UNIT REVIEW Chemical Reactions Coles Notes CHEMICAL REACTIONS









- Slides: 27

UNIT REVIEW: Chemical Reactions (Coles Notes!)

CHEMICAL REACTIONS Chemicals & Their Properties Chemicals & Their Reactions Acids & Bases

Chemicals & Their Properties

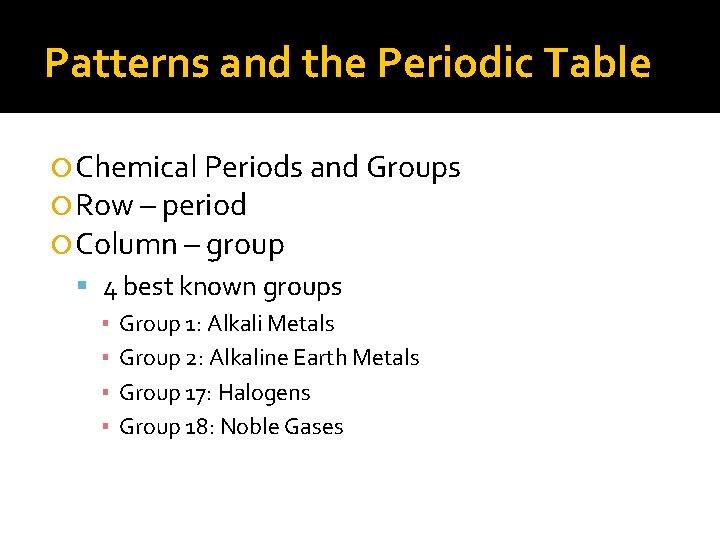
Patterns and the Periodic Table Chemical Periods and Groups Row – period Column – group 4 best known groups ▪ Group 1: Alkali Metals ▪ Group 2: Alkaline Earth Metals ▪ Group 17: Halogens ▪ Group 18: Noble Gases

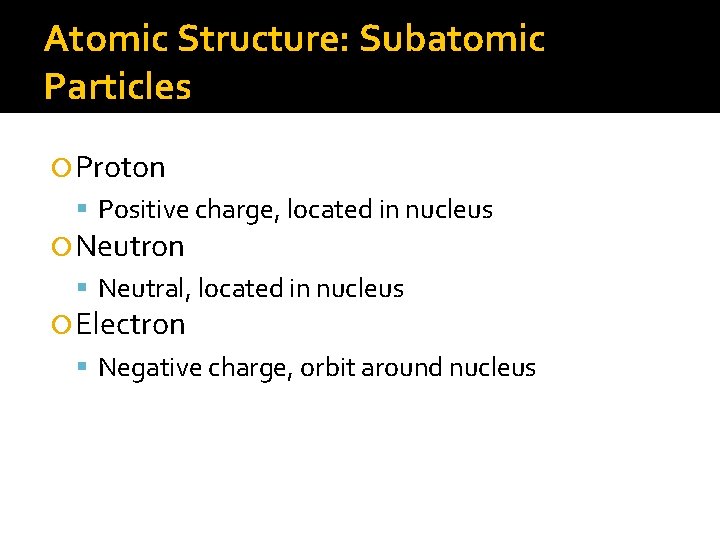
Atomic Structure: Subatomic Particles Proton Positive charge, located in nucleus Neutron Neutral, located in nucleus Electron Negative charge, orbit around nucleus

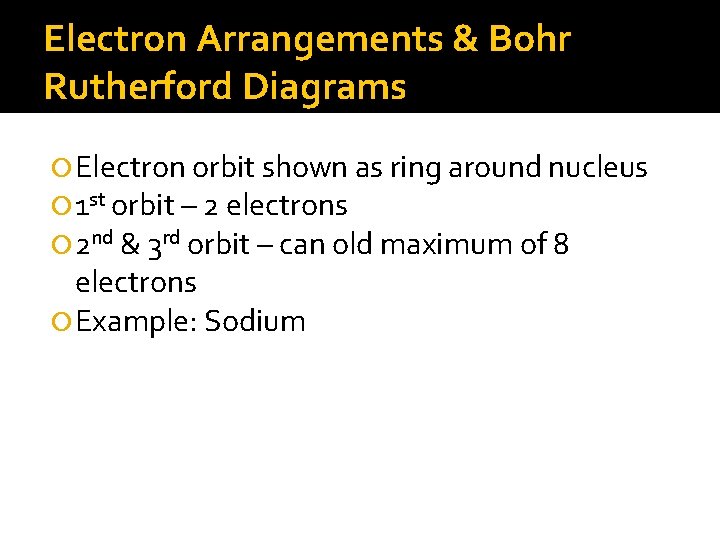
Electron Arrangements & Bohr Rutherford Diagrams Electron orbit shown as ring around nucleus 1 st orbit – 2 electrons 2 nd & 3 rd orbit – can old maximum of 8 electrons Example: Sodium

