Transcatheter Aortic Valve Replacement with the Lotus Valve








- Slides: 42

Transcatheter Aortic Valve Replacement with the Lotus Valve for Severe, Symptomatic Aortic Stenosis: St. Luke’s Medical Center Global City Heart Institute Experience from 2012 to 2016 Santos-Tuscano, Sheryll D. MD, Cuenca, FCC MD, Posas, FE MD, Valencia, O

BACKGROUND • Aortic stenosis (AS) is the most prevalent heart disease with incidence of 1 -2% and increased to about 5% in people older than 75 years. • Surgical aortic valve replacement (SAVR) is the traditional treatment modality and is seen to improve symptoms, quality of life and prolong survival. • A substantial number of patients (30% - 40%) are deemed unsuitable for SAVR because of multiple co-morbidities that place them to high periprocedural risk. • In 2002, Alain Cribler et al performed the first successful human transcatheter aortic valve replacement (TAVR); providing a new treatment option for patients with inoperable, symptomatic severe AS patients. Ortega-Paz, L, Giacchi, G, et al. (2015) Percutaneous Transcatheter Aortic Valve Implantation: A Review Focus on Outcomes and Safety. AIMS Medical Science 2(3): 200 -221. Bourantas, CV and Serruys, PW. (2014) Evolution of Transcatheter Aortic Valve Replacement. Circulation Research. American Heart Association 114(302292): 1037 -1047.

BACKGROUND • The self-expanding and balloon expandable systems have dominated the clinical scene and large, randomized clinical trials have established their safety and efficacy. • Paravalvular leak (PVL) and malposition of the valve are two problems that are associated with adverse outcomes and poor prognosis • Newer valves have been designed to address some of these issues and recently, a second-generation TAVR device was made which addressed both PVL and malposition. • The repositionable and fully retrievable Lotus Valve was designed to facilitate accurate primary positioning, early valve function, and hemodynamic stability during deployment resulting in minimal paravalvular regurgitation. Bjursten, H, Shahab, N, et al. (2017) The safety of introducing a new generation TAVR device: one department’s experience from introducing a second generation repositionable TAVR. Bio. Med Central Cardiovascular Disorders 17(25): 1 -7. Meredith, I, Walters, D, et al. (2016) 1 -Year Outcomes with the Fully Repositionable and Retrievable Lotus Transcather Aortic Replacement Valve in 120 High-Risk Surgical Patients with Severe Aortic Stenosis. Journal of American College of Cardiology. Cardiovascular Interventions 9(4): 376 -384.

OBJECTIVES • To evaluate the safety and performance of the Lotus Valve TAVR system in symptomatic patients with severe, calcific native aortic stenosis. • To compare the outcomes in terms of device success, safety, and clinical efficacy as defined by the Valve Academic Research Consortium-2 (VARC-2) with those of Core. Valve treated patients at 30 days.

METHODOLOGY

DEVICE DESCRIPTION • The Boston Scientific Lotus Valve system consists of a woven nitinolframed bioprosthesis with bovine pericardium leaflets, which is premounted on a transfemoral catheter delivery system • It is designed to provide more precise release and the possibility of its repositioning or recovery after implantation • Minimizes the risk of paravalvular aortic regurgitation with its new-age sealing system (urethane membrane) that adapts to the irregular surface of the ring • For the present study, three valve sizes were used: 23 mm, 25 mm and 27 mm

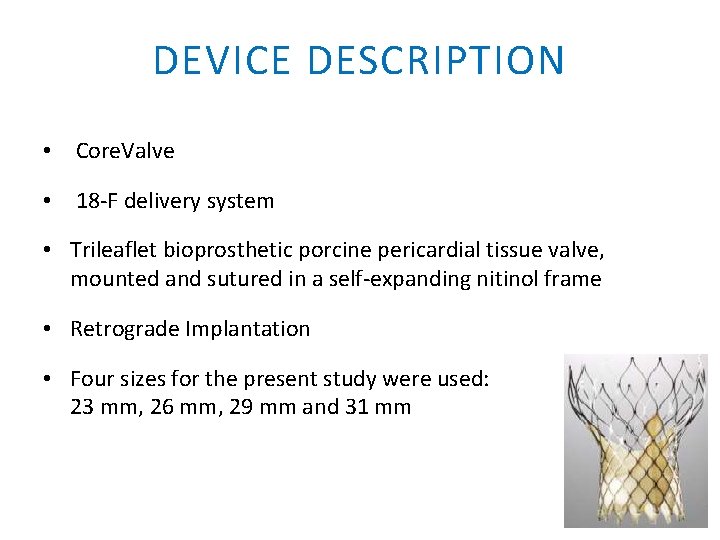
DEVICE DESCRIPTION • Core. Valve • 18 -F delivery system • Trileaflet bioprosthetic porcine pericardial tissue valve, mounted and sutured in a self-expanding nitinol frame • Retrograde Implantation • Four sizes for the present study were used: 23 mm, 26 mm, 29 mm and 31 mm

STUDY DESIGN • This study is a retro-prospective cohort, single-center study and includes all TAVR performed from February, 2012 to November, 2016 at St. Luke’s Medical Center Global City, Taguig, Philippines • The TAVR program started in February, 2012 and since its beginning, the center has used the self-expandable Medtronic Core. Valve and Lotus valve in 2015

PATIENT SELECTION • The patients included all had severe symptomatic aortic stenosis, defined as aortic valve area of < 1. 0 cm 2 or aortic valve area index of <0. 6 cm 2/m 2, mean pressure gradient of > 40 mm. Hg or a jet velocity of > 4 m/s. • All candidates for TAVR were decided upon by the Heart Team (Cardiac Interventionalists, TCVS, Interventional Echocardiographer, Cardiac Anesthesiologist, Critical Care & Pulmonologist and Cardiac Rehab Specialist) • Additional High Risk Features: - Porcelain aorta - Cachexia, defined as BMI < 18 kg/m 2 - Frailty - Hostile chest or other anatomic contraindication to open chest surgery - Morbid obesity

PATIENT SELECTION • Inclusion Criteria – Life expectancy of > 1 year – STS > 10 and Logistic Euroscore of >15 – Signed informed consent • Exclusion Criteria – Estimated life expectancy <1 year – Liver cirrhosis (Child Pugh C) – Sepsis or ongoing severe infectious processes – Stroke within the previous month – Untreated, symptomatic carotid or vertebral artery disease – Inability or refusal to sign informed consent – No vascular access.

