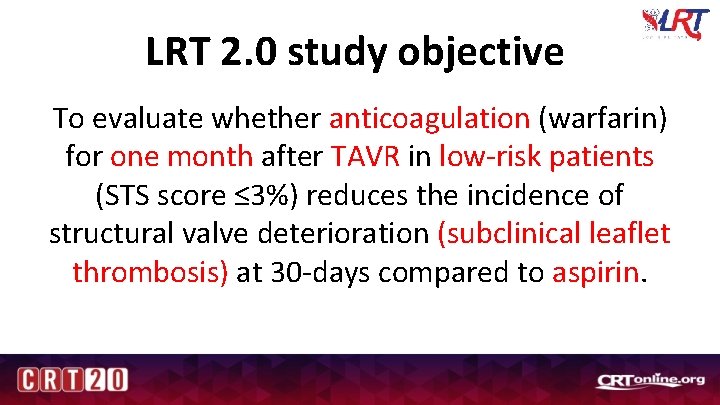
LRT 2. 0 study objective To evaluate whether anticoagulation (warfarin) for one month after TAVR in low-risk patients (STS score ≤ 3%) reduces the incidence of structural valve deterioration (subclinical leaflet thrombosis) at 30 -days compared to aspirin.

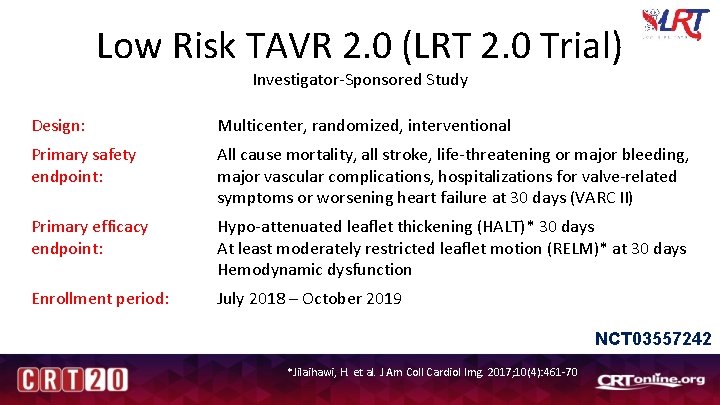
Low Risk TAVR 2. 0 (LRT 2. 0 Trial) Investigator-Sponsored Study Design: Multicenter, randomized, interventional Primary safety endpoint: All cause mortality, all stroke, life-threatening or major bleeding, major vascular complications, hospitalizations for valve-related symptoms or worsening heart failure at 30 days (VARC II) Primary efficacy endpoint: Hypo-attenuated leaflet thickening (HALT)* 30 days At least moderately restricted leaflet motion (RELM)* at 30 days Hemodynamic dysfunction Enrollment period: July 2018 – October 2019 NCT 03557242 *Jilaihawi, H. et al. J Am Coll Cardiol Img. 2017; 10(4): 461 -70

Trial administration National Principal Investigators Toby Rogers, MD, Ph. D Ron Waksman, MD Christian Shults, MD Med. Star Cardiovascular Research Network Director of Clinical Research Operations Rebecca Torguson, MPH Clinical Review Committee Interventional Cardiology Itsik Ben-Dor, MD Anees Musallam, MD Manager, Data Management Paige E. Craig, MPH Cardiothoracic Surgery Christian Shults, MD Independent Clinical Events Committee Chairman Hector Garcia-Garcia, MD Database Technology Director Alan Monath Core Laboratories Director Echo Core Lab Federico Asch, MD Director CT Core Lab Gaby Weissman, MD Data & Safety Monitoring Board Chairman William Weintraub, MD

Enrolling sites Site Name Med. Star Washington Hospital Center Rhode Island Hospital Stony Brook Hospital Maine Medical Center Henrico Doctors’ Hospital Foundation for Cardiovascular Medicine St. John Heart Institute The Valley Hospital Sentara Norfolk General Hospital Principal Investigators Ron Waksman, MD Christian Shults, MD Paul Gordon, MD Afshin Ehsan, MD Puja Parikh, MD Thomas Bilfinger, MD David Butzel, MD Scott Buchanan, MD Robert Levitt, MD Chiwon Hahn, MD Maurice Buchbinder, MD Ricardo Moreno, MD Nicholas Hanna, MD George Comas, MD Scott Wilson, MD Mariano Brizzio, MD Paul Mahoney, MD Joseph Newton, MD Location Washington, DC Providence, RI Stony Brook, NY Portland, ME Richmond, VA San Diego, CA Tulsa, OK Ridgewood, NJ Norfolk, VA

