Newgeneration drugeluting stents and dual antiplatelet therapy overview






















- Slides: 50

New-generation drug-eluting stents and dual antiplatelet therapy: overview Giuseppe Biondi Zoccai, MD Department of Medico-Surgical Sciences and Biotechnologies Sapienza University of Rome, Italy giuseppe. biondizoccai@uniroma 1. it

Our original sin…

An ongoing challenge after stenting: preventing both… THROMBOSIS BLEEDING

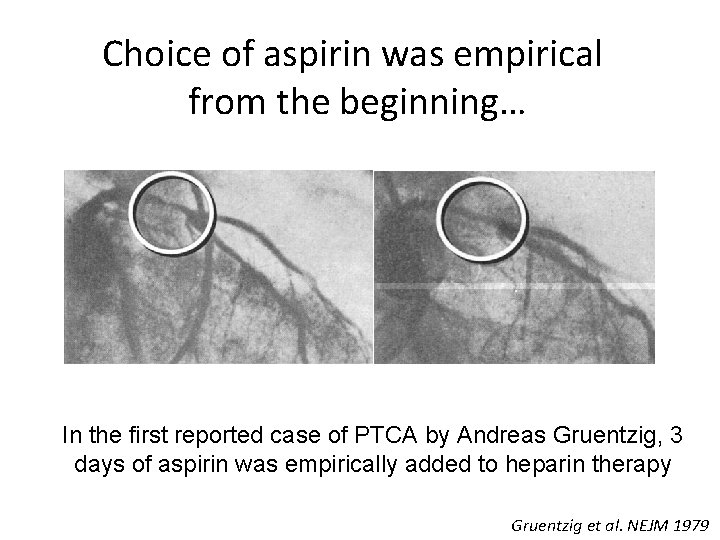
Choice of aspirin was empirical from the beginning… In the first reported case of PTCA by Andreas Gruentzig, 3 days of aspirin was empirically added to heparin therapy Gruentzig et al. NEJM 1979

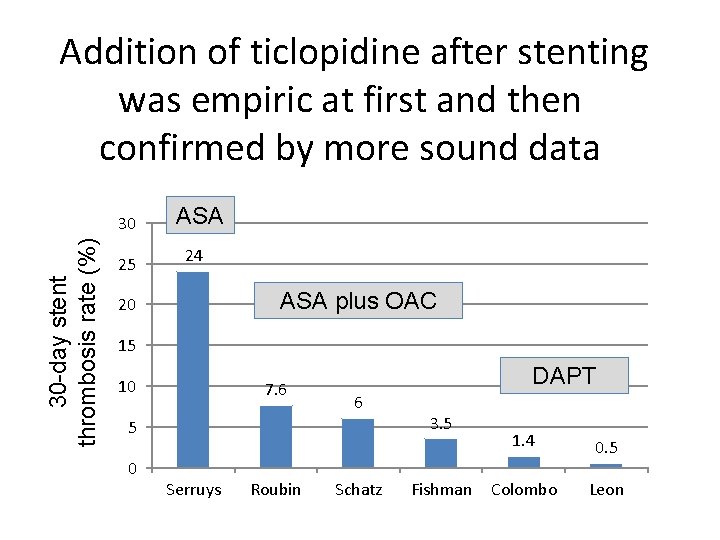
Addition of ticlopidine after stenting was empiric at first and then confirmed by more sound data 30 -day stent thrombosis rate (%) 30 25 ASA 24 ASA plus OAC 20 15 10 7. 6 DAPT 6 5 0 Serruys Roubin Schatz 3. 5 Fishman 1. 4 0. 5 Colombo Leon

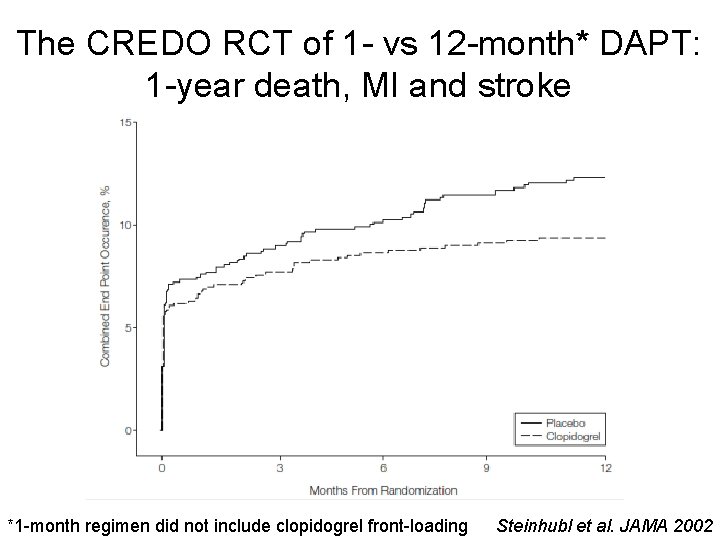
The CREDO RCT of 1 - vs 12 -month* DAPT: 1 -year death, MI and stroke *1 -month regimen did not include clopidogrel front-loading Steinhubl et al. JAMA 2002

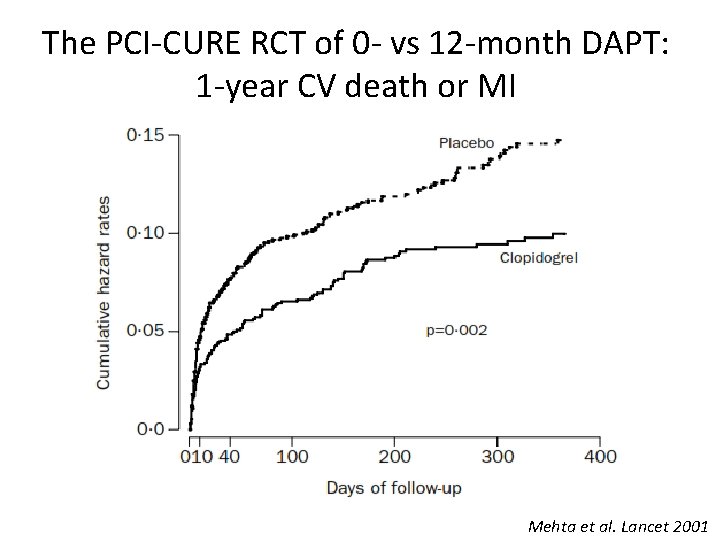
The PCI-CURE RCT of 0 - vs 12 -month DAPT: 1 -year CV death or MI Mehta et al. Lancet 2001