PRE-OPERATIVE WORK-UP • Coronary angiography Pre TAVR revascularization if indicated • CT scan of the thoraco-abdominal aorta Vascular access , Aortic anatomy , coronary take off height Annular sizing – diameter and perimeters Leaflet and annular calcification - severity / pattern • Transthoracic Echocardiography LV/RV function, AVA, valve morphology, leaflet & annular calcification Annular dimensions at hinge points All echoes read and measured by 3 independent readers

VALVE SIZING & IMPLANTATION TECHNIQUE • • Valve size based on CTA perimeter measurements Intra-op TEE used confirmation of optimal valve size All procedures done at cardiac catheterization laboratory General anesthesia / endotracheal intubation Conscious sedation 5 F balloon tipped temporary pacemaker Femoral approach whenever possible - Left subclavian or direct aortic implantation if unsuitable

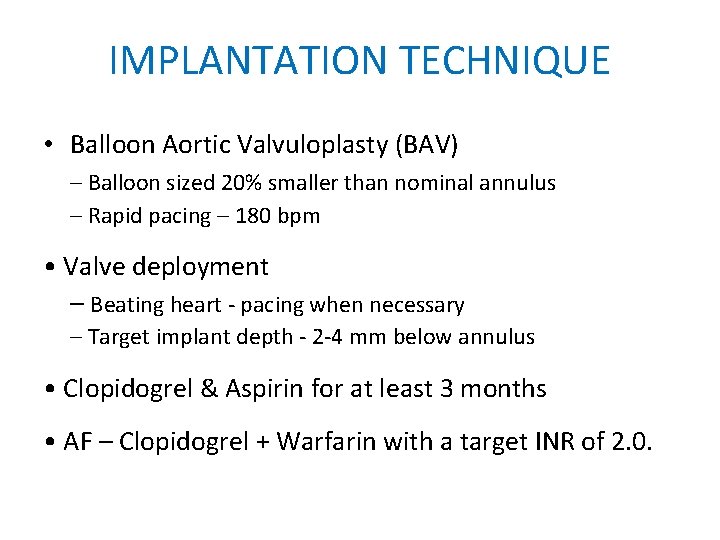
IMPLANTATION TECHNIQUE • Balloon Aortic Valvuloplasty (BAV) – Balloon sized 20% smaller than nominal annulus – Rapid pacing – 180 bpm • Valve deployment – Beating heart - pacing when necessary – Target implant depth - 2 -4 mm below annulus • Clopidogrel & Aspirin for at least 3 months • AF – Clopidogrel + Warfarin with a target INR of 2. 0.

DATA SOURCES • Peri-operative data (all clinical and laboratory) are recorded in standard data collection sheets. • Early safety according to VARC-2 criteria was registered prospectively. • Pre and post-operative echocardiographic examinations are likewise included in the data bank.

STATISTICAL ANALYSIS • Discrete variables were summarized as counts and percentages while continuous variables were summarized by their mean, standard deviation and 95% confidence interval • Comparison of the normally distributed outcomes before and after the procedure was analyzed using a paired t test • For non normal outcomes, Wilcoxon signed ranks test was used • The level of significance was set at α < 0. 05

RESULTS

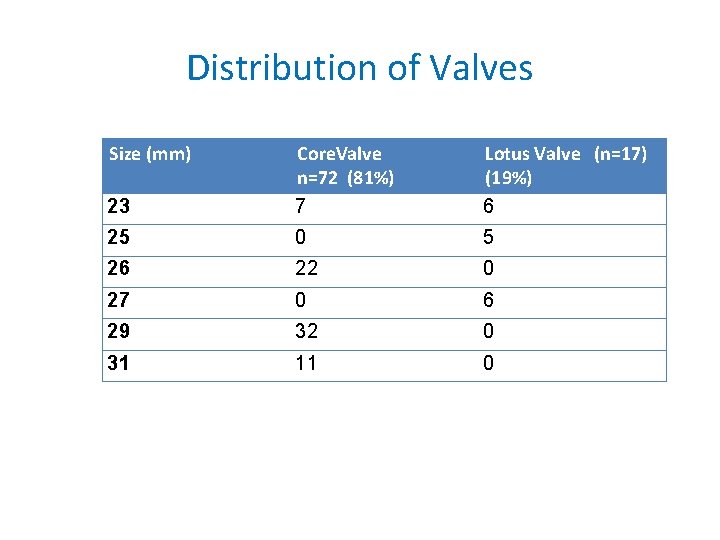
Distribution of Valves Size (mm) 23 Core. Valve n=72 (81%) 7 Lotus Valve (n=17) (19%) 6 25 0 5 26 22 0 27 0 6 29 32 0 31 11 0

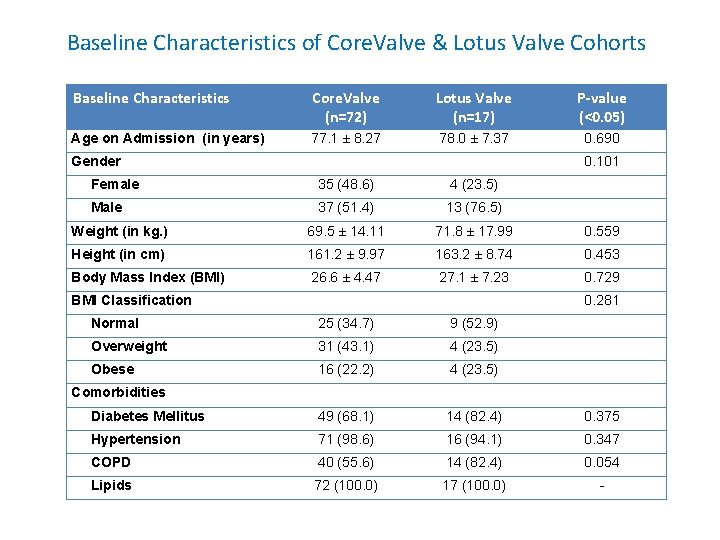
Baseline Characteristics of Core. Valve & Lotus Valve Cohorts Baseline Characteristics Core. Valve (n=72) Lotus Valve (n=17) P-value (<0. 05) Age on Admission (in years) 77. 1 ± 8. 27 78. 0 ± 7. 37 0. 690 Gender 0. 101 Female 35 (48. 6) 4 (23. 5) Male 37 (51. 4) 13 (76. 5) Weight (in kg. ) 69. 5 ± 14. 11 71. 8 ± 17. 99 0. 559 Height (in cm) 161. 2 ± 9. 97 163. 2 ± 8. 74 0. 453 Body Mass Index (BMI) 26. 6 ± 4. 47 27. 1 ± 7. 23 0. 729 BMI Classification 0. 281 Normal 25 (34. 7) 9 (52. 9) Overweight 31 (43. 1) 4 (23. 5) Obese 16 (22. 2) 4 (23. 5) Diabetes Mellitus 49 (68. 1) 14 (82. 4) 0. 375 Hypertension 71 (98. 6) 16 (94. 1) 0. 347 COPD 40 (55. 6) 14 (82. 4) 0. 054 Lipids 72 (100. 0) 17 (100. 0) - Comorbidities

