Transcatheter aortic valve implantation for failed surgical aortic






- Slides: 27

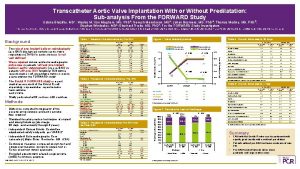
Transcatheter aortic valve implantation for failed surgical aortic bioprostheses using a self-expanding device Early results from the prospective VIVA post-market study Ran Kornowski, MD, FESC, FACC Rabin Medical Center, Petah Tikva, Israel

Disclosure Statement of Financial Interest Within the past 12 months, I have had a financial interest/arrangement or affiliation with the organization listed below. Affiliation/Financial Relationship Company • • Research grant for the conducting the VIVA trial Medtronic

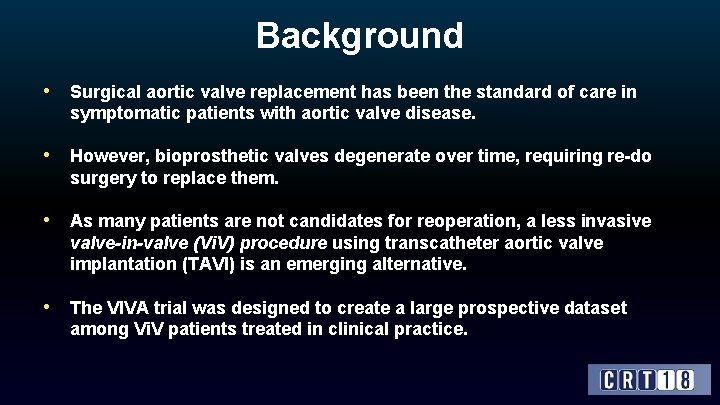
Background • Surgical aortic valve replacement has been the standard of care in symptomatic patients with aortic valve disease. • However, bioprosthetic valves degenerate over time, requiring re-do surgery to replace them. • As many patients are not candidates for reoperation, a less invasive valve-in-valve (Vi. V) procedure using transcatheter aortic valve implantation (TAVI) is an emerging alternative. • The VIVA trial was designed to create a large prospective dataset among Vi. V patients treated in clinical practice.

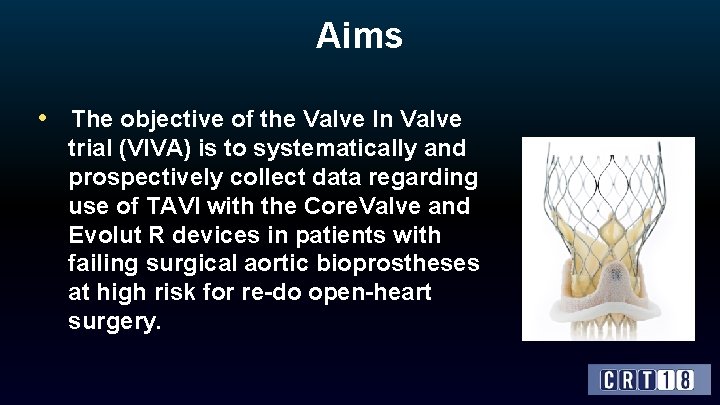
Aims • The objective of the Valve In Valve trial (VIVA) is to systematically and prospectively collect data regarding use of TAVI with the Core. Valve and Evolut R devices in patients with failing surgical aortic bioprostheses at high risk for re-do open-heart surgery.

