Unit 4 Review Chemical Reactions Compounds The image


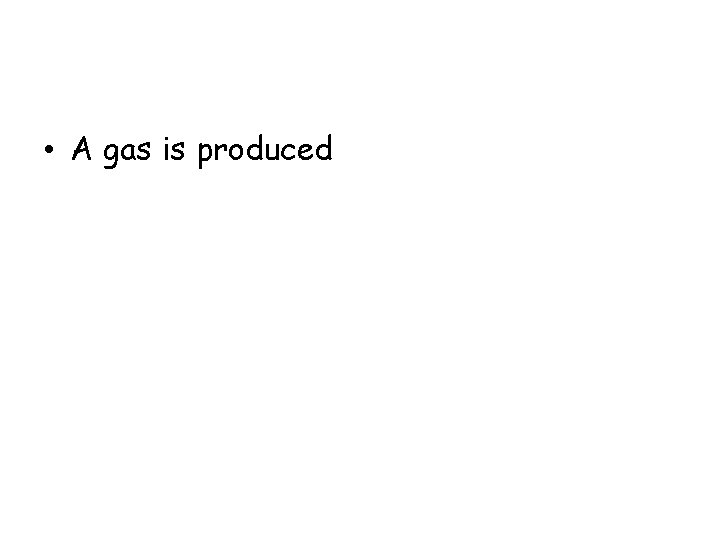
























































- Slides: 83

Unit 4 Review Chemical Reactions & Compounds

The image shows what happens when cold medicine tablets are placed in room-temperature water. What evidence would indicate that a new substance is produced?
• A gas is produced

A student measures out 100 m. L of two different clear liquids and then pours one into the other. The mixture, which has a volume of 200 m. L, turns bright red, then begins to become quite cold. In this process, the first evidence that a new substance had formed was observed when the -
• Changing colors

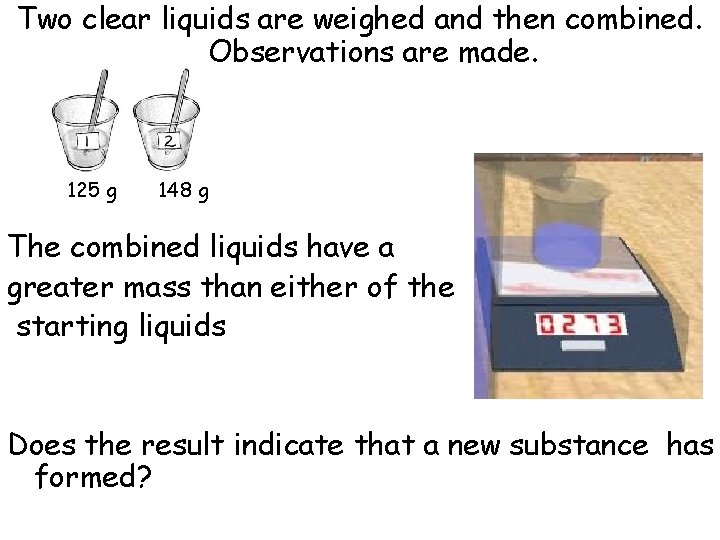
Two clear liquids are weighed and then combined. Observations are made. 125 g 148 g The combined liquids have a greater mass than either of the starting liquids Does the result indicate that a new substance has formed?

• Physical, there should be more mass when two substances combine – nothing was produced

Two clear liquids are combined and observations are made. Does the following result indicate that a new substance has formed? + = The liquids, when mixed, produce a white solid which settles to the bottom

• Chemical, precipitate

A mixture is made by combining two clear liquids and observations are made. Does the following result indicate that a new substance has formed? The mixture of the two liquids takes the shape of the container they are in + =
• Characteristics of a liquid – not a chemical reaction

A mixture is made by combining four clear liquids and observations are made. Does the following result indicate that a new substance has formed? The denser of the two liquids lies below the other less dense liquid

• No new substance, evidence of density

Does this indicate a new substance has been formed? A solid has become a liquid

• Change in state of matter, no new substance

Does this indicate a new substance has been formed? A substance has permanently changed color

• Chemical change – permanent change in color

Does this indicate a new substance has been formed? A solid has dissolved in a liquid

• No, can be undone by evaporation, or distilling

Does this indicate a new substance has been formed? The volume of a substance increased.