Atoms & Ions An ion is an atom that has become charged by gaining or losing electrons Positive ion - cation Negative ion - anion

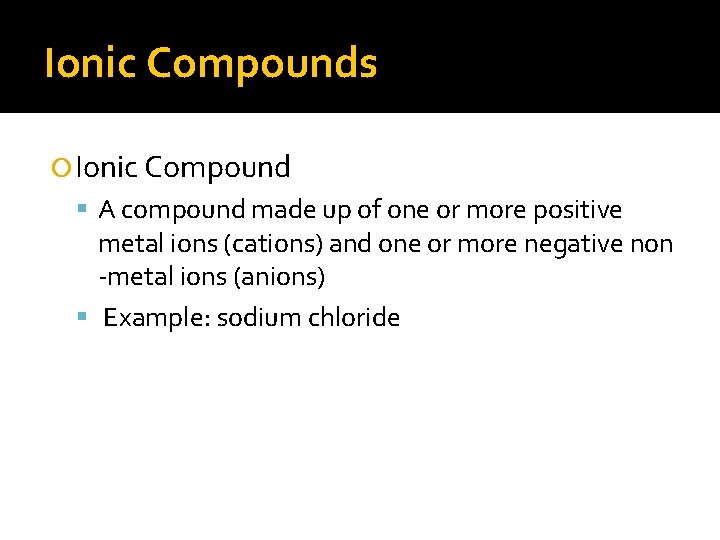
Ionic Compounds Ionic Compound A compound made up of one or more positive metal ions (cations) and one or more negative non -metal ions (anions) Example: sodium chloride

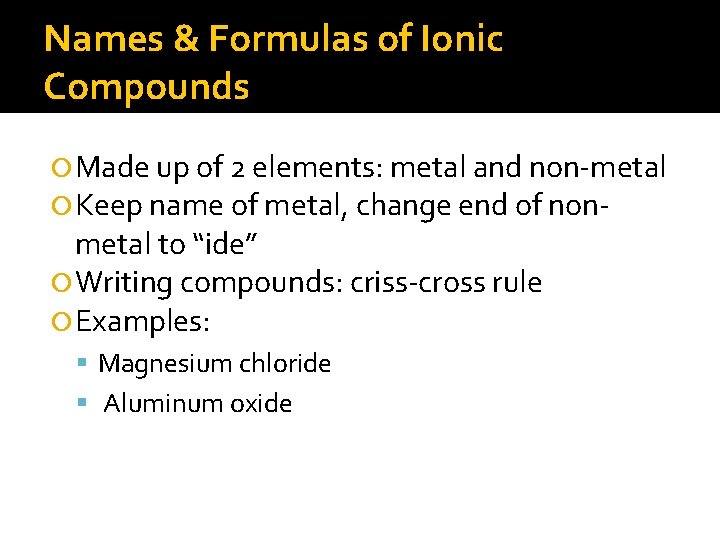
Names & Formulas of Ionic Compounds Made up of 2 elements: metal and non-metal Keep name of metal, change end of non- metal to “ide” Writing compounds: criss-cross rule Examples: Magnesium chloride Aluminum oxide

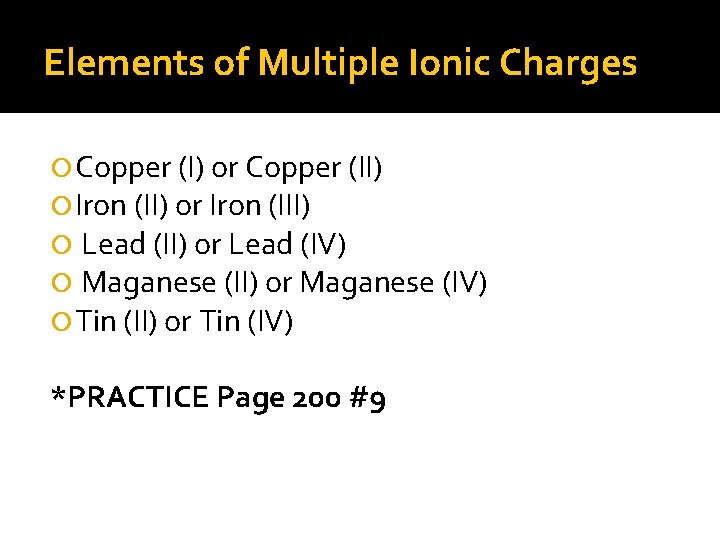
Elements of Multiple Ionic Charges Copper (I) or Copper (II) Iron (II) or Iron (III) Lead (II) or Lead (IV) Maganese (II) or Maganese (IV) Tin (II) or Tin (IV) *PRACTICE Page 200 #9