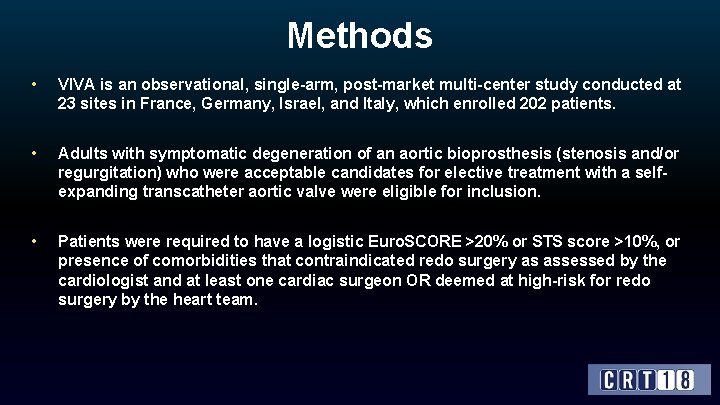
Methods • VIVA is an observational, single-arm, post-market multi-center study conducted at 23 sites in France, Germany, Israel, and Italy, which enrolled 202 patients. • Adults with symptomatic degeneration of an aortic bioprosthesis (stenosis and/or regurgitation) who were acceptable candidates for elective treatment with a selfexpanding transcatheter aortic valve were eligible for inclusion. • Patients were required to have a logistic Euro. SCORE >20% or STS score >10%, or presence of comorbidities that contraindicated redo surgery as assessed by the cardiologist and at least one cardiac surgeon OR deemed at high-risk for redo surgery by the heart team.

Trial Organization Executive Committee/PIs: Publication Committee: R. Kornowski, D. Tchétché, JP Verhoye, B. Chevalier CERC, Massy, France CRO: Sponsor: 1) CEC: P. Ménasché (Chair), G. Sardella, N. Löffelhardt 2) Echo Corelab: M. Poupineau 3) Site management Medtronic

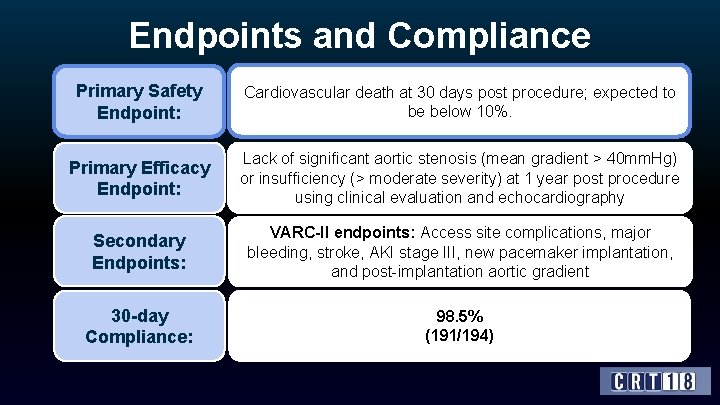
Endpoints and Compliance Primary Safety Endpoint: Cardiovascular death at 30 days post procedure; expected to be below 10%. Primary Efficacy Endpoint: Lack of significant aortic stenosis (mean gradient > 40 mm. Hg) or insufficiency (> moderate severity) at 1 year post procedure using clinical evaluation and echocardiography Secondary Endpoints: VARC-II endpoints: Access site complications, major bleeding, stroke, AKI stage III, new pacemaker implantation, and post-implantation aortic gradient 30 -day Compliance: 98. 5% (191/194)

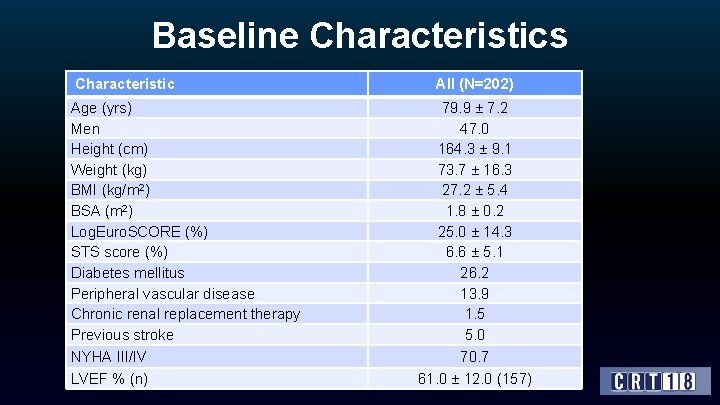
Baseline Characteristics Characteristic Age (yrs) Men Height (cm) Weight (kg) BMI (kg/m 2) BSA (m 2) Log. Euro. SCORE (%) STS score (%) Diabetes mellitus Peripheral vascular disease Chronic renal replacement therapy Previous stroke NYHA III/IV LVEF % (n) All (N=202) 79. 9 ± 7. 2 47. 0 164. 3 ± 9. 1 73. 7 ± 16. 3 27. 2 ± 5. 4 1. 8 ± 0. 2 25. 0 ± 14. 3 6. 6 ± 5. 1 26. 2 13. 9 1. 5 5. 0 70. 7 61. 0 ± 12. 0 (157)

