Decalcification of Calcified Aortic Valve with Shockwave Therapy












- Slides: 17

Decalcification of Calcified Aortic Valve with Shockwave Therapy Todd J. Brinton, MD Clinical Professor of Medicine Adjunct Professor of Bioengineering Stanford University

Todd Brinton, MD I have relevant financial relationships Founder, Board of Directors, Shareholder. Shockwave Medical, Inc. Jump Medical, Inc. Consultant Endoluminal Sciences, Inc. Shareholder Qool Therapeutics, Inc.

Lithotripsy for Structural Heart: Clinical Programs FIH I Study FIH III Study Feasibility Acute, Open Procedure Acute, Direct Aortic Chronic, Trans-femoral N=3 N=4 N ~ 20 Study Completed Planned 2018

Lithotripsy for Aortic Valve Description Power Pulses Working Length FIM I Directional, 3 balloons, 2 emitters/balloon 3 kv 50 per balloon 55 cm FIM II Helical, 3 loops, 3 emitters/loop 5 kv 50 per loop 53 cm FIM II

Lithotripsy: Loop In Action

Aortic Valve Lithotripsy – FIM II • Prospective, single-arm, single-center, clinical study • Aortic Valve Lithotripsy followed by assessment of: – Impact on safety parameters – Change in pressure gradient – Leaflet mobility • Subjects will undergo aortic valve replacement following excision of aortic valve leaflets Adrian Ebner, MD Italian Hospital Asuncion, Paraguay

FIM II Study: Key Inclusion / Exclusion Inclusion • Age of subject is >18. • Patient with severe degenerative aortic valve stenosis – AV area (EOA) of <1 cm 2, Mean AV gradient >40 mm. Hg, LVEF > 40% • Patients must be symptomatic from the aortic valve stenosis (dyspnea in NYHAclass II or greater, angina pectoris, or syncope) • Patients eligible for surgical valve replacement Exclusion • Aortic valve insufficiency > than 2+ • Coronary artery co-morbidity requiring revascularization • Previous median sternotomy or cardiac surgery • Myocardial infarction or percutaneous coronary intervention within the last 6 months • Stroke or TIA within last 90 days

FIM II Study: Baseline Characteristics Echocardiographic Parameters Age Aortic Valve Type Mean AV Grad. mm. Hg Peak AV Grad. mm. Hg AV area LVEF PGY-001 70 Bicuspid 42 69 0. 75 60% PGY-002 71 Tricuspid 43 79 0. 64 68% PGY-003 56 Tricuspid 60 91 0. 68 67% PGY-004 62 Tricuspid 55 97 0. 86 65% Baseline transthoracic echo parameters

FIM II Study: Acute Procedural Results† Pre 68 80 60 40 55 48 Post 72 Mean Av Gradient 61 40 47 36 20 Post 34 31 19 80 43 42 30 20 34 21 60 24 67 Pre Post 48 37 40 25 0 0 mm HG PGY-001 PGY-002 PGY-003 PGY-004 Average Change in Gradient 20 10 0 mm HG Pre 50 72 PGY-001 PGY-002 PGY-003 PGY-004 mm HG Peak Av Gradient Peak Mean †Core Lab Reported Measurements by TEE

FIM II Study: Acute Procedural Results† AV Area Pre 1. 4 Post 1. 2 1. 1 1. 2 1. 3 Pre Post PGY-001 Trace PGY-002 Trace 0. 4 PGY-003 Trace 0. 2 PGY-004 Mild Central 1. 0 Sq. cm Aortic Regurgitation 0. 8 0. 6 0. 7 0. 0 PGY-001 PGY-002 PGY-003 PGY-004 †Core Lab Reported

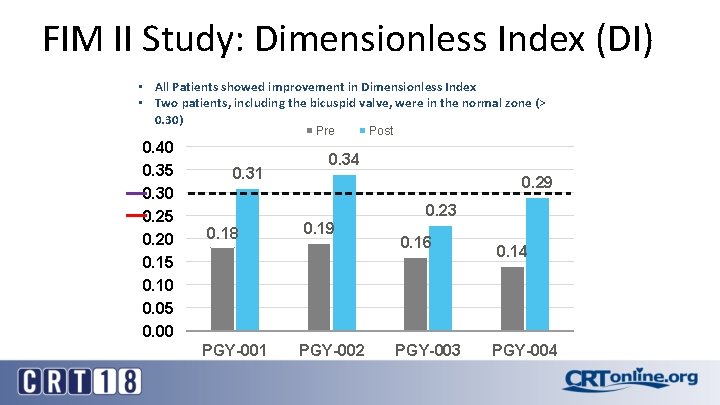
FIM II Study: Dimensionless Index (DI) • All Patients showed improvement in Dimensionless Index • Two patients, including the bicuspid valve, were in the normal zone (> 0. 30) Pre 0. 40 0. 35 0. 30 0. 25 0. 20 0. 15 0. 10 0. 05 0. 00 0. 31 Post 0. 34 0. 29 0. 23 0. 18 PGY-001 0. 19 PGY-002 0. 16 PGY-003 0. 14 PGY-004

FIM II Study: Echocardiography PRE POST Leaflet morphology: Trileaflet with calcification at leaflet margins. Restricted leaflet opening, left cusp moves best. Baseline: True calcified trileaflet aortic stenosis. Trace Tricuspid Regurgitation

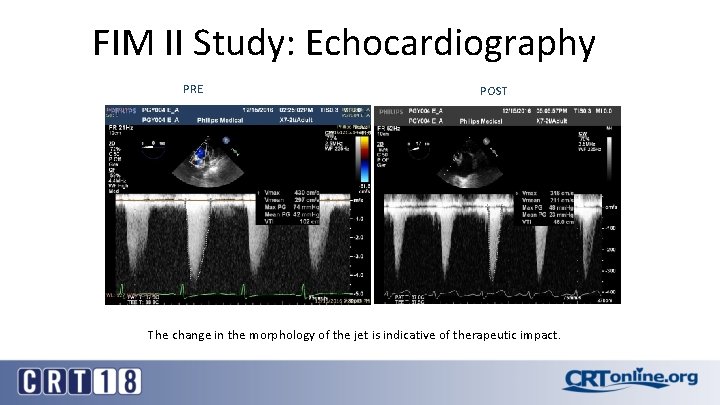
FIM II Study: Echocardiography PRE POST The change in the morphology of the jet is indicative of therapeutic impact.