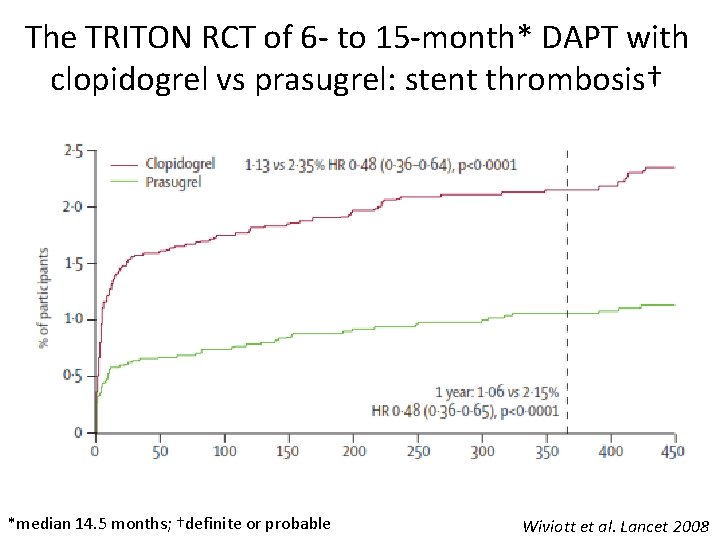
The TRITON RCT of 6 - to 15 -month* DAPT with clopidogrel vs prasugrel: stent thrombosis† *median 14. 5 months; †definite or probable Wiviott et al. Lancet 2008

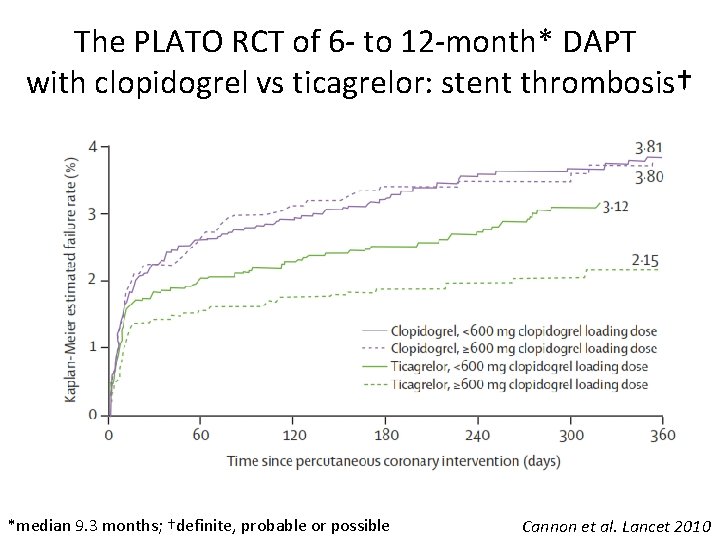
The PLATO RCT of 6 - to 12 -month* DAPT with clopidogrel vs ticagrelor: stent thrombosis† *median 9. 3 months; †definite, probable or possible Cannon et al. Lancet 2010

The PLATO RCT of 6 - to 12 -month* DAPT with clopidogrel vs ticagrelor: all cause death *median 14. 5 months Cannon et al. Lancet 2010

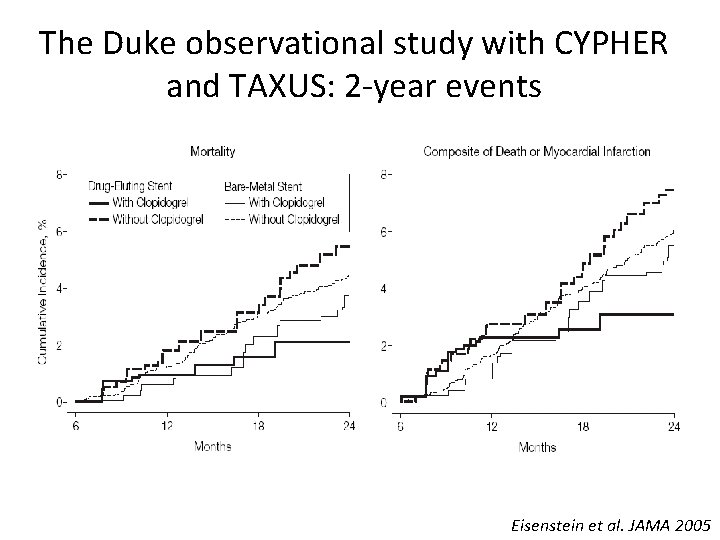
The Duke observational study with CYPHER and TAXUS: 2 -year events Eisenstein et al. JAMA 2005

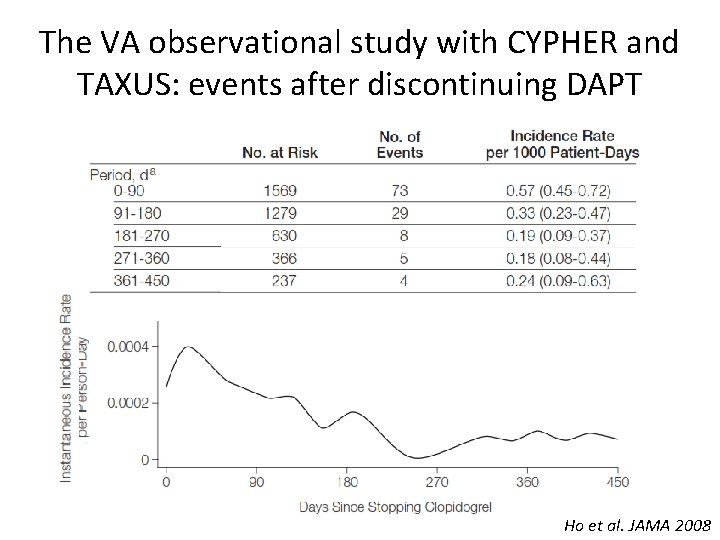
The VA observational study with CYPHER and TAXUS: events after discontinuing DAPT Ho et al. JAMA 2008

The J-CYPHER observational study: 2 -year stent thrombosis Kimura et al. Circulation 2009