Devices Utilized Core. Valve Enrolled: n=19 Evolut R Enrolled: n=183

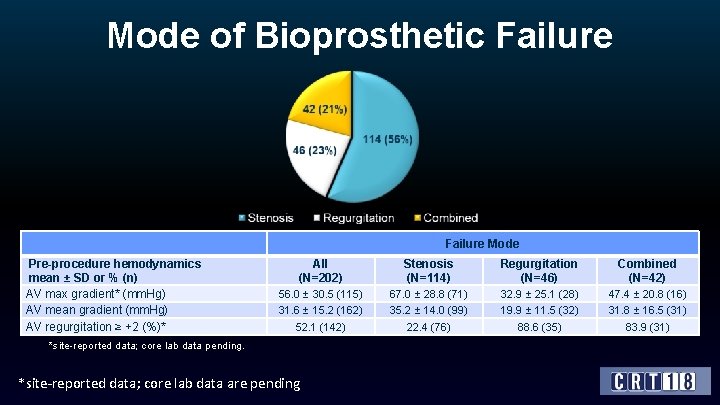
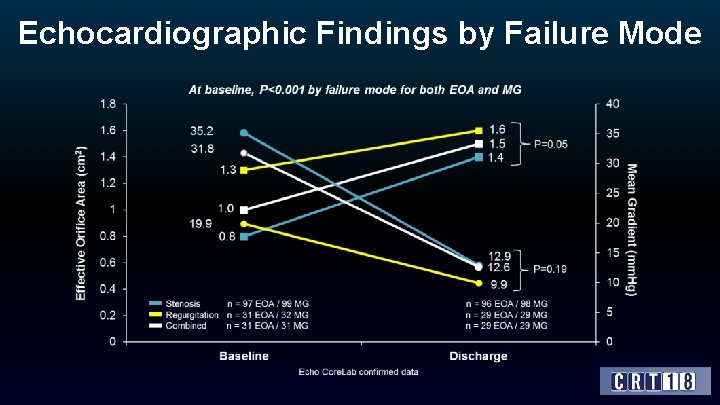
Mode of Bioprosthetic Failure Mode Pre-procedure hemodynamics mean ± SD or % (n) AV max gradient* (mm. Hg) AV mean gradient (mm. Hg) AV regurgitation ≥ +2 (%)* All (N=202) Stenosis (N=114) Regurgitation (N=46) Combined (N=42) 56. 0 ± 30. 5 (115) 67. 0 ± 28. 8 (71) 32. 9 ± 25. 1 (28) 47. 4 ± 20. 8 (16) 31. 6 ± 15. 2 (162) 35. 2 ± 14. 0 (99) 19. 9 ± 11. 5 (32) 31. 8 ± 16. 5 (31) 52. 1 (142) 22. 4 (76) 88. 6 (35) 83. 9 (31) *site-reported data; core lab data pending. *site-reported data; core lab data are pending