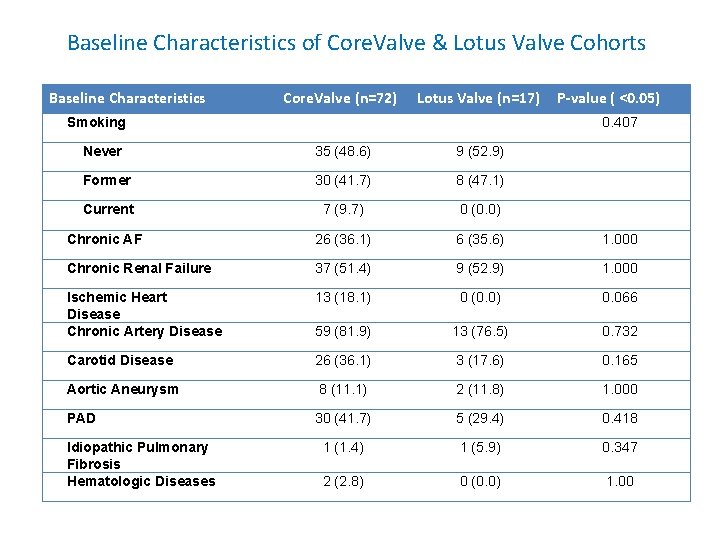
Baseline Characteristics of Core. Valve & Lotus Valve Cohorts Baseline Characteristics Core. Valve (n=72) Lotus Valve (n=17) Smoking P-value ( <0. 05) 0. 407 Never 35 (48. 6) 9 (52. 9) Former 30 (41. 7) 8 (47. 1) Current 7 (9. 7) 0 (0. 0) Chronic AF 26 (36. 1) 6 (35. 6) 1. 000 Chronic Renal Failure 37 (51. 4) 9 (52. 9) 1. 000 Ischemic Heart Disease Chronic Artery Disease 13 (18. 1) 0 (0. 0) 0. 066 59 (81. 9) 13 (76. 5) 0. 732 Carotid Disease 26 (36. 1) 3 (17. 6) 0. 165 Aortic Aneurysm 8 (11. 1) 2 (11. 8) 1. 000 PAD 30 (41. 7) 5 (29. 4) 0. 418 1 (1. 4) 1 (5. 9) 0. 347 2 (2. 8) 0 (0. 0) 1. 00 Idiopathic Pulmonary Fibrosis Hematologic Diseases

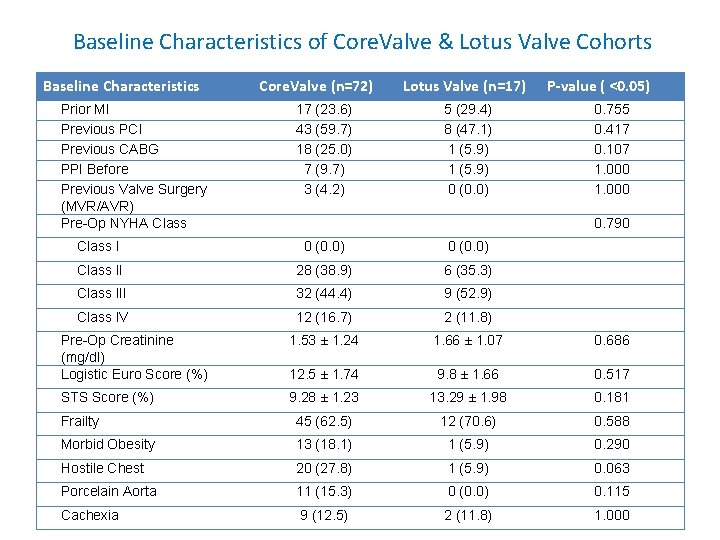
Baseline Characteristics of Core. Valve & Lotus Valve Cohorts Baseline Characteristics Prior MI Previous PCI Previous CABG PPI Before Previous Valve Surgery (MVR/AVR) Pre-Op NYHA Class Core. Valve (n=72) 17 (23. 6) 43 (59. 7) 18 (25. 0) 7 (9. 7) 3 (4. 2) Lotus Valve (n=17) 5 (29. 4) 8 (47. 1) 1 (5. 9) 0 (0. 0) P-value ( <0. 05) 0. 755 0. 417 0. 107 1. 000 0. 790 Class I 0 (0. 0) Class II 28 (38. 9) 6 (35. 3) Class III 32 (44. 4) 9 (52. 9) Class IV 12 (16. 7) 2 (11. 8) Pre-Op Creatinine (mg/dl) Logistic Euro Score (%) 1. 53 ± 1. 24 1. 66 ± 1. 07 0. 686 12. 5 ± 1. 74 9. 8 ± 1. 66 0. 517 STS Score (%) 9. 28 ± 1. 23 13. 29 ± 1. 98 0. 181 Frailty 45 (62. 5) 12 (70. 6) 0. 588 Morbid Obesity 13 (18. 1) 1 (5. 9) 0. 290 Hostile Chest 20 (27. 8) 1 (5. 9) 0. 063 Porcelain Aorta 11 (15. 3) 0 (0. 0) 0. 115 Cachexia 9 (12. 5) 2 (11. 8) 1. 000