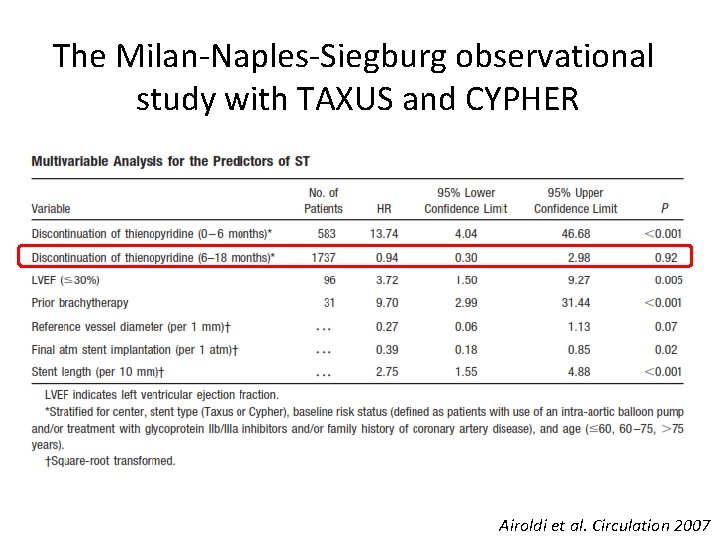
The Milan-Naples-Siegburg observational study with TAXUS and CYPHER Airoldi et al. Circulation 2007

The LATE RCTs of 12 - vs 24 -month DAPT: 2 -year death, MI and stroke Park et al. NEJM 2010

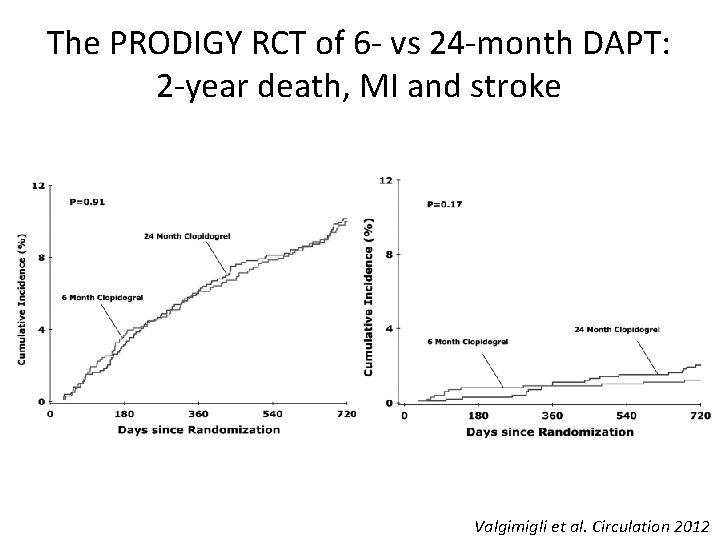
The PRODIGY RCT of 6 - vs 24 -month DAPT: 2 -year death, MI and stroke Valgimigli et al. Circulation 2012

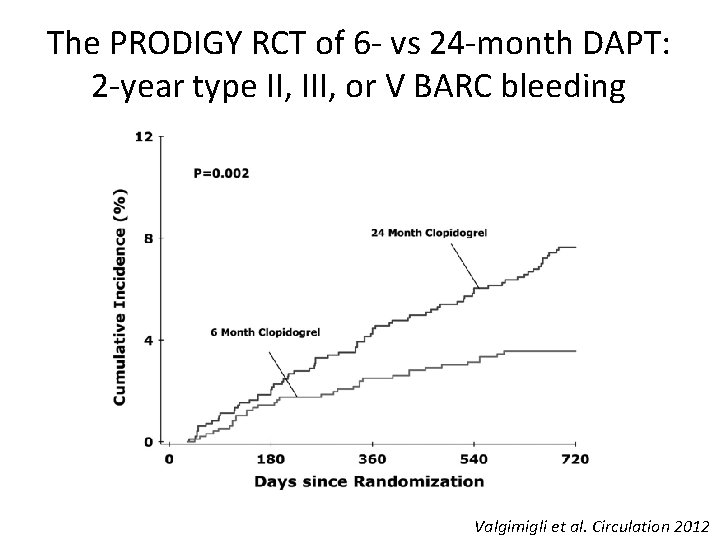
The PRODIGY RCT of 6 - vs 24 -month DAPT: 2 -year type II, III, or V BARC bleeding Valgimigli et al. Circulation 2012

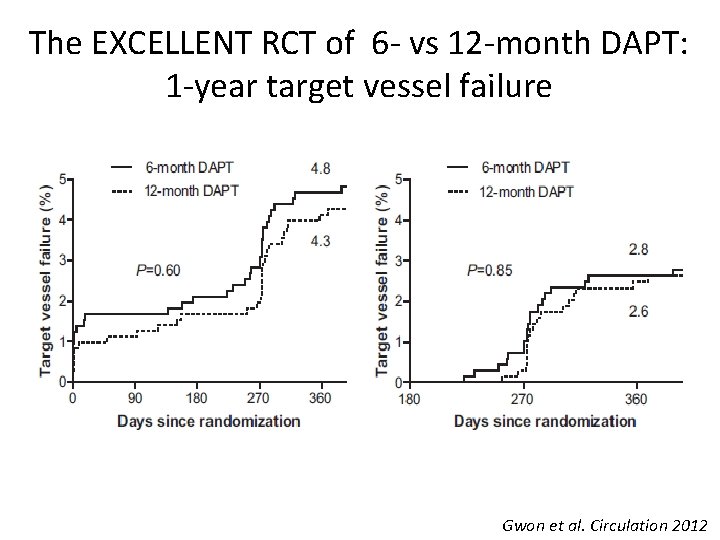
The EXCELLENT RCT of 6 - vs 12 -month DAPT: 1 -year target vessel failure Gwon et al. Circulation 2012

The EXCELLENT RCT of 6 - vs 12 -month DAPT: other 1 -year events Gwon et al. Circulation 2012