Polyatomic Ions An ion made up of more than one atom that acts as a single particle Page 202 - Table 1: Formulas and Names of Common Polyatomic Ions Examples: Sodium carbonate Iron (III) nitrate Ca(OH)2 *PRACTICE Page 205 #1, 2

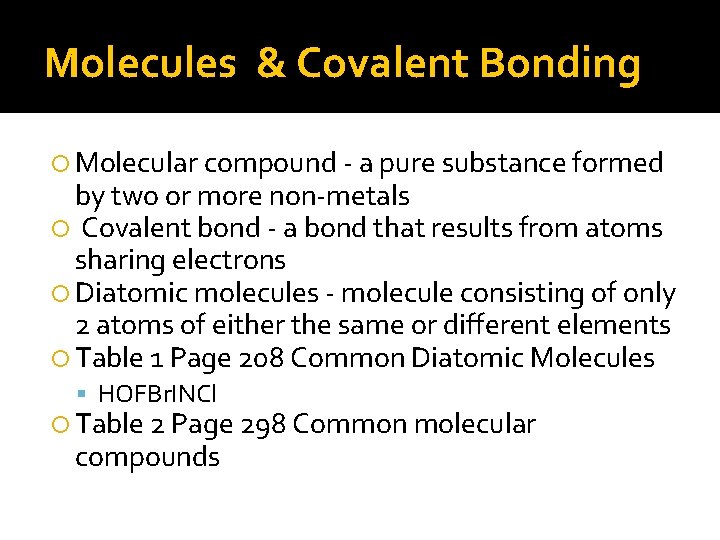
Molecules & Covalent Bonding Molecular compound - a pure substance formed by two or more non-metals Covalent bond - a bond that results from atoms sharing electrons Diatomic molecules - molecule consisting of only 2 atoms of either the same or different elements Table 1 Page 208 Common Diatomic Molecules HOFBr. INCl Table 2 Page 298 Common molecular compounds

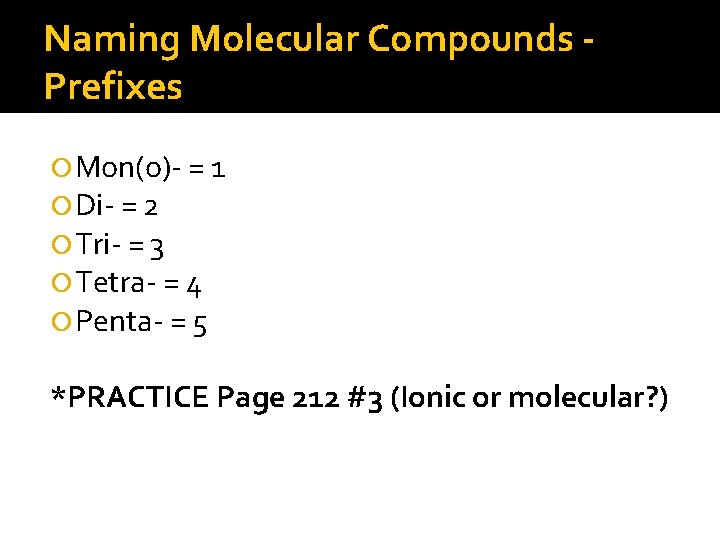
Naming Molecular Compounds Prefixes Mon(o)- = 1 Di- = 2 Tri- = 3 Tetra- = 4 Penta- = 5 *PRACTICE Page 212 #3 (Ionic or molecular? )