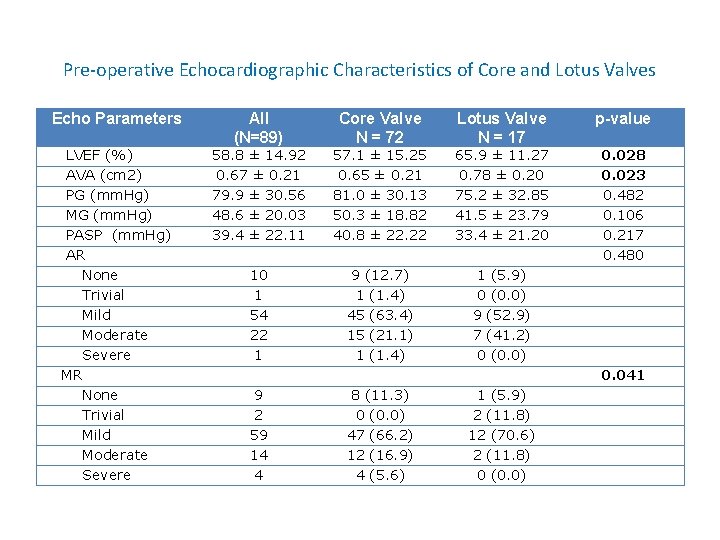
Pre-operative Echocardiographic Characteristics of Core and Lotus Valves Echo Parameters All (N=89) Core Valve N = 72 Lotus Valve N = 17 p-value LVEF (%) AVA (cm 2) PG (mm. Hg) MG (mm. Hg) PASP (mm. Hg) AR None Trivial Mild Moderate Severe MR None Trivial Mild Moderate Severe 58. 8 ± 14. 92 0. 67 ± 0. 21 79. 9 ± 30. 56 48. 6 ± 20. 03 39. 4 ± 22. 11 57. 1 ± 15. 25 0. 65 ± 0. 21 81. 0 ± 30. 13 50. 3 ± 18. 82 40. 8 ± 22. 22 65. 9 ± 11. 27 0. 78 ± 0. 20 75. 2 ± 32. 85 41. 5 ± 23. 79 33. 4 ± 21. 20 0. 028 0. 023 0. 482 0. 106 0. 217 0. 480 10 1 54 22 1 9 (12. 7) 1 (1. 4) 45 (63. 4) 15 (21. 1) 1 (1. 4) 1 (5. 9) 0 (0. 0) 9 (52. 9) 7 (41. 2) 0 (0. 0) 0. 041 9 2 59 14 4 8 (11. 3) 0 (0. 0) 47 (66. 2) 12 (16. 9) 4 (5. 6) 1 (5. 9) 2 (11. 8) 12 (70. 6) 2 (11. 8) 0 (0. 0)

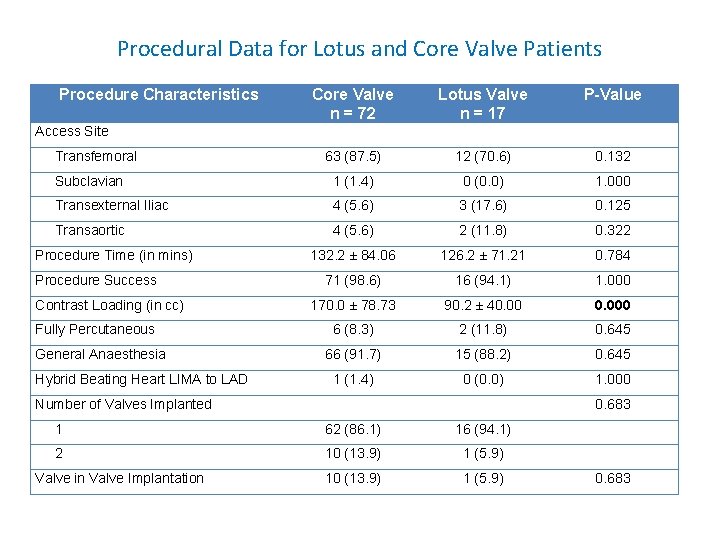
Procedural Data for Lotus and Core Valve Patients Procedure Characteristics Core Valve n = 72 Lotus Valve n = 17 P-Value 63 (87. 5) 12 (70. 6) 0. 132 Subclavian 1 (1. 4) 0 (0. 0) 1. 000 Transexternal Iliac 4 (5. 6) 3 (17. 6) 0. 125 Transaortic 4 (5. 6) 2 (11. 8) 0. 322 132. 2 ± 84. 06 126. 2 ± 71. 21 0. 784 71 (98. 6) 16 (94. 1) 1. 000 170. 0 ± 78. 73 90. 2 ± 40. 000 6 (8. 3) 2 (11. 8) 0. 645 66 (91. 7) 15 (88. 2) 0. 645 1 (1. 4) 0 (0. 0) 1. 000 Access Site Transfemoral Procedure Time (in mins) Procedure Success Contrast Loading (in cc) Fully Percutaneous General Anaesthesia Hybrid Beating Heart LIMA to LAD Number of Valves Implanted 0. 683 1 62 (86. 1) 16 (94. 1) 2 10 (13. 9) 1 (5. 9) Valve in Valve Implantation 0. 683

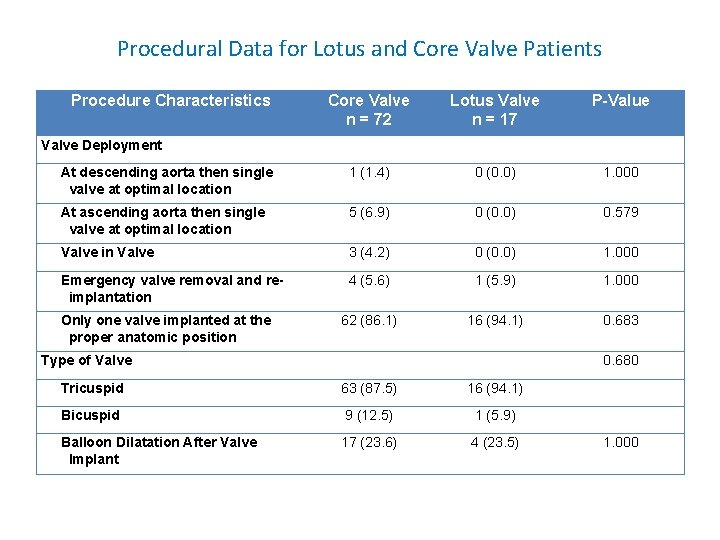
Procedural Data for Lotus and Core Valve Patients Procedure Characteristics Core Valve n = 72 Lotus Valve n = 17 P-Value At descending aorta then single valve at optimal location 1 (1. 4) 0 (0. 0) 1. 000 At ascending aorta then single valve at optimal location 5 (6. 9) 0 (0. 0) 0. 579 Valve in Valve 3 (4. 2) 0 (0. 0) 1. 000 Emergency valve removal and reimplantation 4 (5. 6) 1 (5. 9) 1. 000 62 (86. 1) 16 (94. 1) 0. 683 Valve Deployment Only one valve implanted at the proper anatomic position Type of Valve 0. 680 Tricuspid 63 (87. 5) 16 (94. 1) Bicuspid 9 (12. 5) 1 (5. 9) Balloon Dilatation After Valve Implant 17 (23. 6) 4 (23. 5) 1. 000