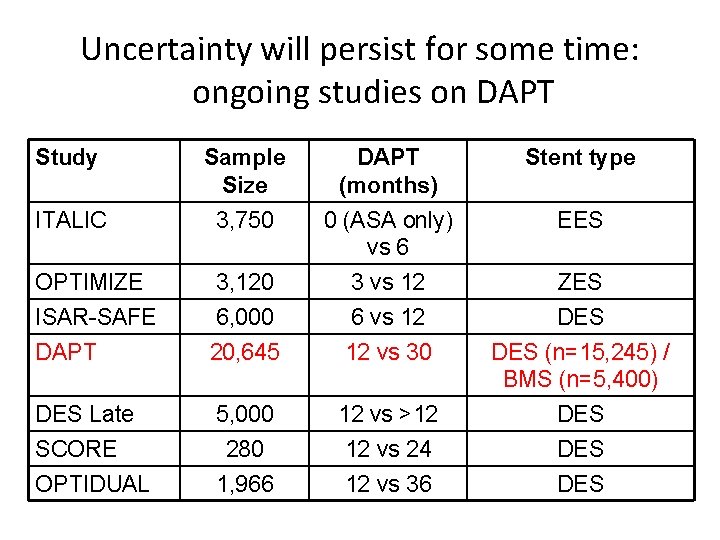
Uncertainty will persist for some time: ongoing studies on DAPT Study Sample Size DAPT (months) Stent type ITALIC 3, 750 0 (ASA only) vs 6 EES OPTIMIZE 3, 120 3 vs 12 ZES ISAR-SAFE 6, 000 6 vs 12 DES DAPT 20, 645 12 vs 30 DES (n=15, 245) / BMS (n=5, 400) DES Late 5, 000 12 vs >12 DES 280 12 vs 24 DES 1, 966 12 vs 36 DES SCORE OPTIDUAL

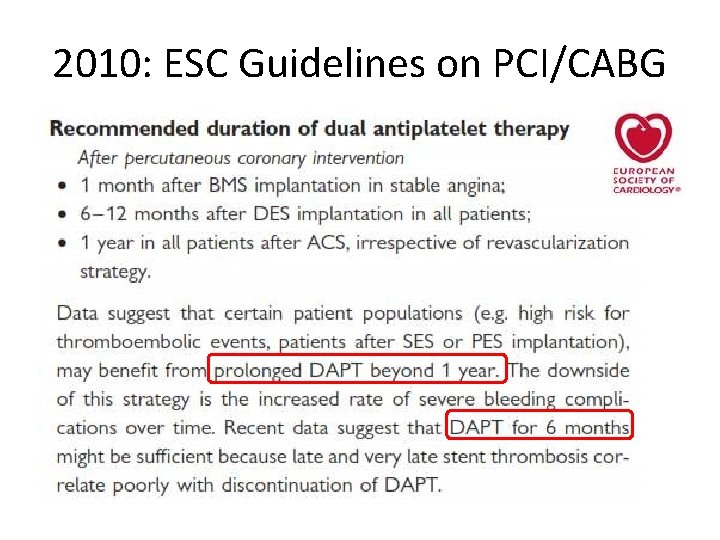
2010: ESC Guidelines on PCI/CABG

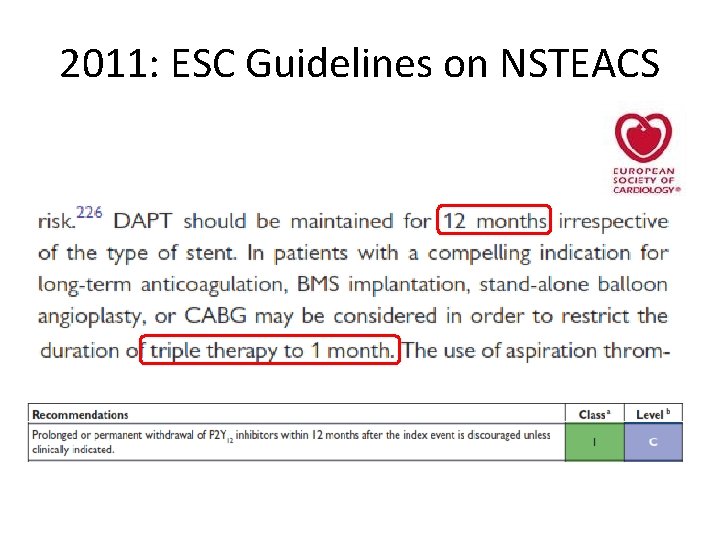
2011: ESC Guidelines on NSTEACS

The XIENCE V USA study including 5, 054 unselected real-world patients AMI 18. 1% Left Main 1. 6% ACS 37. 5% Graft Lesion 4. 8% Renal Insufficiency 11. 1% EF < 30% 3. 4% CTO Lesion 2. 5% A Real-World Population Diabetes 35. 6% Multivessel Disease 40. 8% Multivessel Treated 13. 8% Direct Stenting 38. 7% Restenosis Lesion 9. 5% Bifurcation 9. 0% Ostial 11. 9% Hermiller, PCR 2010

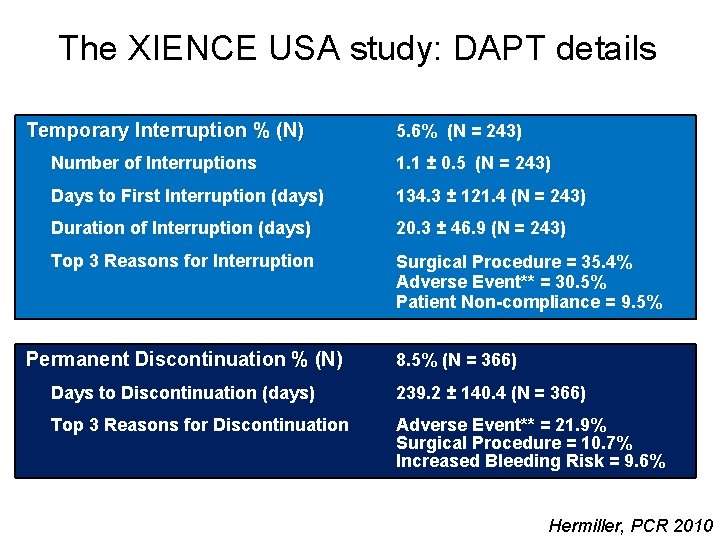
The XIENCE USA study: DAPT details Temporary Interruption % (N) 5. 6% (N = 243) Number of Interruptions 1. 1 ± 0. 5 (N = 243) Days to First Interruption (days) 134. 3 ± 121. 4 (N = 243) Duration of Interruption (days) 20. 3 ± 46. 9 (N = 243) Top 3 Reasons for Interruption Surgical Procedure = 35. 4% Adverse Event** = 30. 5% Patient Non-compliance = 9. 5% Permanent Discontinuation % (N) 8. 5% (N = 366) Days to Discontinuation (days) 239. 2 ± 140. 4 (N = 366) Top 3 Reasons for Discontinuation Adverse Event** = 21. 9% Surgical Procedure = 10. 7% Increased Bleeding Risk = 9. 6% Hermiller, PCR 2010