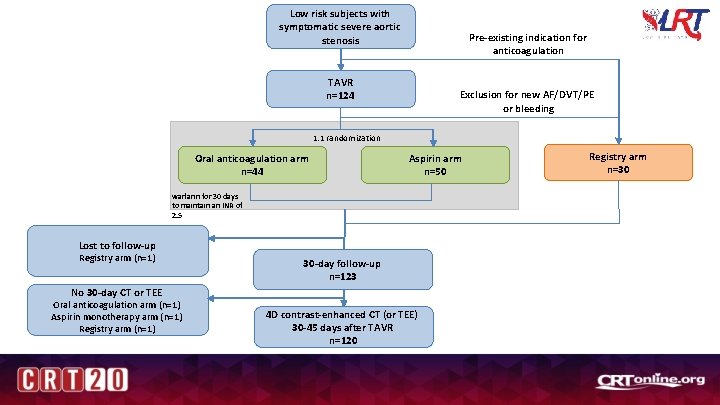
Low risk subjects with symptomatic severe aortic stenosis Pre-existing indication for anticoagulation TAVR n=124 Exclusion for new AF/DVT/PE or bleeding 1: 1 randomization Oral anticoagulation arm n=44 Aspirin arm n=50 warfarin for 30 days to maintain an INR of 2. 5 Lost to follow-up Registry arm (n=1) 30 -day follow-up n=123 No 30 -day CT or TEE Oral anticoagulation arm (n=1) Aspirin monotherapy arm (n=1) Registry arm (n=1) 4 D contrast-enhanced CT (or TEE) 30 -45 days after TAVR n=120 Registry arm n=30

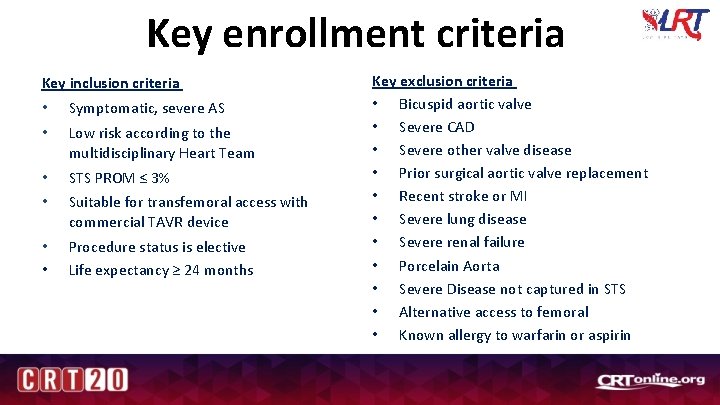
Key enrollment criteria Key inclusion criteria • • Symptomatic, severe AS • • STS PROM ≤ 3% • Procedure status is elective Life expectancy ≥ 24 months • Low risk according to the multidisciplinary Heart Team Suitable for transfemoral access with commercial TAVR device Key exclusion criteria • Bicuspid aortic valve • Severe CAD • Severe other valve disease • Prior surgical aortic valve replacement • Recent stroke or MI • Severe lung disease • Severe renal failure • Porcelain Aorta • Severe Disease not captured in STS • Alternative access to femoral • Known allergy to warfarin or aspirin

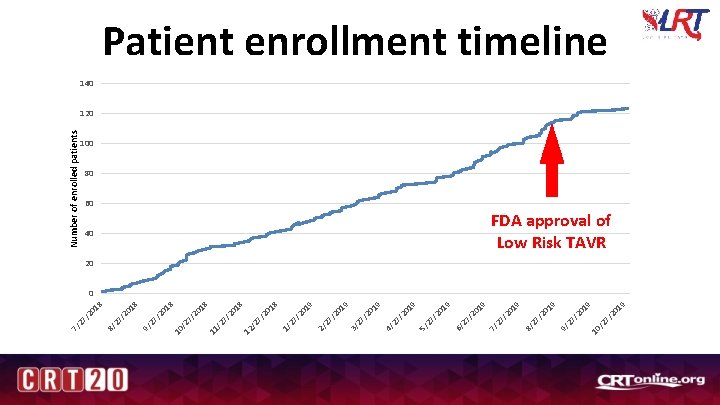
/2 7 10 19 /2 0 27 9/ 19 /2 0 27 8/ 19 Number of enrolled patients 40 /2 0 27 7/ 19 19 /2 0 27 6/ 19 /2 0 27 5/ /2 0 27 4/ 19 19 /2 0 27 3/ /2 0 27 2/ 19 18 /2 0 27 1/ /2 7 12 /2 7 11 /2 7 10 /2 0 27 9/ 18 18 /2 0 27 8/ /2 0 27 7/ Patient enrollment timeline 140 120 100 80 60 FDA approval of Low Risk TAVR 20 0