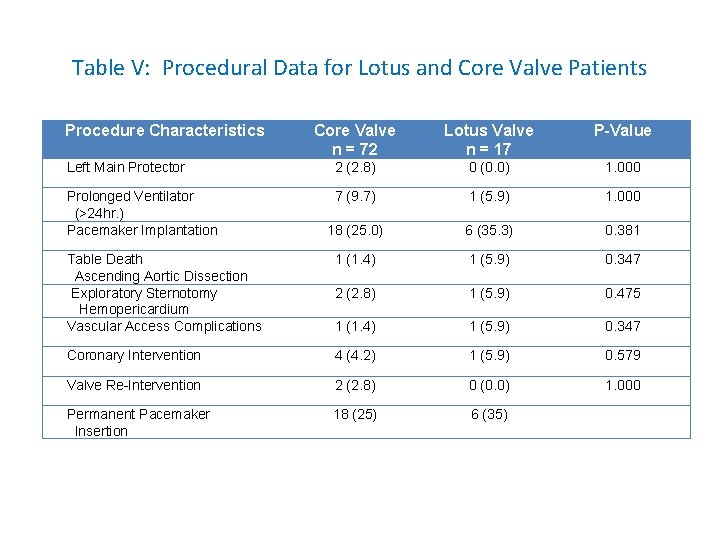
Table V: Procedural Data for Lotus and Core Valve Patients Procedure Characteristics Core Valve n = 72 Lotus Valve n = 17 P-Value Left Main Protector 2 (2. 8) 0 (0. 0) 1. 000 Prolonged Ventilator (>24 hr. ) Pacemaker Implantation 7 (9. 7) 1 (5. 9) 1. 000 18 (25. 0) 6 (35. 3) 0. 381 Table Death Ascending Aortic Dissection Exploratory Sternotomy Hemopericardium Vascular Access Complications 1 (1. 4) 1 (5. 9) 0. 347 2 (2. 8) 1 (5. 9) 0. 475 1 (1. 4) 1 (5. 9) 0. 347 Coronary Intervention 4 (4. 2) 1 (5. 9) 0. 579 Valve Re-Intervention 2 (2. 8) 0 (0. 0) 1. 000 Permanent Pacemaker Insertion 18 (25) 6 (35)

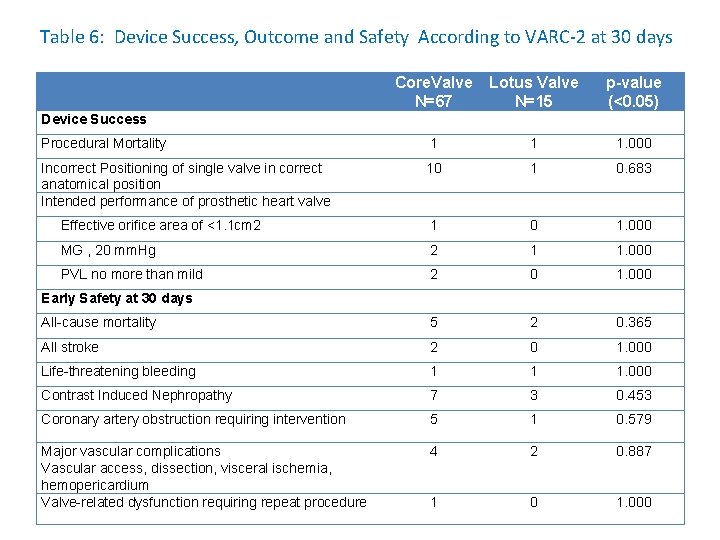
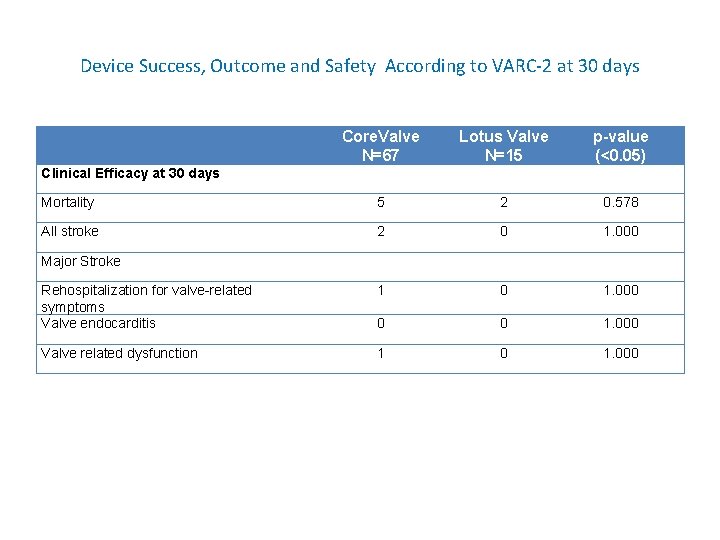
Table 6: Device Success, Outcome and Safety According to VARC-2 at 30 days Core. Valve N=67 Lotus Valve N=15 p-value (<0. 05) Procedural Mortality 1 1 1. 000 Incorrect Positioning of single valve in correct anatomical position Intended performance of prosthetic heart valve 10 1 0. 683 Effective orifice area of <1. 1 cm 2 1 0 1. 000 MG , 20 mm. Hg 2 1 1. 000 PVL no more than mild 2 0 1. 000 All-cause mortality 5 2 0. 365 All stroke 2 0 1. 000 Life-threatening bleeding 1 1 1. 000 Contrast Induced Nephropathy 7 3 0. 453 Coronary artery obstruction requiring intervention 5 1 0. 579 Major vascular complications Vascular access, dissection, visceral ischemia, hemopericardium Valve-related dysfunction requiring repeat procedure 4 2 0. 887 1 0 1. 000 Device Success Early Safety at 30 days