The XIENCE USA study: stent thrombosis according to DAPT interruption Hermiller, PCR 2010

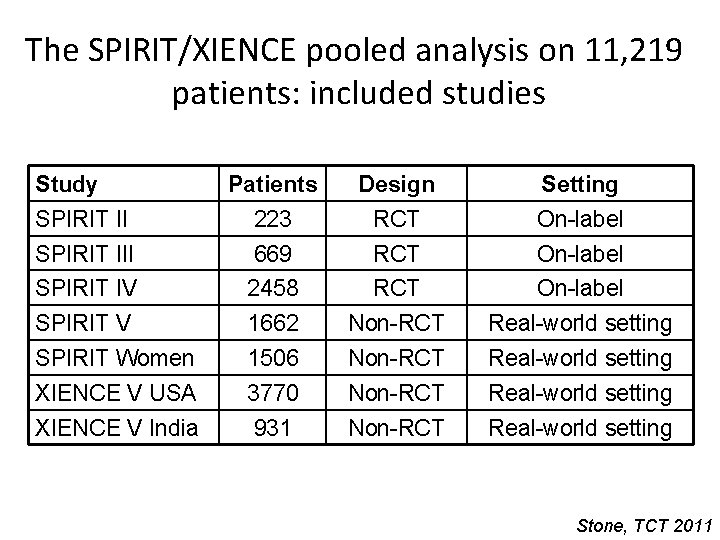
The SPIRIT/XIENCE pooled analysis on 11, 219 patients: included studies Study Patients Design Setting SPIRIT II 223 RCT On-label SPIRIT III 669 RCT On-label SPIRIT IV 2458 RCT On-label SPIRIT V 1662 Non-RCT Real-world setting SPIRIT Women 1506 Non-RCT Real-world setting XIENCE V USA 3770 Non-RCT Real-world setting XIENCE V India 931 Non-RCT Real-world setting Stone, TCT 2011

The SPIRIT/XIENCE pooled analysis on 11, 219 patients: 2 -year stent thrombosis Stone, TCT 2011

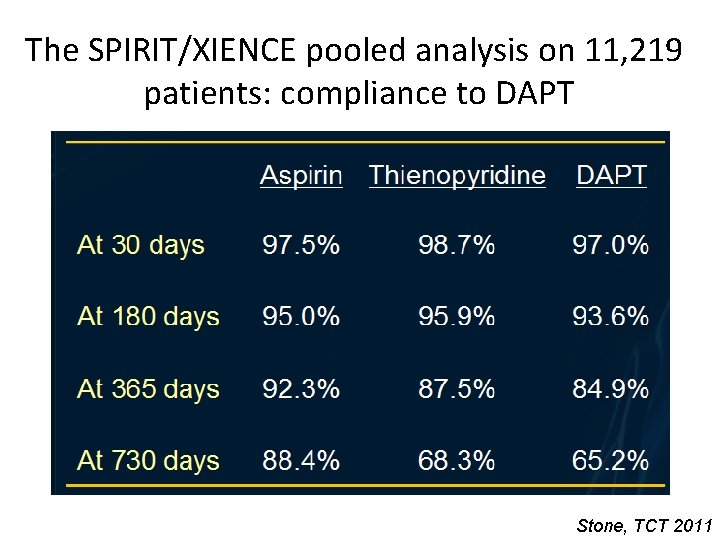
The SPIRIT/XIENCE pooled analysis on 11, 219 patients: compliance to DAPT Stone, TCT 2011

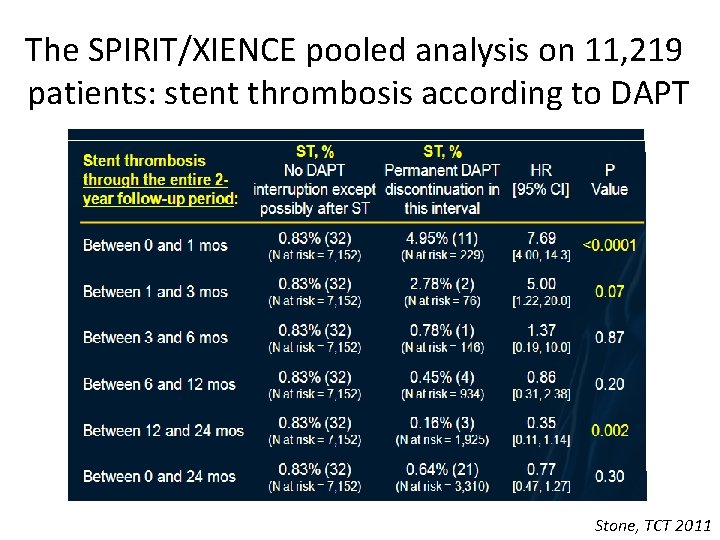
The SPIRIT/XIENCE pooled analysis on 11, 219 patients: stent thrombosis according to DAPT No DAPT interruption within 90 days DAPT interruption after 90 days Stone, TCT 2011

The SPIRIT/XIENCE pooled analysis on 11, 219 patients: days of DAPT discontinuation Stone, TCT 2011

The SPIRIT/XIENCE pooled analysis on 11, 219 patients: stent thrombosis according to DAPT Stone, TCT 2011

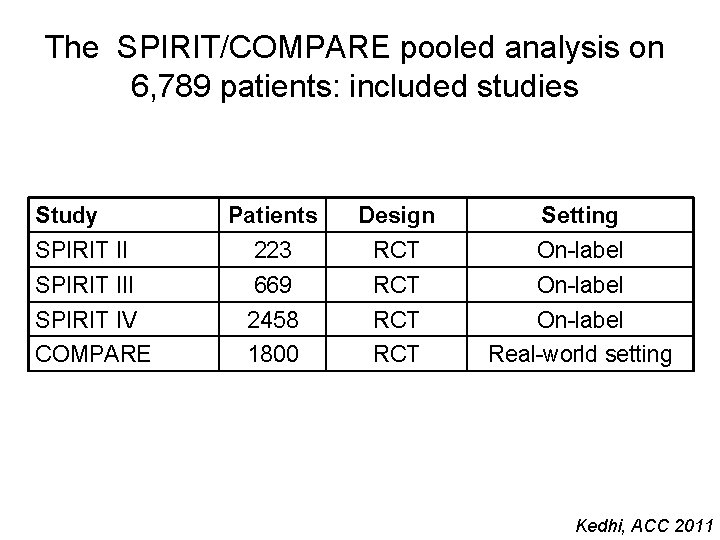
The SPIRIT/COMPARE pooled analysis on 6, 789 patients: included studies Study Patients Design Setting SPIRIT II 223 RCT On-label SPIRIT III 669 RCT On-label SPIRIT IV 2458 RCT On-label COMPARE 1800 RCT Real-world setting Kedhi, ACC 2011