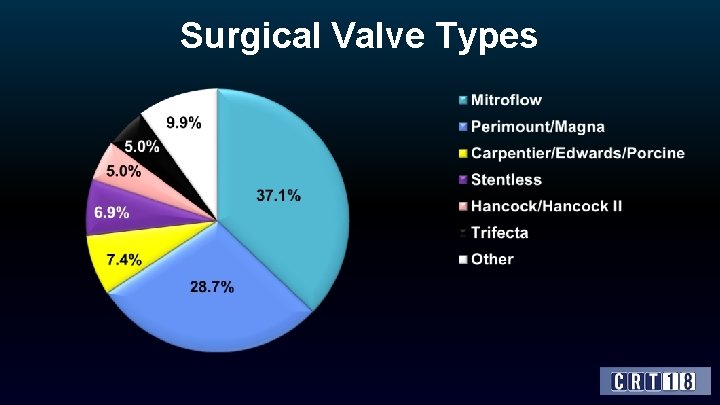
Surgical Valve Types

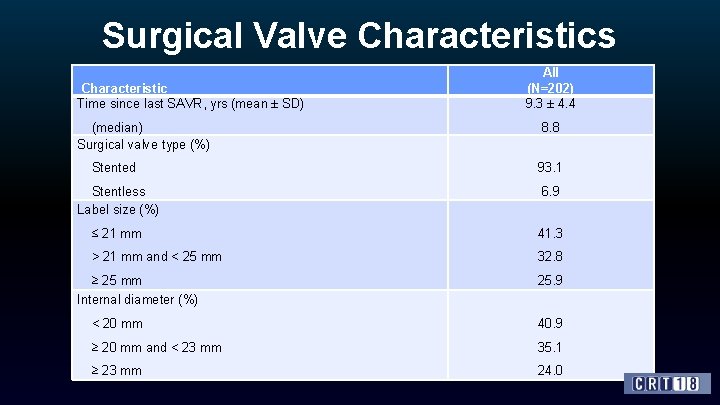
Surgical Valve Characteristics Characteristic Time since last SAVR, yrs (mean ± SD) All (N=202) 9. 3 ± 4. 4 (median) Surgical valve type (%) 8. 8 Stented 93. 1 Stentless Label size (%) 6. 9 ≤ 21 mm 41. 3 > 21 mm and < 25 mm 32. 8 ≥ 25 mm Internal diameter (%) 25. 9 < 20 mm 40. 9 ≥ 20 mm and < 23 mm 35. 1 ≥ 23 mm 24. 0

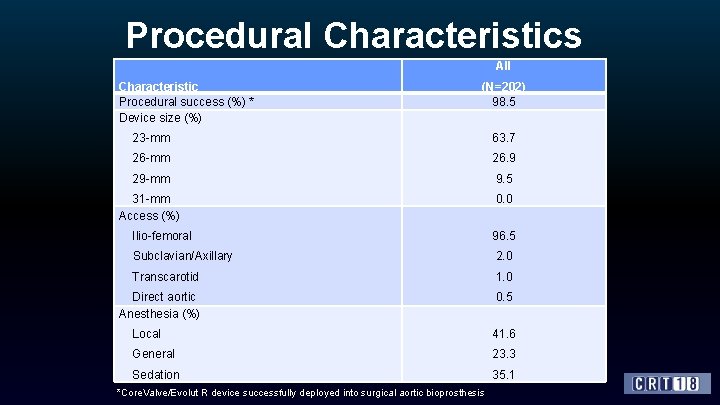
Procedural Characteristics All Characteristic Procedural success (%) * Device size (%) (N=202) 98. 5 23 -mm 63. 7 26 -mm 26. 9 29 -mm 9. 5 31 -mm Access (%) 0. 0 Ilio-femoral 96. 5 Subclavian/Axillary 2. 0 Transcarotid 1. 0 Direct aortic Anesthesia (%) 0. 5 Local 41. 6 General 23. 3 Sedation 35. 1 *Core. Valve/Evolut R device successfully deployed into surgical aortic bioprosthesis