Device Success, Outcome and Safety According to VARC-2 at 30 days Core. Valve N=67 Lotus Valve N=15 p-value (<0. 05) Mortality 5 2 0. 578 All stroke 2 0 1. 000 Rehospitalization for valve-related symptoms Valve endocarditis 1 0 1. 000 0 0 1. 000 Valve related dysfunction 1 0 1. 000 Clinical Efficacy at 30 days Major Stroke

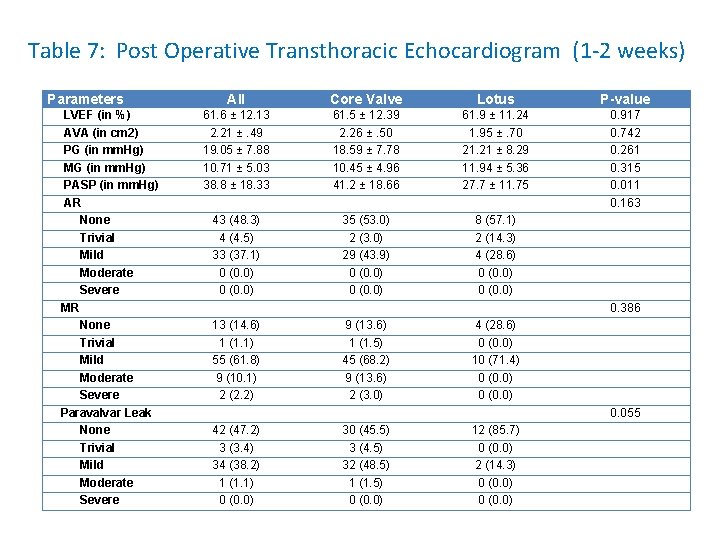
Table 7: Post Operative Transthoracic Echocardiogram (1 -2 weeks) Parameters LVEF (in %) AVA (in cm 2) PG (in mm. Hg) MG (in mm. Hg) PASP (in mm. Hg) AR None Trivial Mild Moderate Severe MR None Trivial Mild Moderate Severe Paravalvar Leak None Trivial Mild Moderate Severe All Core Valve Lotus P-value 61. 6 ± 12. 13 2. 21 ±. 49 19. 05 ± 7. 88 10. 71 ± 5. 03 38. 8 ± 18. 33 61. 5 ± 12. 39 2. 26 ±. 50 18. 59 ± 7. 78 10. 45 ± 4. 96 41. 2 ± 18. 66 61. 9 ± 11. 24 1. 95 ±. 70 21. 21 ± 8. 29 11. 94 ± 5. 36 27. 7 ± 11. 75 0. 917 0. 742 0. 261 0. 315 0. 011 0. 163 43 (48. 3) 4 (4. 5) 33 (37. 1) 0 (0. 0) 35 (53. 0) 2 (3. 0) 29 (43. 9) 0 (0. 0) 8 (57. 1) 2 (14. 3) 4 (28. 6) 0 (0. 0) 0. 386 13 (14. 6) 1 (1. 1) 55 (61. 8) 9 (10. 1) 2 (2. 2) 9 (13. 6) 1 (1. 5) 45 (68. 2) 9 (13. 6) 2 (3. 0) 4 (28. 6) 0 (0. 0) 10 (71. 4) 0 (0. 0) 0. 055 42 (47. 2) 3 (3. 4) 34 (38. 2) 1 (1. 1) 0 (0. 0) 30 (45. 5) 3 (4. 5) 32 (48. 5) 1 (1. 5) 0 (0. 0) 12 (85. 7) 0 (0. 0) 2 (14. 3) 0 (0. 0)

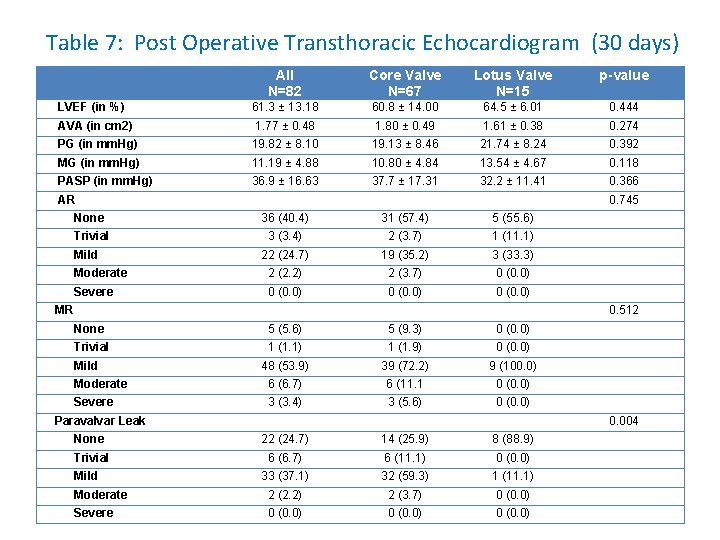
Table 7: Post Operative Transthoracic Echocardiogram (30 days) All N=82 Core Valve N=67 Lotus Valve N=15 p-value LVEF (in %) 61. 3 ± 13. 18 60. 8 ± 14. 00 64. 5 ± 6. 01 0. 444 AVA (in cm 2) 1. 77 ± 0. 48 1. 80 ± 0. 49 1. 61 ± 0. 38 0. 274 PG (in mm. Hg) 19. 82 ± 8. 10 19. 13 ± 8. 46 21. 74 ± 8. 24 0. 392 MG (in mm. Hg) 11. 19 ± 4. 88 10. 80 ± 4. 84 13. 54 ± 4. 67 0. 118 PASP (in mm. Hg) 36. 9 ± 16. 63 37. 7 ± 17. 31 32. 2 ± 11. 41 0. 366 AR 0. 745 None 36 (40. 4) 31 (57. 4) 5 (55. 6) Trivial 3 (3. 4) 2 (3. 7) 1 (11. 1) 22 (24. 7) 19 (35. 2) 3 (33. 3) Moderate 2 (2. 2) 2 (3. 7) 0 (0. 0) Severe 0 (0. 0) Mild MR 0. 512 None 5 (5. 6) 5 (9. 3) 0 (0. 0) Trivial 1 (1. 1) 1 (1. 9) 0 (0. 0) 48 (53. 9) 39 (72. 2) 9 (100. 0) Moderate 6 (6. 7) 6 (11. 1 0 (0. 0) Severe 3 (3. 4) 3 (5. 6) 0 (0. 0) Mild Paravalvar Leak 0. 004 None 22 (24. 7) 14 (25. 9) 8 (88. 9) Trivial 6 (6. 7) 6 (11. 1) 0 (0. 0) 33 (37. 1) 32 (59. 3) 1 (11. 1) Moderate 2 (2. 2) 2 (3. 7) 0 (0. 0) Severe 0 (0. 0) Mild