The SPIRIT/COMPARE pooled analysis on 6, 789 patients: stent thrombosis according to DAPT 1 -6 mo DAPT 6 -12 mo DAPT 12 -24 mo DAPT >24 mo DAPT Kedhi, ACC 2011

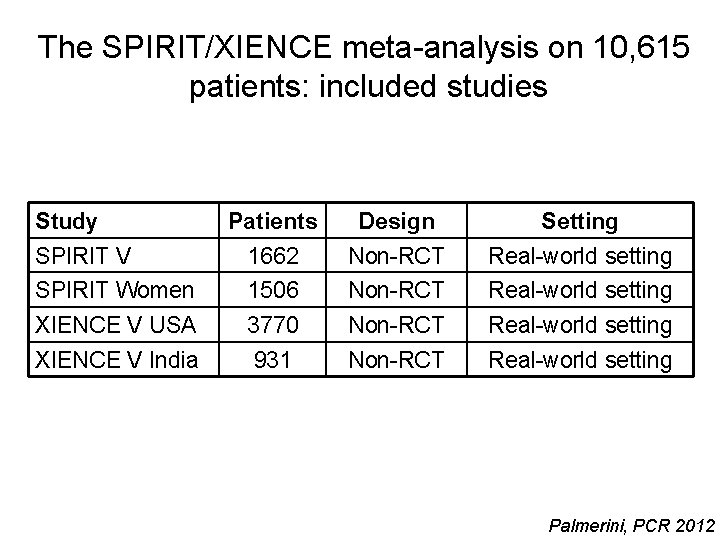
The SPIRIT/XIENCE meta-analysis on 10, 615 patients: included studies Study Patients Design Setting SPIRIT V 1662 Non-RCT Real-world setting SPIRIT Women 1506 Non-RCT Real-world setting XIENCE V USA 3770 Non-RCT Real-world setting XIENCE V India 931 Non-RCT Real-world setting Palmerini, PCR 2012

The SPIRIT/XIENCE meta-analysis on 10, 615 patients: stent thrombosis according to DAPT Palmerini, PCR 2012

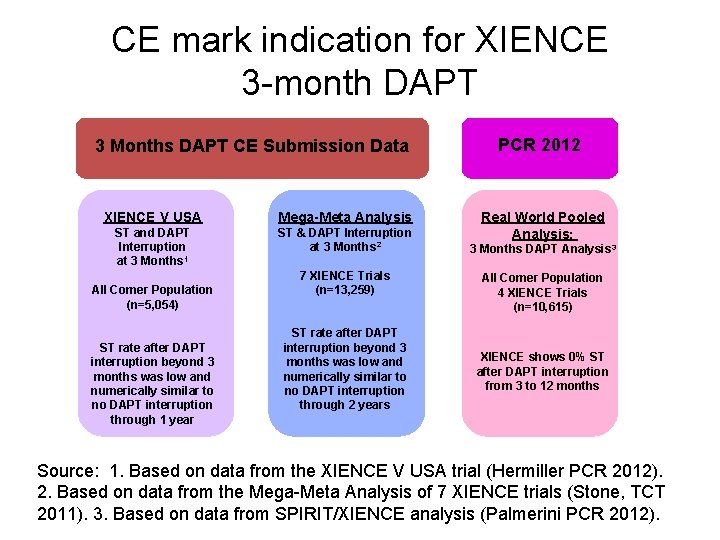
CE mark indication for XIENCE 3 -month DAPT Language from CE IFU Section 8. 1 for XIENCE: XIENCE demonstrated low stent thrombosis rates in patients who either discontinued or interrupted Dual Antiplatelet Therapy (DAPT) after 3 months post stent implantation. It is therefore recommended that patients treated with XIENCE stents remain on DAPT for at least 3 months after stent implantation. New indication underlines the XIENCE safety outcomes even when patients interrupt DAPT after 3 months

CE mark indication for XIENCE 3 -month DAPT 3 Months DAPT CE Submission Data XIENCE V USA Mega-Meta Analysis ST and DAPT Interruption at 3 Months 1 ST & DAPT Interruption at 3 Months 2 All Comer Population (n=5, 054) ST rate after DAPT interruption beyond 3 months was low and numerically similar to no DAPT interruption through 1 year 7 XIENCE Trials (n=13, 259) ST rate after DAPT interruption beyond 3 months was low and numerically similar to no DAPT interruption through 2 years PCR 2012 Real World Pooled Analysis: 3 Months DAPT Analysis 3 All Comer Population 4 XIENCE Trials (n=10, 615) XIENCE shows 0% ST after DAPT interruption from 3 to 12 months Source: 1. Based on data from the XIENCE V USA trial (Hermiller PCR 2012). 2. Based on data from the Mega-Meta Analysis of 7 XIENCE trials (Stone, TCT 2011). 3. Based on data from SPIRIT/XIENCE analysis (Palmerini PCR 2012).