• Yes, a permanent change has occurred

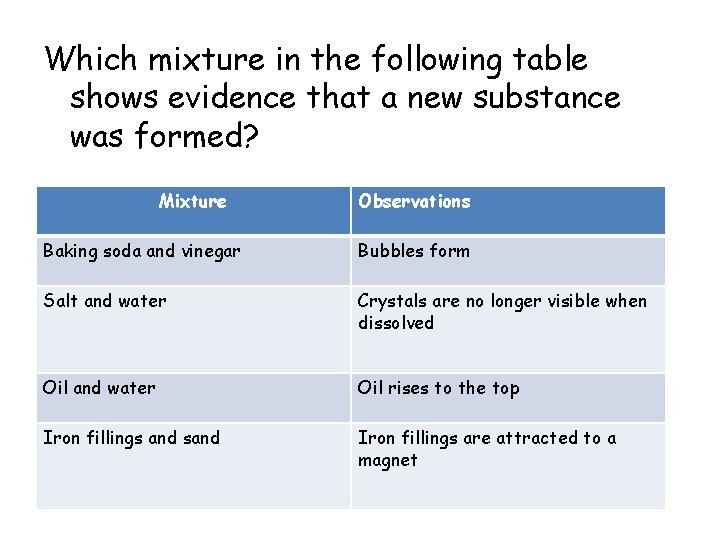
Which mixture in the following table shows evidence that a new substance was formed? Mixture Observations Baking soda and vinegar Bubbles form Salt and water Crystals are no longer visible when dissolved Oil and water Oil rises to the top Iron fillings and sand Iron fillings are attracted to a magnet
• Baking soda and vinegar

Students combine several chemicals and record the observations in their science notebooks. A list of the observed results is given here. How many of the observations are evidence that a new substance was formed? • • • Production of a gas (bubbles) Phase change (solid to liquid) A precipitate forms Change in shape Crystals dissolving Heat produced

• Production of a gas (bubbles) • A precipitate forms • Heat produced

Bubbles are evidence of _______

• A gas is being produced

Chemical or Physical? Gives off heat

• chemical

Chemical or Physical? Granules dissolve

• physical

Chemical or Physical? Volume increases

• Chemical

Does volume of a substance increasing indicate a new substance has been formed?

• Yes, chemical

Chemical or Physical? Granules seem to get smaller

• No, dissolving

Chemical or Physical? Two substances are mixed, would a decrease in temperature indicate a new substance is formed?

• Yes, endothermic reaction

Chemical or Physical? Two substances are mixed, would an increase in mass indicate a new substance is formed?
• No, would be expected when two masses are combined

Chemical or Physical? Two substances are mixed, if one of the substances dissolved indicate a new substance is formed?

• No, can be undone by evaporation, or distilling

Chemical or Physical? Two clear liquid substances are mixed, a white powder can be seen settling on the bottom of the test tube. The white powder is called?

• precipitate

What is always a characteristic of a chemical reaction?