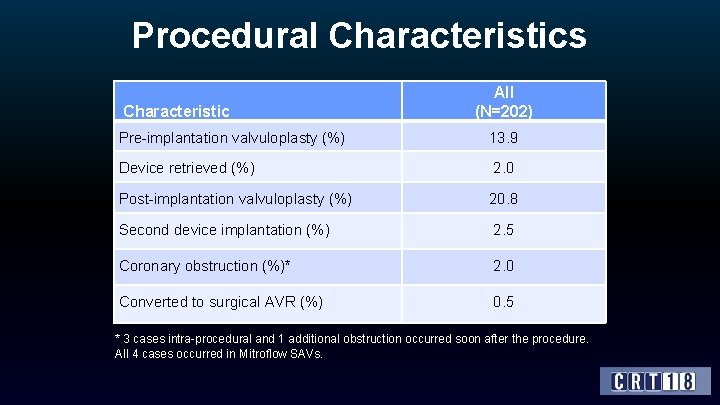
Procedural Characteristics Characteristic All (N=202) Pre-implantation valvuloplasty (%) 13. 9 Device retrieved (%) 2. 0 Post-implantation valvuloplasty (%) 20. 8 Second device implantation (%) 2. 5 Coronary obstruction (%)* 2. 0 Converted to surgical AVR (%) 0. 5 * 3 cases intra-procedural and 1 additional obstruction occurred soon after the procedure. All 4 cases occurred in Mitroflow SAVs.

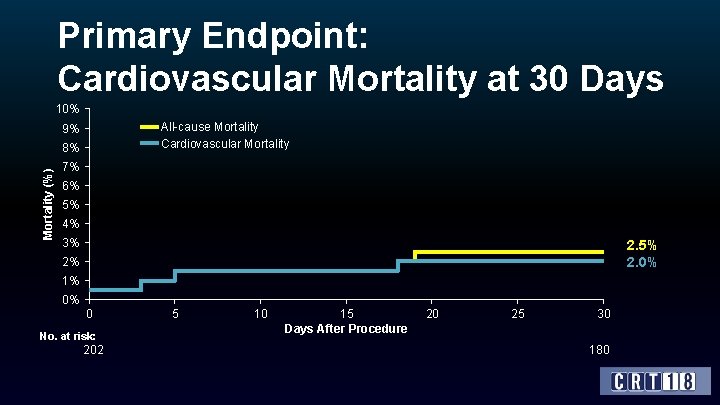
Primary Endpoint: Cardiovascular Mortality at 30 Days 10% All-cause Mortality Cardiovascular Mortality 9% Mortality (%) 8% 7% 6% 5% 4% 3% 2. 5% 2. 0% 2% 1% 0% 0 No. at risk: 202 5 10 15 Days After Procedure 20 25 30 180

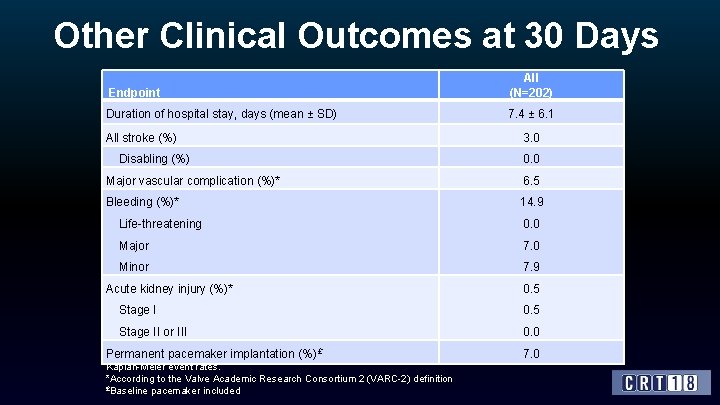
Other Clinical Outcomes at 30 Days Endpoint All (N=202) Duration of hospital stay, days (mean ± SD) 7. 4 ± 6. 1 All stroke (%) 3. 0 Disabling (%) 0. 0 Major vascular complication (%)* 6. 5 Bleeding (%)* 14. 9 Life-threatening 0. 0 Major 7. 0 Minor 7. 9 Acute kidney injury (%)* 0. 5 Stage II or III 0. 0 Permanent pacemaker implantation (%) £ 7. 0 Kaplan-Meier event rates. *According to the Valve Academic Research Consortium 2 (VARC-2) definition £Baseline pacemaker included