Additional insights from a comprehensive network meta-analysis Palmerini, Biondi-Zoccai et al, Lancet 2012

Evidence network 9 studies 1 s tud y 9 s tud ies es udi 8 st s die u t 4 s PES es udi 2 st 5 st udi es BMS 6 studies End-ZES SES dies 6 stu Co. Cr-EES 2 Res-ZES ies d stu 1 st ud y Pt. Cr-EES Palmerini, Biondi-Zoccai et al, Lancet 2012

Additional insights from a comprehensive network meta-analysis 1 -Year Definite Stent Thrombosis Odds Ratio [95%] Co. Cr-EES vs BMS 0. 23 (0. 13 -0. 41) Co. Cr-EES vs PES 0. 28 (0. 16 -0. 48) Co. Cr-EES vs SES 0. 41 (0. 24 -0. 70) Co. Cr-EES vs Res-ZES 0. 14 (0. 03 -0. 47) Co. Cr-EES vs End-ZES 0. 21 (0. 10 -0. 44) SES vs BMS 0. 57 (0. 36 -0. 88) End-ZES vs SES 1. 92 (1. 07 -3. 90) 0. 01 1 0. 1 Favors Stent 1 10 Favors Stent 2 Palmerini, Biondi-Zoccai et al, Lancet 2012

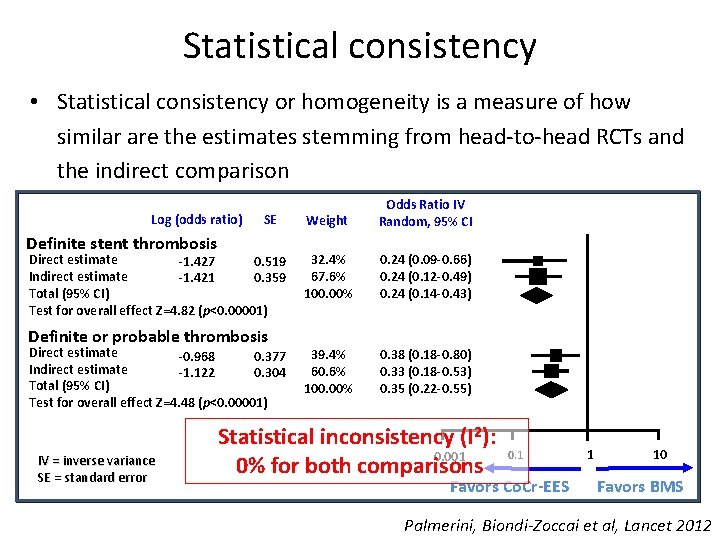
Statistical consistency • Statistical consistency or homogeneity is a measure of how similar are the estimates stemming from head-to-head RCTs and the indirect comparison Log (odds ratio) SE Definite stent thrombosis Direct estimate -1. 427 0. 519 Indirect estimate -1. 421 0. 359 Total (95% CI) Test for overall effect Z=4. 82 (p<0. 00001) Definite or probable thrombosis Direct estimate -0. 968 0. 377 Indirect estimate -1. 122 0. 304 Total (95% CI) Test for overall effect Z=4. 48 (p<0. 00001) IV = inverse variance SE = standard error Weight Odds Ratio IV Random, 95% CI 32. 4% 67. 6% 100. 00% 0. 24 (0. 09 -0. 66) 0. 24 (0. 12 -0. 49) 0. 24 (0. 14 -0. 43) 39. 4% 60. 6% 100. 00% 0. 38 (0. 18 -0. 80) 0. 33 (0. 18 -0. 53) 0. 35 (0. 22 -0. 55) Statistical inconsistency (I 2): 0. 001 0% for both comparisons 0. 1 Favors Co. Cr-EES 1 10 Favors BMS Palmerini, Biondi-Zoccai et al, Lancet 2012

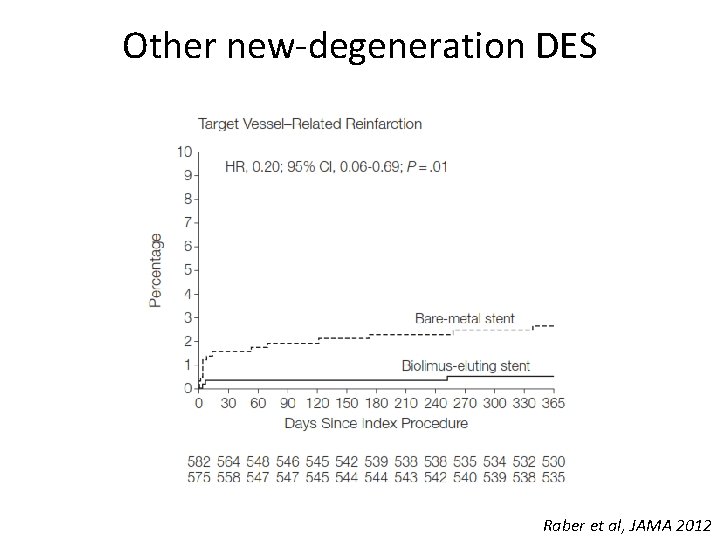
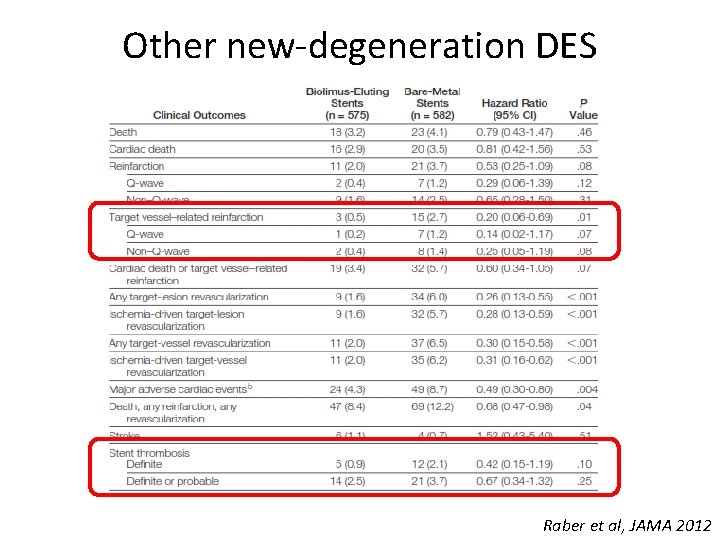
Other new-degeneration DES Raber et al, JAMA 2012

Other new-degeneration DES Raber et al, JAMA 2012

Prototypical clinical cases

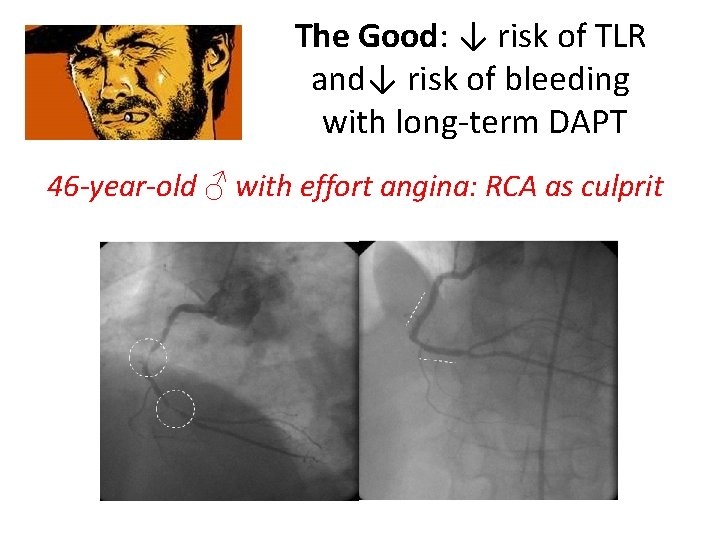
The Good: ↓ risk of TLR and↓ risk of bleeding with long-term DAPT 46 -year-old ♂ with effort angina: RCA as culprit