• A new substance is formed

Physical or Chemical Frying an egg

Physical or Chemical

Physical or Chemical Toast

Physical or Chemical Toast Chemical

Physical or Chemical Bread Rising

Physical or Chemical

Physical or Chemical Cracking an Egg

Physical or Chemical Cracking an Egg Physical

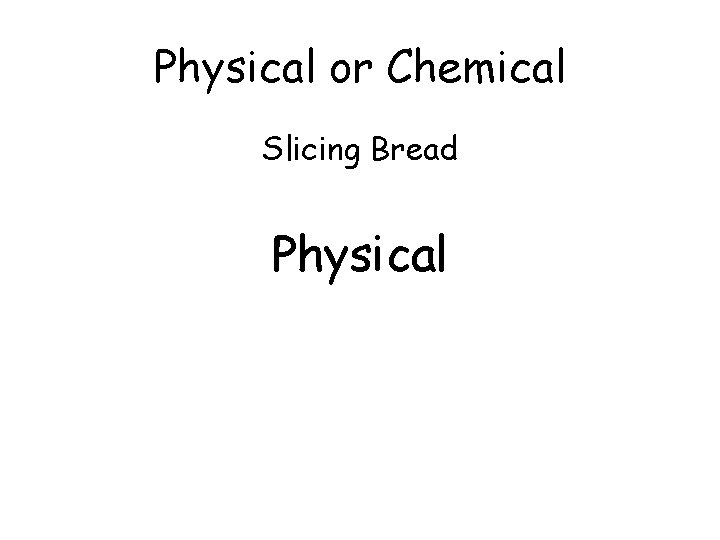
Physical or Chemical Slicing Bread

Physical or Chemical Slicing Bread Physical

Physical or Chemical Lighting a Match

Physical or Chemical Lighting a Match Chemical

Physical or Chemical Candle wax melting

Physical or Chemical Physical

Physical or Chemical Candle wick burning

Physical or Chemical

Physical or Chemical Roasting marshmallows

Physical or Chemical Roasting marshmallows Chemical

Physical or Chemical Rusty Cans

Physical or Chemical Rusty Cans Chemical

Physical or Chemical Ice Melting

Physical or Chemical Ice Melting Physical

Physical or Chemical Glass Breaking

Physical or Chemical Glass Breaking Physical

Physical or Chemical Boiling Water

Physical or Chemical Boiling Water Physical

Physical or Chemical Fresh Lemonade

Physical or Chemical Fresh Lemonade Physical

Physical or Chemical Baking a Cake

Physical or Chemical Baking a Cake Chemical

Physical or Chemical Mowing the Lawn

Physical or Chemical Mowing the Lawn Physical

Physical or Chemical Fireworks

Physical or Chemical Fireworks Chemical

Physical or Chemical Digesting food

Physical or Chemical Digesting food Chemical
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Empirical formula pogil
Empirical formula pogil 7-1 practice problems chemistry answers
7-1 practice problems chemistry answers Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Balancing equations chapter 8
Balancing equations chapter 8 Writing names for ionic compounds
Writing names for ionic compounds Examples of chemical change
Examples of chemical change Unit 11 chemical reactions
Unit 11 chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Unit chemical bonding forming ionic compounds ws 2
Unit chemical bonding forming ionic compounds ws 2 Unit 6 review questions
Unit 6 review questions Reduction half reaction
Reduction half reaction Maltose and cellobiose difference
Maltose and cellobiose difference Non metals examples
Non metals examples Covalent and ionic bond venn diagram
Covalent and ionic bond venn diagram Stoichiometry island diagram
Stoichiometry island diagram Balancing redox reactions
Balancing redox reactions Types of reactions
Types of reactions 4 types of chemical reactions
4 types of chemical reactions What are the 5 types of chemical reactions
What are the 5 types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Principles of immunochemical reaction
Principles of immunochemical reaction Predicting products of chemical reactions
Predicting products of chemical reactions Synthesis reaction predicting products
Synthesis reaction predicting products Combination reaction equation
Combination reaction equation Toxic reactions chemical equations lesson 68 answers
Toxic reactions chemical equations lesson 68 answers Four types of chemical reactions
Four types of chemical reactions What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Chemical reactions in everyday life
Chemical reactions in everyday life 5 general types of chemical reactions
5 general types of chemical reactions Chemical reactions reactants and products
Chemical reactions reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Chemical reactions study guide
Chemical reactions study guide Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Equilibrium of chemical reactions
Equilibrium of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions 5 chemical reactions
5 chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Types of reactions
Types of reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems How to name carboxylic acid
How to name carboxylic acid Summary of chemical reactions
Summary of chemical reactions Chemical reaction of bread
Chemical reaction of bread Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Solvent in chemical reactions
Solvent in chemical reactions Three types of chemical reactions
Three types of chemical reactions Mass relationships in chemical reactions
Mass relationships in chemical reactions Indications of chemical reaction
Indications of chemical reaction Describing chemical reactions
Describing chemical reactions Rules of chemical reaction
Rules of chemical reaction Rearrangement of atoms
Rearrangement of atoms Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations Classification of chemical reactions worksheet
Classification of chemical reactions worksheet Combustion chemical reaction
Combustion chemical reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Limiting reactant lab report answers
Limiting reactant lab report answers Chemical reactions in welding
Chemical reactions in welding Chemical reactions grade 11
Chemical reactions grade 11 A balanced chemical reaction obeys the law of
A balanced chemical reaction obeys the law of Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 2 chemical reactions answer key
Section 2 chemical reactions answer key Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Macromolecules
Macromolecules Mnvii
Mnvii Are all chemical reactions reversible
Are all chemical reactions reversible