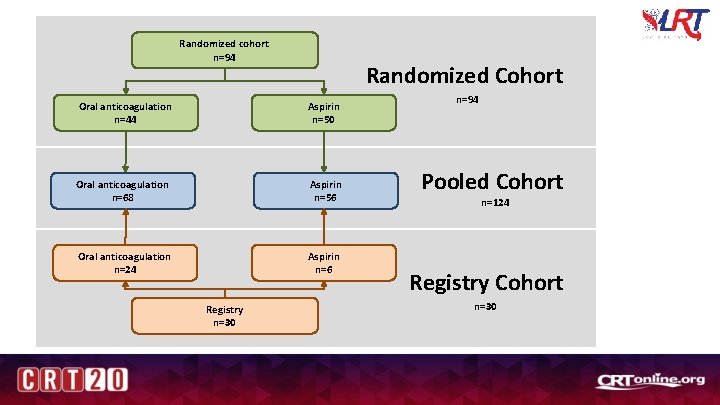
Randomized cohort n=94 Randomized Cohort Oral anticoagulation n=44 Aspirin n=50 Oral anticoagulation n=68 Aspirin n=56 Oral anticoagulation n=24 Aspirin n=6 Registry n=30 n=94 Pooled Cohort n=124 Registry Cohort n=30

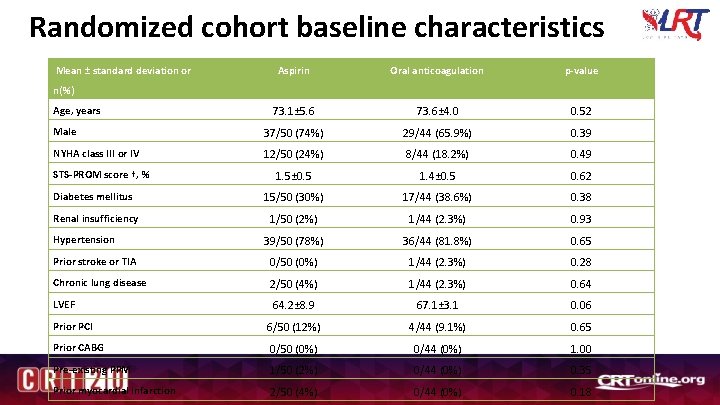
Randomized cohort baseline characteristics Mean ± standard deviation or Aspirin Oral anticoagulation p-value 73. 1± 5. 6 73. 6± 4. 0 0. 52 Male 37/50 (74%) 29/44 (65. 9%) 0. 39 NYHA class III or IV 12/50 (24%) 8/44 (18. 2%) 0. 49 1. 5± 0. 5 1. 4± 0. 5 0. 62 Diabetes mellitus 15/50 (30%) 17/44 (38. 6%) 0. 38 Renal insufficiency 1/50 (2%) 1/44 (2. 3%) 0. 93 39/50 (78%) 36/44 (81. 8%) 0. 65 Prior stroke or TIA 0/50 (0%) 1/44 (2. 3%) 0. 28 Chronic lung disease 2/50 (4%) 1/44 (2. 3%) 0. 64 LVEF 64. 2± 8. 9 67. 1± 3. 1 0. 06 Prior PCI 6/50 (12%) 4/44 (9. 1%) 0. 65 Prior CABG 0/50 (0%) 0/44 (0%) 1. 00 Pre-existing PPM 1/50 (2%) 0/44 (0%) 0. 35 Prior myocardial infarction 2/50 (4%) 0/44 (0%) 0. 18 n(%) Age, years STS-PROM score †, % Hypertension