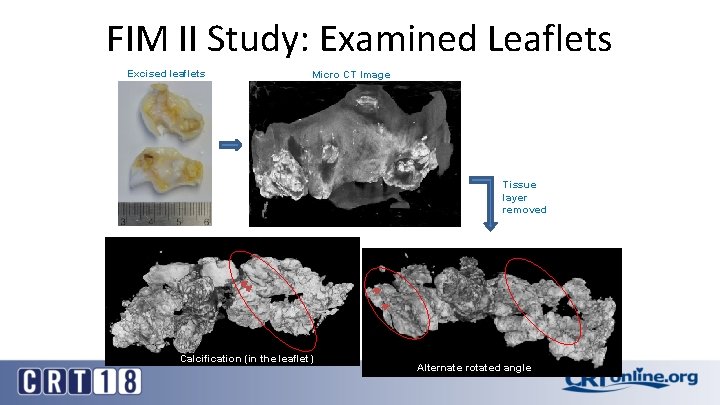
FIM II Study: Examined Leaflets Excised leaflets Micro CT Image Tissue layer removed Calcification (in the leaflet) Alternate rotated angle

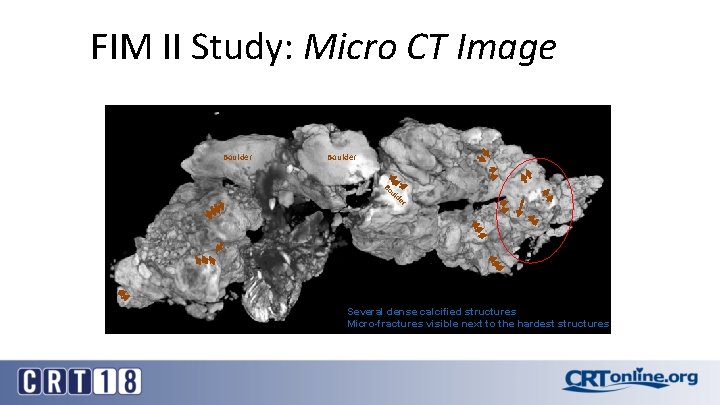
FIM II Study: Micro CT Image Boulder Bo ul d er Several dense calcified structures Micro-fractures visible next to the hardest structures

Design Updates for FIM III Description Power Pulses Working Length FIM I Directional, 3 balloons, 2 emitters/balloon 3 kv 50 per balloon 55 cm FIM II Helical, 3 loops, 3 emitters/loop 5 kv 50 per loop 53 cm FIM III Helical, 3 loops, 6 emitters/loop 5 kv 50 per loop 110 cm FIH III

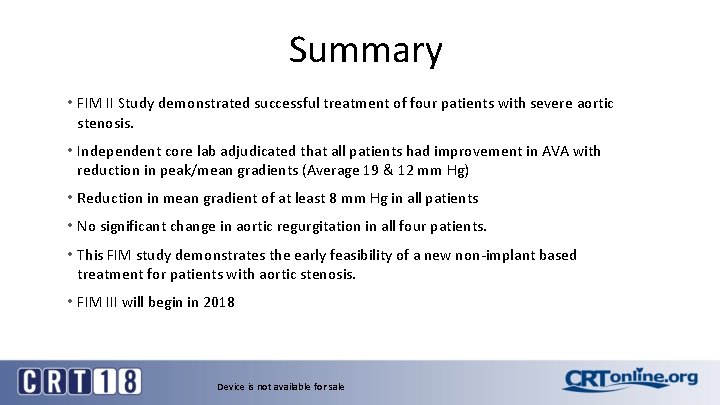
Summary • FIM II Study demonstrated successful treatment of four patients with severe aortic stenosis. • Independent core lab adjudicated that all patients had improvement in AVA with reduction in peak/mean gradients (Average 19 & 12 mm Hg) • Reduction in mean gradient of at least 8 mm Hg in all patients • No significant change in aortic regurgitation in all four patients. • This FIM study demonstrates the early feasibility of a new non-implant based treatment for patients with aortic stenosis. • FIM III will begin in 2018 Device is not available for sale
 Shockwave coronarico
Shockwave coronarico Aortic valve leaflets
Aortic valve leaflets Dvi aortic valve
Dvi aortic valve The pulmonary semilunar valve
The pulmonary semilunar valve Dvi in echo
Dvi in echo Rcc aortic valve
Rcc aortic valve Nicks procedure
Nicks procedure Aortic valve dimensionless index
Aortic valve dimensionless index Aortic semilunar valve sheep heart
Aortic semilunar valve sheep heart Dvi aortic valve
Dvi aortic valve Fcu calcific tendonitis
Fcu calcific tendonitis Bone
Bone Dip and dunk tissue processor
Dip and dunk tissue processor Define decalcification
Define decalcification Difference between proportional valve and servo valve
Difference between proportional valve and servo valve Humanistic therapies aim to boost
Humanistic therapies aim to boost Psychodynamic and humanistic therapies have in common
Psychodynamic and humanistic therapies have in common Bioness bits cost
Bioness bits cost