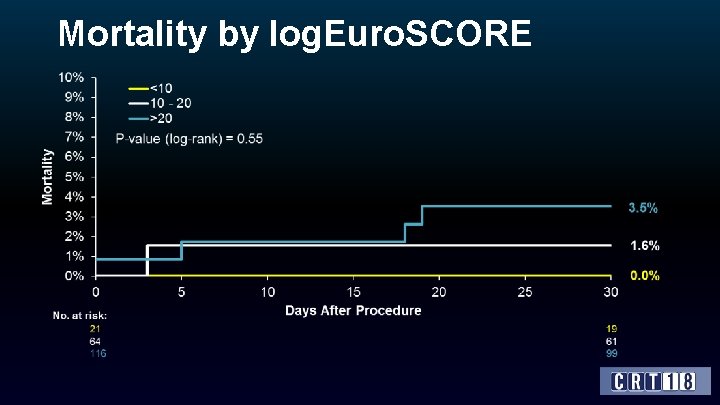
Mortality by log. Euro. SCORE

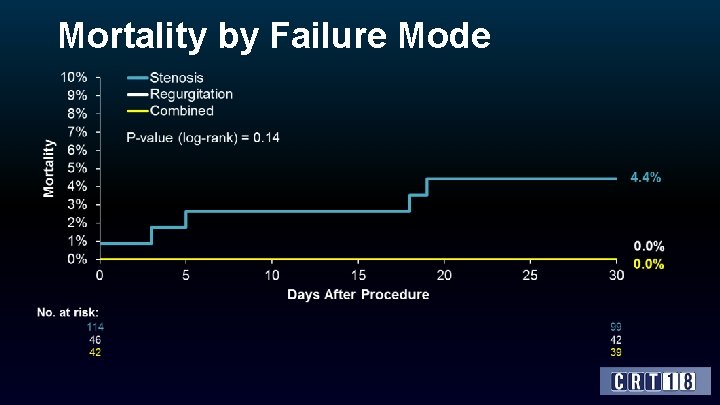
Mortality by Failure Mode

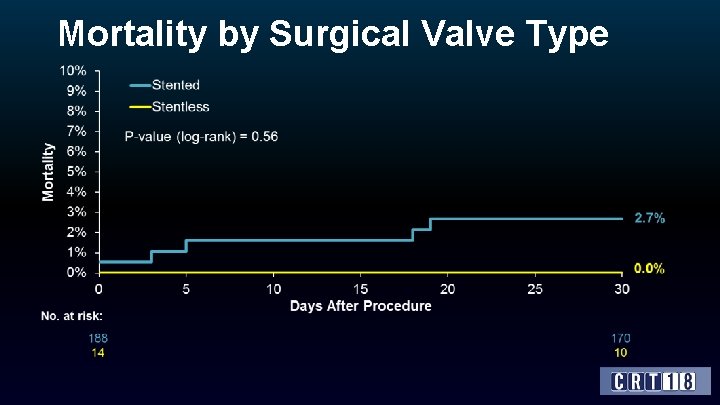
Mortality by Surgical Valve Type

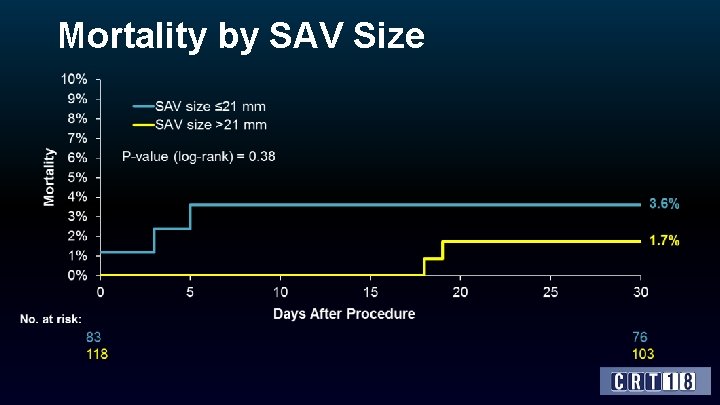
Mortality by SAV Size

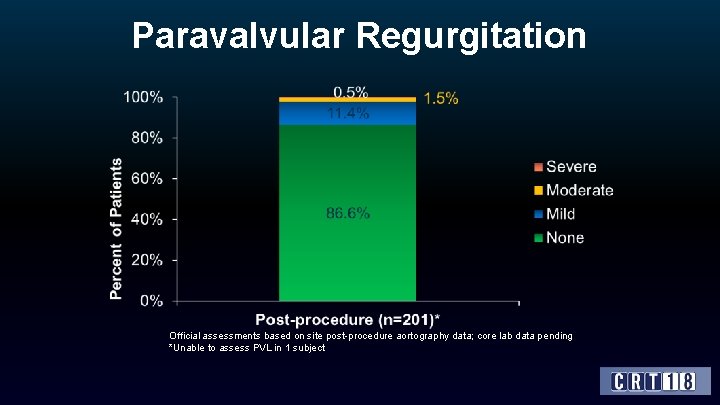
Paravalvular Regurgitation Official assessments based on site post-procedure aortography data; core lab data pending *Unable to assess PVL in 1 subject

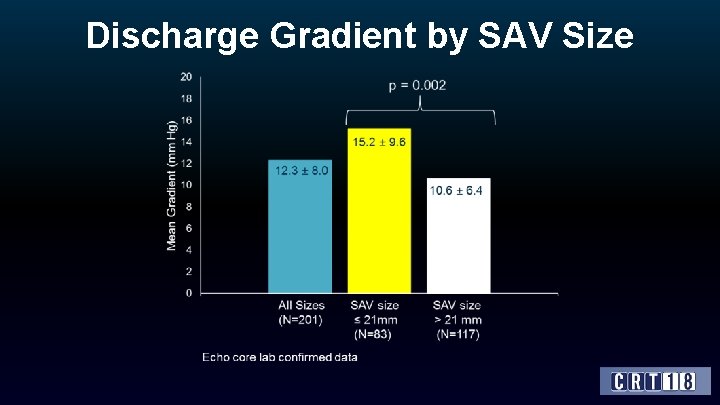
Discharge Gradient by SAV Size

Echocardiographic Findings by Failure Mode

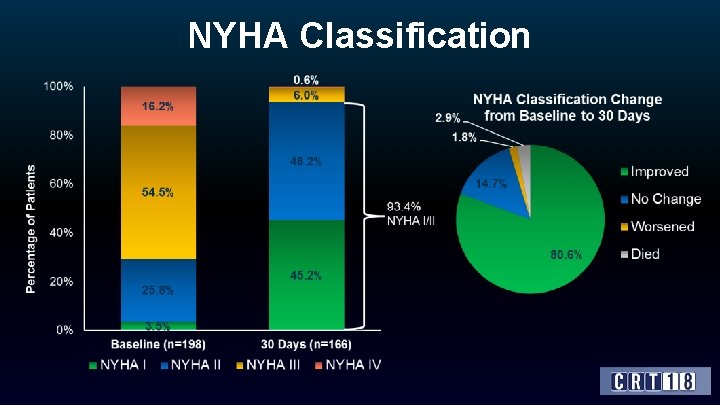
NYHA Classification

Conclusions • The VIVA trial confirmed the feasibility, safety and effectiveness of the TAVI Vi. V intervention using the Core. Valve/Evolut R devices in high-risk patients with failing surgical aortic bioprostheses. • 30 -day mortality/CV mortality was 2. 5%/2. 0% among patients who had average Log. Euro. SCORE 25% and mean STS 6. 6%. In this respect, the study met its primary safety endpoint at 30 -days (which was pre-defined as 30 -day CV mortality rate <10%). • Complications were mostly minor (i. e. not life threatening) and at a relatively low rate. • Echocardiography data at discharge and NYHA functional class after 30 -days are favorable, which indicates the short-term effectiveness of this mode of treatment. • The one-year clinical and echocardiographic efficacy data are awaited and will be reported in the near-future.