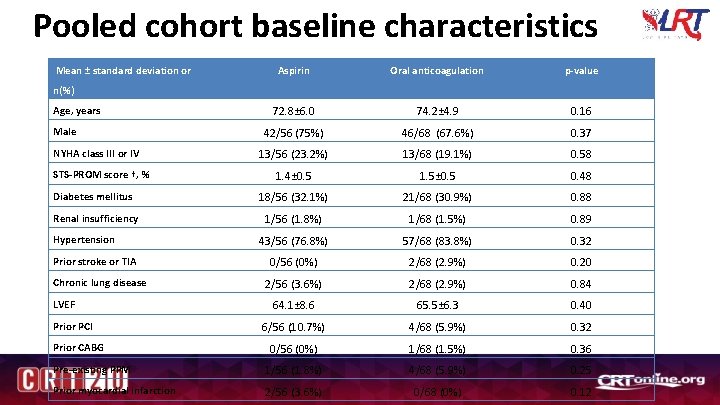
Pooled cohort baseline characteristics Mean ± standard deviation or Aspirin Oral anticoagulation p-value 72. 8± 6. 0 74. 2± 4. 9 0. 16 42/56 (75%) 46/68 (67. 6%) 0. 37 13/56 (23. 2%) 13/68 (19. 1%) 0. 58 1. 4± 0. 5 1. 5± 0. 5 0. 48 Diabetes mellitus 18/56 (32. 1%) 21/68 (30. 9%) 0. 88 Renal insufficiency 1/56 (1. 8%) 1/68 (1. 5%) 0. 89 43/56 (76. 8%) 57/68 (83. 8%) 0. 32 0/56 (0%) 2/68 (2. 9%) 0. 20 2/56 (3. 6%) 2/68 (2. 9%) 0. 84 64. 1± 8. 6 65. 5± 6. 3 0. 40 6/56 (10. 7%) 4/68 (5. 9%) 0. 32 0/56 (0%) 1/68 (1. 5%) 0. 36 Pre-existing PPM 1/56 (1. 8%) 4/68 (5. 9%) 0. 25 Prior myocardial infarction 2/56 (3. 6%) 0/68 (0%) 0. 12 n(%) Age, years Male NYHA class III or IV STS-PROM score †, % Hypertension Prior stroke or TIA Chronic lung disease LVEF Prior PCI Prior CABG

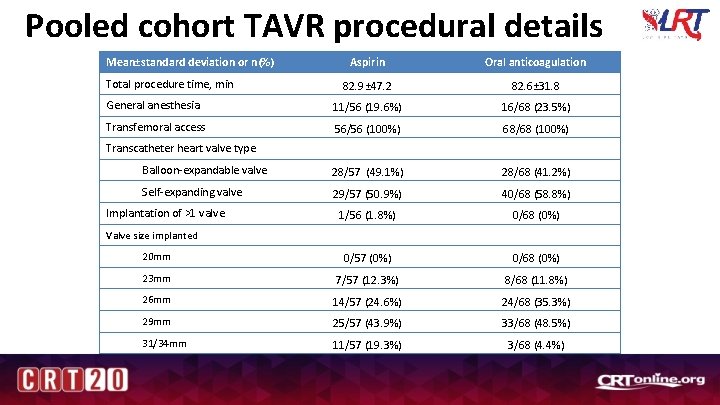
Pooled cohort TAVR procedural details Mean±standard deviation or n(%) Aspirin Oral anticoagulation 82. 9± 47. 2 82. 6± 31. 8 General anesthesia 11/56 (19. 6%) 16/68 (23. 5%) Transfemoral access 56/56 (100%) 68/68 (100%) Balloon-expandable valve 28/57 (49. 1%) 28/68 (41. 2%) Self-expanding valve 29/57 (50. 9%) 40/68 (58. 8%) 1/56 (1. 8%) 0/68 (0%) 20 mm 0/57 (0%) 0/68 (0%) 23 mm 7/57 (12. 3%) 8/68 (11. 8%) 26 mm 14/57 (24. 6%) 24/68 (35. 3%) 29 mm 25/57 (43. 9%) 33/68 (48. 5%) 31/34 mm 11/57 (19. 3%) 3/68 (4. 4%) Total procedure time, min Transcatheter heart valve type Implantation of >1 valve Valve size implanted