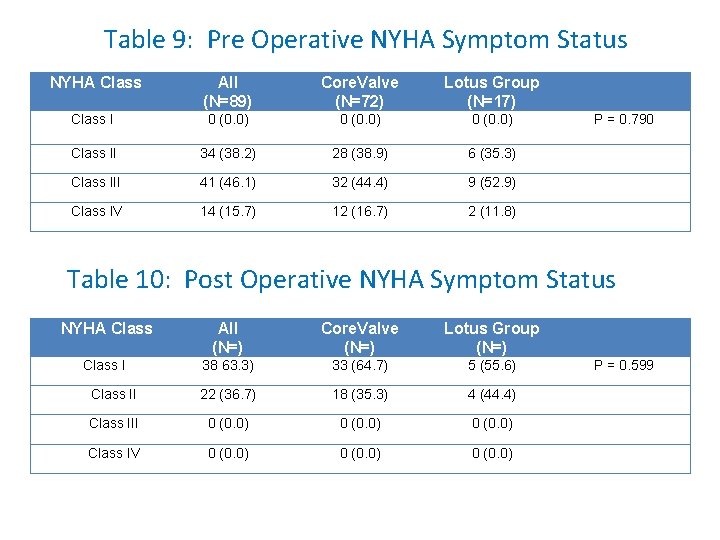
Table 9: Pre Operative NYHA Symptom Status NYHA Class All (N=89) Core. Valve (N=72) Lotus Group (N=17) Class I 0 (0. 0) Class II 34 (38. 2) 28 (38. 9) 6 (35. 3) Class III 41 (46. 1) 32 (44. 4) 9 (52. 9) Class IV 14 (15. 7) 12 (16. 7) 2 (11. 8) P = 0. 790 Table 10: Post Operative NYHA Symptom Status NYHA Class All (N=) Core. Valve (N=) Lotus Group (N=) Class I 38 63. 3) 33 (64. 7) 5 (55. 6) Class II 22 (36. 7) 18 (35. 3) 4 (44. 4) Class III 0 (0. 0) Class IV 0 (0. 0) P = 0. 599

DISCUSSION • The main aim of the study is to evaluate the safety and performance of the Lotus Valve TAVR system in symptomatic patients with severe aortic stenosis and to compare the outcomes in terms of device success, safety, and clinical efficacy as defined by the Valve Academic Research Consortium-2 (VARC-2) with those of Core. Valve treated patients at 30 days. • The study can conclude that implantation of the second generation Lotus valve could be performed safely without affecting outcomes for patients and the safety and efficacy of the Lotus valve system is comparable with the Core. Valve at short term follow-up (30 days).

DISCUSSION • No significant difference in the baseline demographics and echocardiographic characteristics of the two cohorts were seen • At 1 month post TAVR, the aortic valve area increased to 1. 77 cm ± 0. 48 (p=0. 274), mean gradient and peak gradient dropped to 11. 19 mm. Hg ± 4. 88 mm. Hg (p=0. 118) and 19. 82 mm. Hg ± 8. 10 mm. Hg (p=0. 392) respectively with no significant difference in the two groups.

DISCUSSION • Significantly less PVL in the Lotus group compared to the Core. Valve group (p=. 004) at 30 days follow-up. • Eighty-nine percent (89%) of the Lotus treated patients have no PVL as compared to 26% in the Core. Valve group. • No moderate or severe PVL (0%) at 1 month was seen in the Lotus group as compared to 3. 7% in the Core. Valve group.

DISCUSSION • The significant difference in the amount of PVL between the Core. Valve and Lotus group with the Lotus cohort having a better valve performance at 30 days’ follow-up may be secondary to the valve’s ability to be retrieved and repositioned. • The incorporation of the polymeric outer Adaptive Seal to the Lotus valve was designed to minimize PVL.

DISCUSSION • The predictors of PVL: low depth implantation, valve undersizing and high Agatston calcium score. • Valve undersizing is less frequent in this study by careful pre operative CTA measurement with intraoperative TEE being used for confirmation of the optimal valve size. • Low valve implantation on is being avoided in this study as majority of the patients utilized an intraoperative TEE guidance prior to valve deployment. • In the PARTNER trial, it was found that even the presence of mild AR was related to poor 1) Ortega-Paz, L, Giacchi, G, et al. (2015) Percutaneous Transcatheter Aortic Valve Implantation: A Review Focus on Outcome and Safety. AIMS Medical Science 2(3): 200 -221.

DISCUSSION • In terms of procedural outcome, significantly less contrast agent was used in the Lotus treated group as compared to the Core. Valve group (p=0. 000). • This is highly beneficial for patients with chronic renal insufficiency as better outcomes after TAVR were seen in patients who did not develop contrast-induced nephropathy and not pushed to renal replacement therapy. • In this current study, 51% already had chronic renal failure at baseline and 10 patients (11%) developed contrast induced nephropathy and 3 required renal replacement therapy. • In a study done by Barbash(7) regarding incidence and predictors of AKI after TAVR, 14. 6% developed acute AKI according to VARC definition and this is comparable to our present study. Barbash, I, Ben-Dor, I, et al, Incidence and Predictors of Acute Kidney Injury After Transcatheter Aortic Valve Replacement. American Heart Journal. 2012; 163 (6): 1031 -1036.