Please note

Participating Centers France Clinique Pasteur; Toulouse - Dr. Didier Tchétché Hopital Jacques Cartier; Massy – Dr. Bernard Chevalier CHU Mondor; Créteil - Prof. Emmanuel Teiger CHU de Nantes - Dr. Thibaut Manigold CHU Lille – Dr. Thomas Modine CHU Clermont; Clermont-Ferrand – Dr. Geraud Souteyrand Tonkin Clinic; Villeurbanne – Dr. Didier Champagnac CHU Rennes - Prof. Jean Philippe Verhoye CHU Bordeaux; Pessac - Dr Lionel Leroux CHU Brest - Prof. Martine Gilard CHU Rangueil; Toulouse - Dr. Bertrand Marcheix Clinique Parly 2; Le Chesnay - Dr. Gregoire Dambrin CHU La Timone; Marseille - Dr. Dominique Grisoli Israel Rabin Medical Center; Petah Tikva - Prof. Ran Kornowski Sheba Medical Center; Tel Hashomer - Prof. Victor Guetta Germany Herzzentrum Leipzig - Dr. David Holzhey Sana-Herzzentrum Cottbus - Dr. Axel Harnath UK Hamburg Eppendorf - Prof. Ulrich Schäfer Herz-und Diabeteszentrum NRW; Bad Oeynhausen - Dr. Werner Scholtz Kerckhoff Klinik; Bad Nauheim - Dr. Won-Keun Kim Italy Brescia Hospital - Dr. Federica Ettori Pisa Hospital - Prof. Anna Sonia Petronio San Donato; Milano - Prof. Francesco Bedogni
 Failed to execute 'fetch' on 'window': failed to parse url
Failed to execute 'fetch' on 'window': failed to parse url Dvi aortic valve
Dvi aortic valve Continuity equation echo
Continuity equation echo Aortic valve anatomy
Aortic valve anatomy Aortic valve dimensionless index
Aortic valve dimensionless index Endocardium sheep heart
Endocardium sheep heart Dvi aortic valve
Dvi aortic valve Aortic valve leaflets
Aortic valve leaflets Dvi aortic valve
Dvi aortic valve Crista terminalis
Crista terminalis Mechanical servo valve
Mechanical servo valve Implantation
Implantation Meryem berrak
Meryem berrak Definition of spotting during pregnancy
Definition of spotting during pregnancy Pickwickian syndrome in dogs
Pickwickian syndrome in dogs Ion implantation
Ion implantation Implantation batiment methode
Implantation batiment methode Ion implantation
Ion implantation Implantation cadenassage
Implantation cadenassage Physiological function of estrogen
Physiological function of estrogen Stages of implantation pregnancy
Stages of implantation pregnancy The perverse implantation summary
The perverse implantation summary Implantation
Implantation Implantation
Implantation Implantation verticale et horizontale
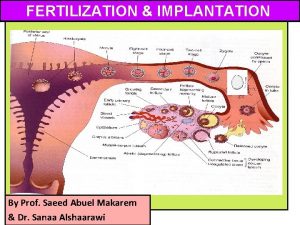
Implantation verticale et horizontale Lesson 15.4 ovulation fertilization and implantation
Lesson 15.4 ovulation fertilization and implantation Hormone levels during pregnancy
Hormone levels during pregnancy What does a testosterone pellet look like
What does a testosterone pellet look like