Primary safety endpoint Zero mortality at 30 days

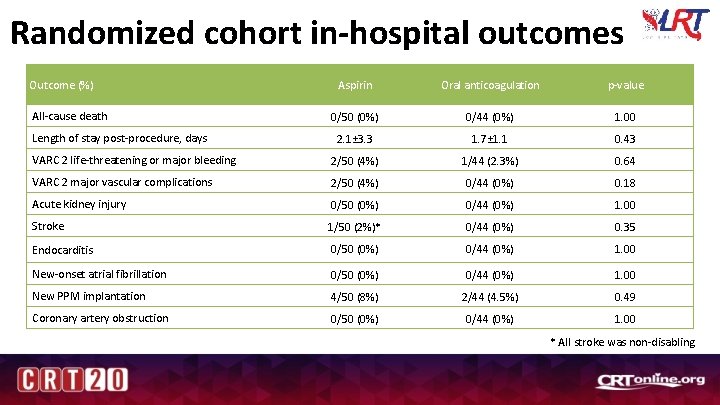
Randomized cohort in-hospital outcomes Outcome (%) Aspirin Oral anticoagulation p-value 0/50 (0%) 0/44 (0%) 1. 00 2. 1± 3. 3 1. 7± 1. 1 0. 43 VARC 2 life-threatening or major bleeding 2/50 (4%) 1/44 (2. 3%) 0. 64 VARC 2 major vascular complications 2/50 (4%) 0/44 (0%) 0. 18 Acute kidney injury 0/50 (0%) 0/44 (0%) 1. 00 Stroke 1/50 (2%)* 0/44 (0%) 0. 35 Endocarditis 0/50 (0%) 0/44 (0%) 1. 00 New-onset atrial fibrillation 0/50 (0%) 0/44 (0%) 1. 00 New PPM implantation 4/50 (8%) 2/44 (4. 5%) 0. 49 Coronary artery obstruction 0/50 (0%) 0/44 (0%) 1. 00 All-cause death Length of stay post-procedure, days * All stroke was non-disabling

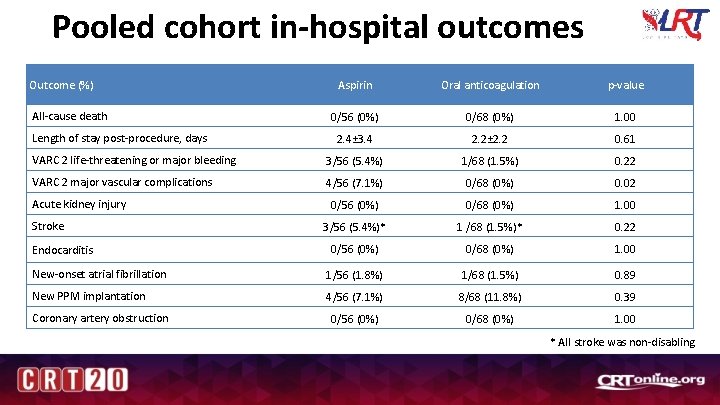
Pooled cohort in-hospital outcomes Outcome (%) Aspirin Oral anticoagulation p-value 0/56 (0%) 0/68 (0%) 1. 00 2. 4± 3. 4 2. 2± 2. 2 0. 61 VARC 2 life-threatening or major bleeding 3/56 (5. 4%) 1/68 (1. 5%) 0. 22 VARC 2 major vascular complications 4/56 (7. 1%) 0/68 (0%) 0. 02 0/56 (0%) 0/68 (0%) 1. 00 3/56 (5. 4%)* 1 /68 (1. 5%)* 0. 22 0/56 (0%) 0/68 (0%) 1. 00 New-onset atrial fibrillation 1/56 (1. 8%) 1/68 (1. 5%) 0. 89 New PPM implantation 4/56 (7. 1%) 8/68 (11. 8%) 0. 39 0/56 (0%) 0/68 (0%) 1. 00 All-cause death Length of stay post-procedure, days Acute kidney injury Stroke Endocarditis Coronary artery obstruction * All stroke was non-disabling