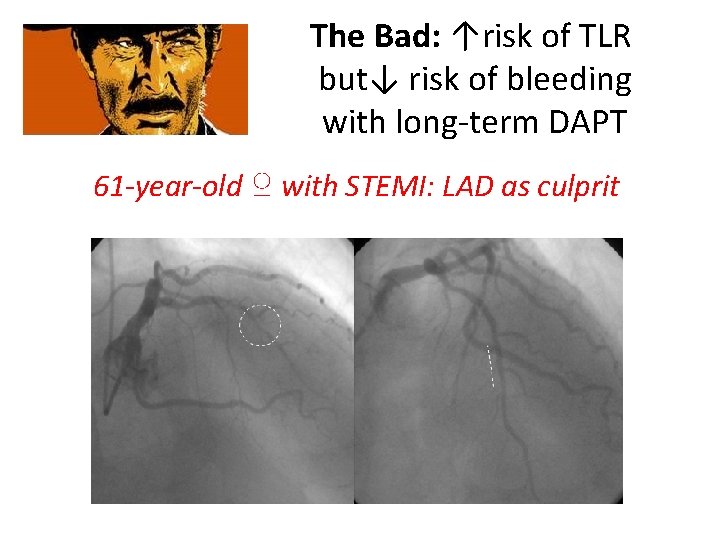
The Bad: ↑risk of TLR but↓ risk of bleeding with long-term DAPT 61 -year-old ♀ with STEMI: LAD as culprit

The Ugly: ↑risk of TLR and ↑ risk of bleeding with long-term DAPT 74 -year-old ♂ with NSTEMI & AF requiring oral anticoagulants: LM-LAD as culprit

Take home messages • DAPT aims to prevent two different events: stent thrombosis and non-target lesion thrombosis. • Long-term DAPT reduces the risk of non-target lesion events. • However, there is mounting uncertainty on the impact of long-term DAPT on stent thrombosis. • EES have a unique safety profile among coronary stents: – After 3 months, patients with EES discontinuing DAPT have a risk of stent thrombosis similar to those not discontinuing;

Take home messages – Accordingly, 3 -month DAPT appears adequate to reduce the risk of stent thrombosis in patients receiving EES; – Favorable yet much less thorough results have also been reported for BES. • I personally do not recommend 3 -month DAPT in all patients, but surely do in carefully selected ones. • Moreover, I can be truly confident that any of my patients who has received a EES and discontinue DAPT ≥ 3 months is not put at a higher risk of stent thrombosis. • This property cannot so far be inferred for any other DES, and thus makes EES a unique treatment opportunity to maximize efficacy and safety.

Thank you for your attention For any correspondence: giuseppe. biondizoccai@uniroma 1. it For these and further slides on these topics feel free to visit the metcardio. org website: http: //www. metcardio. org/slides. html Meta-analysis and Evidence-based medicine Training in Cardiology
 Guidelines for antiplatelet and fibrinolytic therapy
Guidelines for antiplatelet and fibrinolytic therapy Antiplatelet mechanism of action
Antiplatelet mechanism of action Tavr antiplatelet guidelines
Tavr antiplatelet guidelines Dual ii magnum vs dual magnum
Dual ii magnum vs dual magnum Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Bioness integrated therapy system price
Bioness integrated therapy system price Humanistic therapies aim to boost
Humanistic therapies aim to boost Dual language immersion programs pros cons
Dual language immersion programs pros cons Dual slope adc advantages and disadvantages
Dual slope adc advantages and disadvantages Dual bar charts worksheet
Dual bar charts worksheet The dual symbol for a nand gate is a negative-and symbol.
The dual symbol for a nand gate is a negative-and symbol. Multimode operation in os
Multimode operation in os Negative and positive voltage from a dual power supply
Negative and positive voltage from a dual power supply Overview of transcription and translation
Overview of transcription and translation Data quality and data cleaning an overview
Data quality and data cleaning an overview The two rows of elements that seem to be disconnected
The two rows of elements that seem to be disconnected Chicago time
Chicago time What is bioinformatics an introduction and overview
What is bioinformatics an introduction and overview An overview of data warehousing and olap technology
An overview of data warehousing and olap technology Data quality and data cleaning an overview
Data quality and data cleaning an overview Data quality and data cleaning an overview
Data quality and data cleaning an overview Overview of storage and indexing
Overview of storage and indexing Chapter 17 overview elements and their properties
Chapter 17 overview elements and their properties What does compounding the brakes mean
What does compounding the brakes mean Phsc porter campus
Phsc porter campus Dual enrollment nova
Dual enrollment nova Eku merit scholarships
Eku merit scholarships Ggc dual enrollment
Ggc dual enrollment 4118 cont
4118 cont Valencia college withdrawal
Valencia college withdrawal Posgl
Posgl Ung dual enrollment requirements
Ung dual enrollment requirements The dual court system in the u.s. means
The dual court system in the u.s. means Dual laminate system
Dual laminate system Constante de planck
Constante de planck Dual career path
Dual career path Dual sun solar system
Dual sun solar system Yp valuation
Yp valuation Dual pathway inhibition
Dual pathway inhibition Método dual simplex exercícios resolvidos
Método dual simplex exercícios resolvidos Fungsi boolean
Fungsi boolean Dual cvvt
Dual cvvt Describe the trichromatic theory of color vision.
Describe the trichromatic theory of color vision. Architecture of operating system
Architecture of operating system What is dual mode in os
What is dual mode in os Dual aspect monism
Dual aspect monism Dual characteristics of light
Dual characteristics of light Dual gate mosfet mixer
Dual gate mosfet mixer Mission college registration
Mission college registration Simpleks dual
Simpleks dual Contoh primal dan dual
Contoh primal dan dual