DISCUSSION • Moreover, patients who developed AKI have a higher inhospital and 30 -day mortality as compared to patients without AKI. • With a very significant difference (p=0. 000) in the amount of contrast used between Core. Valve and Lotus valve with the Lotus valve requiring less contrast agent, it is very prudent to consider choosing a valve requiring less contrast agent in patients with chronic renal insufficiency.

DISCUSSION • The permanent pacemaker rate insertion was numerically higher in the Lotus group than the Core. Valve group (35% vs 25%) although not statistically significant (p=0. 381). • High grade AVB and development of CRBBB are the two main indications for PPI. • The Lotus group has a pacemaker rate of 35%, comparable to reported 36% in the REPRISE I Study and 29% in the REPRISE II Study. • Other studies have reported a lower PPI insertion rate of 15% (3) and they have attributed it to a new deployment method for the valve, where it is kept in higher position during the entire deployment, never allowing the Lotus valve to drop down into the outflow tract that can potentially scrape the septum and damage the conduction system (3).

DISCUSSION • Another explanation for lower incidence of PPI in the aforementioned study is a careful preoperative assessment with gated CT to avoid oversizing of the valve. This practice was already being used in this present study. • One possible explanation for a higher PPI rate of the Lotus valve in our center compared to the aforementioned study is that we have a smaller sample size (n=17) as compared to 53 in the comparative study.

DISCUSSION • No significant difference between the two cohorts in terms of VARC -2 criteria of Device Success, Early Safety and Clinical Efficacy at 30 days was seen. • The procedural success in this study is 98% and two table deaths were reported, 1 from the Lotus group and 1 from the Core. Valve group. • The 30 -day all cause mortality is 8. 5% (7/89), 5 patients (6. 9%) from the Core. Valve cohort and 2 (11. 8%) from the Lotus group (p=0. 365). • The procedural outcomes compare well with the reported 30 -day mortality in the Canadian registry (10. 4%), Source registry (8. 5%), French registry (12. 7%) and German registry. • Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis : acute and late outcomesof the multicenter Canadian experience. J Am Coll Cardiol. 2010; 55: 1080 – 1090. • Thomas M, Schymik G, et a. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010; 122: 62 – 69. • Eltchaninoff H, Prat A, Gilard M, et sl. FRANCE Registry Investigators. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National Core. Valve and Edwards) registry. Eur Heart J. 2011; 32: 191 – 197. • Zahn R, Gerckens U, et sl. German Transcatheter Aortic Valve Interventions-Registry Investigators. Transcatheter aortic valve implantation: first results from a multicentre real-world registry. Eur Heart J. 2011; 32: 198 – 204.

DISCUSSION • Limitation of the Study – relatively small sample size – lack of randomization between the groups – short term follow-up

CONCLUSION • The present study demonstrates the safety and efficacy of the Lotus valves in patients at high surgical risk with severe aortic stenosis. • The valve is retrievable and repositionable with minimal PVL after deployment. • Marked and significant improvement in functional capacity was likewise seen in this study for both cohorts. • This study showed that no significant difference was seen between the Core. Valve and Lotus valve in terms of device success, safety, and clinical efficacy as defined by the Valve Academic Research Consortium-2 (VARC-2) at 30 days.

Thank You & Enjoy Osaka
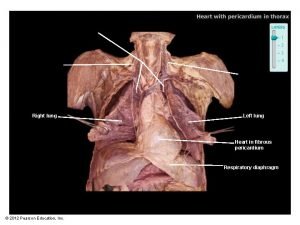
 Fibrous pericardium
Fibrous pericardium Dvi aortic valve
Dvi aortic valve Aortic stenosis echo criteria
Aortic stenosis echo criteria Nicks procedure
Nicks procedure Aortic valve dimensionless index
Aortic valve dimensionless index Aortic semilunar valve sheep heart
Aortic semilunar valve sheep heart Physiological regurgitation
Physiological regurgitation Aortic valve leaflets
Aortic valve leaflets Dvi aortic valve
Dvi aortic valve Combustion reaction equation
Combustion reaction equation Mechanical servo valve
Mechanical servo valve Interruption of the aortic arch
Interruption of the aortic arch Major arteries of the ascending aorta and aortic arch
Major arteries of the ascending aorta and aortic arch Lumbocostal triangle
Lumbocostal triangle Sircumflex
Sircumflex Art-labeling activity: figure 19.26b (4 of 4)
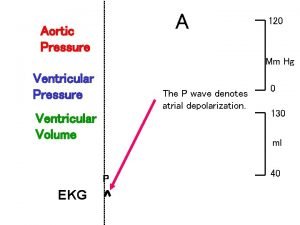
Art-labeling activity: figure 19.26b (4 of 4) Aortic pressure curve
Aortic pressure curve Peripheral signs of aortic regurgitation
Peripheral signs of aortic regurgitation Epididis
Epididis Pathophysiology of aortic aneurysm ppt
Pathophysiology of aortic aneurysm ppt Aortic stanosis
Aortic stanosis Lordotic chest x ray
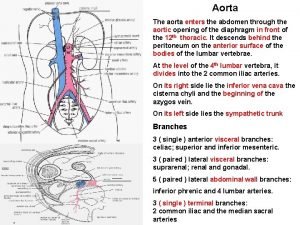
Lordotic chest x ray Aortic branches
Aortic branches Carina respiratory system
Carina respiratory system Robert bersin md
Robert bersin md Anterior cardiac veins of the right ventricle
Anterior cardiac veins of the right ventricle 4th aortic arch derivatives
4th aortic arch derivatives Mitral regurgitation murmur
Mitral regurgitation murmur Korotkoff sound
Korotkoff sound Thorasic aortic aneurysm
Thorasic aortic aneurysm Aortic heart disease
Aortic heart disease 120 mm/hg
120 mm/hg Dr m chadi alraies
Dr m chadi alraies Branches off aortic arch
Branches off aortic arch Aortic vestibule
Aortic vestibule Coronary sulcus
Coronary sulcus Dobutamine dose
Dobutamine dose Aortic body
Aortic body Aortic area 1 and 2
Aortic area 1 and 2 Back view of lungs in body
Back view of lungs in body Acute aortic syndrome
Acute aortic syndrome Parts of the heart diagram
Parts of the heart diagram Transtubercular plane level
Transtubercular plane level