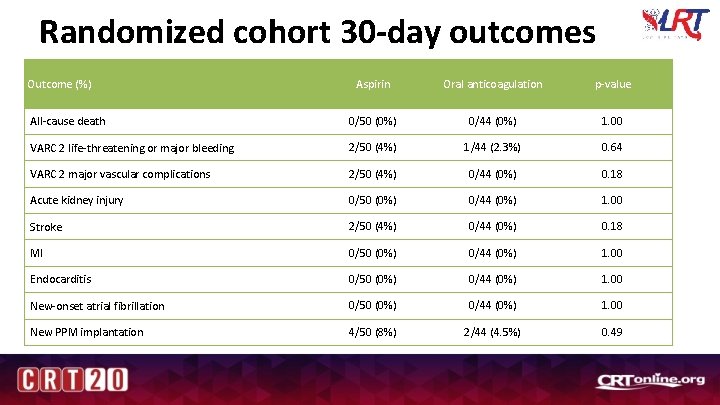
Randomized cohort 30 -day outcomes Outcome (%) Aspirin Oral anticoagulation p-value All-cause death 0/50 (0%) 0/44 (0%) 1. 00 VARC 2 life-threatening or major bleeding 2/50 (4%) 1/44 (2. 3%) 0. 64 VARC 2 major vascular complications 2/50 (4%) 0/44 (0%) 0. 18 Acute kidney injury 0/50 (0%) 0/44 (0%) 1. 00 Stroke 2/50 (4%) 0/44 (0%) 0. 18 MI 0/50 (0%) 0/44 (0%) 1. 00 Endocarditis 0/50 (0%) 0/44 (0%) 1. 00 New-onset atrial fibrillation 0/50 (0%) 0/44 (0%) 1. 00 New PPM implantation 4/50 (8%) 2/44 (4. 5%) 0. 49

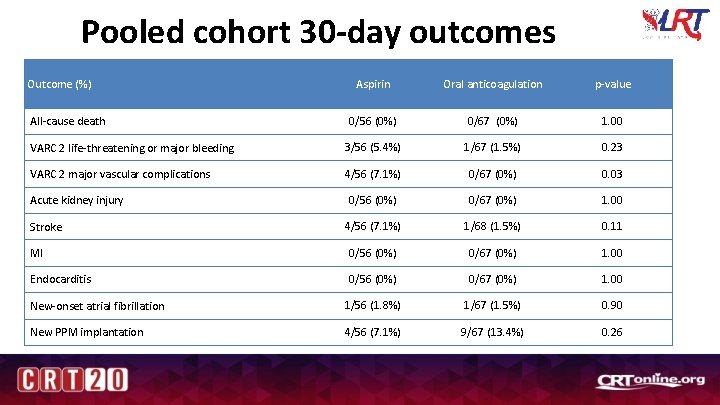
Pooled cohort 30 -day outcomes Outcome (%) Aspirin Oral anticoagulation p-value 0/56 (0%) 0/67 (0%) 1. 00 VARC 2 life-threatening or major bleeding 3/56 (5. 4%) 1/67 (1. 5%) 0. 23 VARC 2 major vascular complications 4/56 (7. 1%) 0/67 (0%) 0. 03 0/56 (0%) 0/67 (0%) 1. 00 4/56 (7. 1%) 1/68 (1. 5%) 0. 11 MI 0/56 (0%) 0/67 (0%) 1. 00 Endocarditis 0/56 (0%) 0/67 (0%) 1. 00 New-onset atrial fibrillation 1/56 (1. 8%) 1/67 (1. 5%) 0. 90 New PPM implantation 4/56 (7. 1%) 9/67 (13. 4%) 0. 26 All-cause death Acute kidney injury Stroke

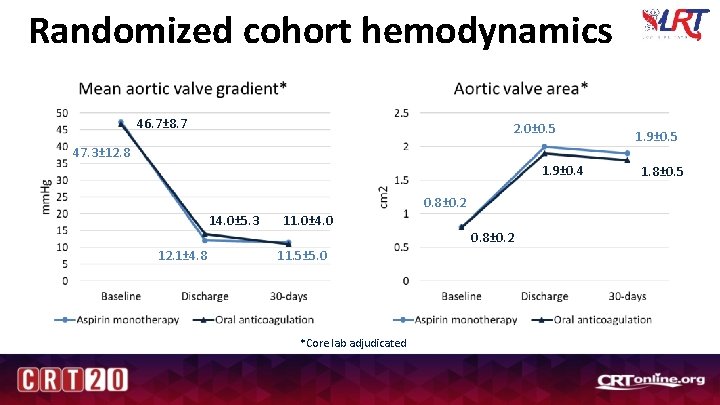
Randomized cohort hemodynamics 46. 7± 8. 7 2. 0± 0. 5 47. 3± 12. 8 1. 9± 0. 4 0. 8± 0. 2 14. 0± 5. 3 11. 0± 4. 0 0. 8± 0. 2 12. 1± 4. 8 11. 5± 5. 0 *Core lab adjudicated 1. 9± 0. 5 1. 8± 0. 5

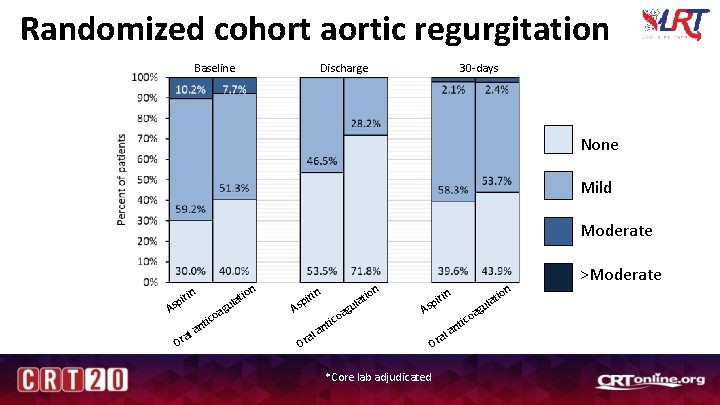
Randomized cohort aortic regurgitation Discharge Baseline 30 -days None Mild Moderate in ir sp A O a ral ic nt ion g oa t ula in ir sp A O g oa tic n a ral ion t ula n io lat u ag in pir As a Or *Core lab adjudicated ico nt l a >Moderate

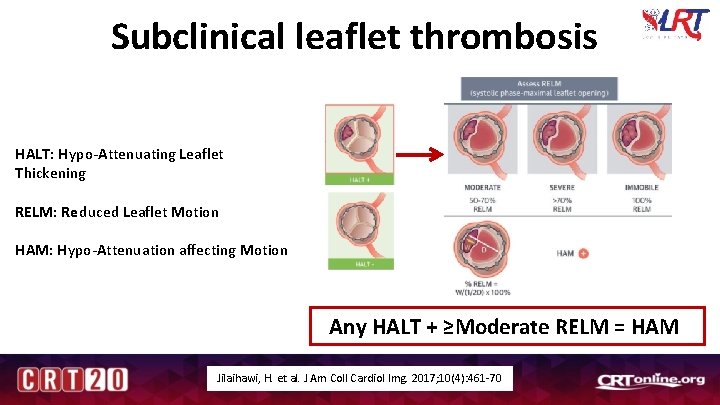
Subclinical leaflet thrombosis HALT: Hypo-Attenuating Leaflet Thickening RELM: Reduced Leaflet Motion HAM: Hypo-Attenuation affecting Motion Any HALT + ≥Moderate RELM = HAM Jilaihawi, H. et al. J Am Coll Cardiol Img. 2017; 10(4): 461 -70

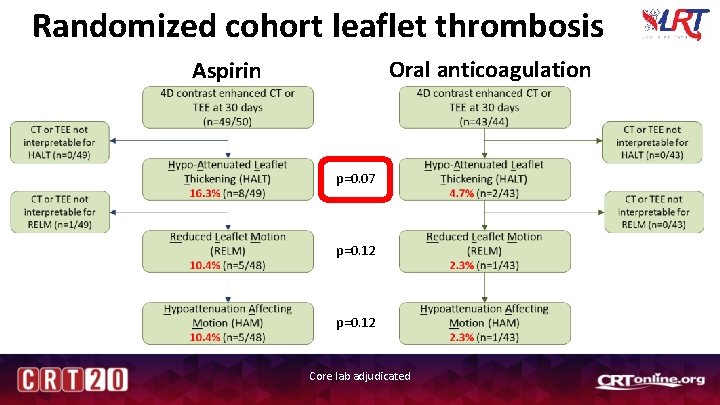
Randomized cohort leaflet thrombosis Oral anticoagulation Aspirin p=0. 07 p=0. 12 Core lab adjudicated

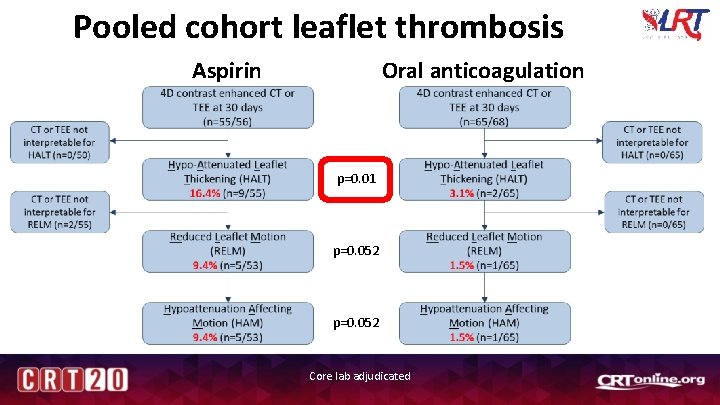
Pooled cohort leaflet thrombosis Aspirin Oral anticoagulation p=0. 01 p=0. 052 Core lab adjudicated

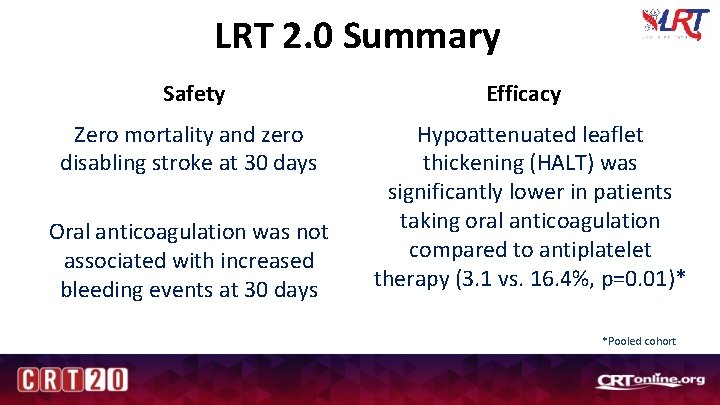
LRT 2. 0 Summary Safety Efficacy Zero mortality and zero disabling stroke at 30 days Hypoattenuated leaflet thickening (HALT) was significantly lower in patients taking oral anticoagulation compared to antiplatelet therapy (3. 1 vs. 16. 4%, p=0. 01)* Oral anticoagulation was not associated with increased bleeding events at 30 days *Pooled cohort

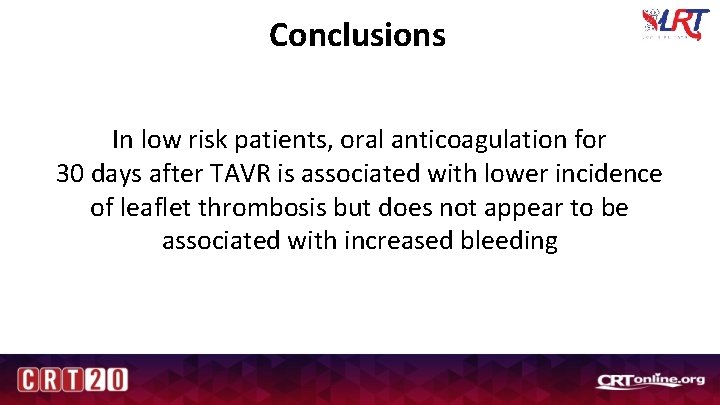
Conclusions In low risk patients, oral anticoagulation for 30 days after TAVR is associated with lower incidence of leaflet thrombosis but does not appear to be associated with increased bleeding

Thank you to all of the LRT 2. 0 patients, site personnel, and investigators who made this trial possible!
 Guidelines for antiplatelet and fibrinolytic therapy
Guidelines for antiplatelet and fibrinolytic therapy Triple therapy anticoagulation
Triple therapy anticoagulation Aortic valve dimensionless index
Aortic valve dimensionless index Aortic semilunar valve sheep heart
Aortic semilunar valve sheep heart Physiological regurgitation
Physiological regurgitation Aortic valve leaflets
Aortic valve leaflets Dvi aortic valve
Dvi aortic valve Aortic semi lunar valve
Aortic semi lunar valve Dvi in echo
Dvi in echo Rcc aortic valve
Rcc aortic valve Aortic root enlargement
Aortic root enlargement Antiplatelet mechanism of action
Antiplatelet mechanism of action Tavr antiplatelet guidelines
Tavr antiplatelet guidelines John 14:1
John 14:1 After me after me after me
After me after me after me Servo valve function
Servo valve function Emory clinic - school of medicine faculty
Emory clinic - school of medicine faculty Anticoagulation
Anticoagulation Anticoagulation
Anticoagulation Wa anticoagulation chart
Wa anticoagulation chart Lahey anticoagulation clinic
Lahey anticoagulation clinic Classification of antiarrhythmic drugs with examples
Classification of antiarrhythmic drugs with examples Heparin drip protocol
Heparin drip protocol Overlake anticoagulation clinic
Overlake anticoagulation clinic Bioness bits cost
Bioness bits cost Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Psychoanalytic vs humanistic
Psychoanalytic vs humanistic Aortic nodes
Aortic nodes