CHEMICALS & THEIR REACTIONS

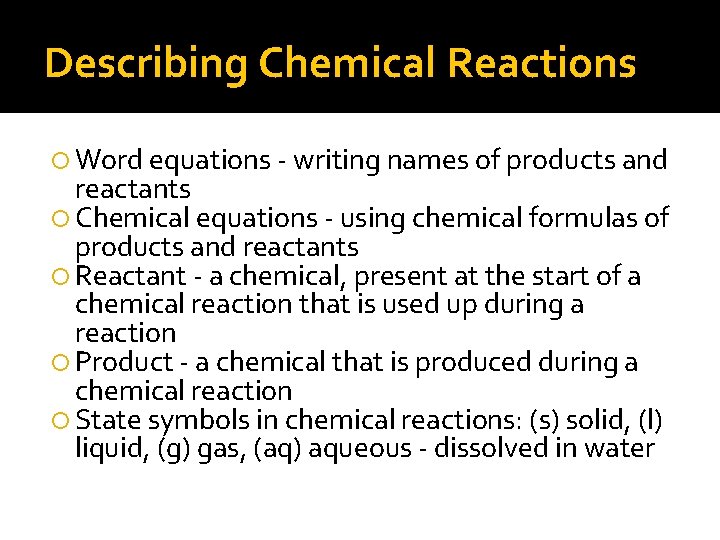
Describing Chemical Reactions Word equations - writing names of products and reactants Chemical equations - using chemical formulas of products and reactants Reactant - a chemical, present at the start of a chemical reaction that is used up during a reaction Product - a chemical that is produced during a chemical reaction State symbols in chemical reactions: (s) solid, (l) liquid, (g) gas, (aq) aqueous - dissolved in water

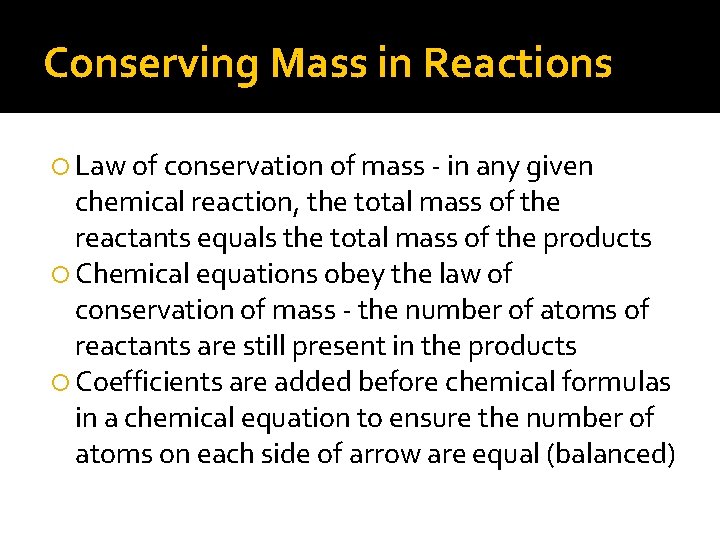
Conserving Mass in Reactions Law of conservation of mass - in any given chemical reaction, the total mass of the reactants equals the total mass of the products Chemical equations obey the law of conservation of mass - the number of atoms of reactants are still present in the products Coefficients are added before chemical formulas in a chemical equation to ensure the number of atoms on each side of arrow are equal (balanced)

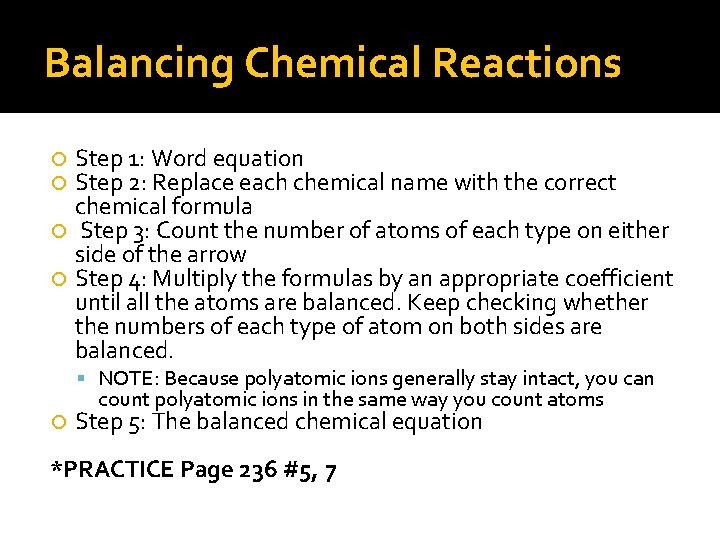
Balancing Chemical Reactions Step 1: Word equation Step 2: Replace each chemical name with the correct chemical formula Step 3: Count the number of atoms of each type on either side of the arrow Step 4: Multiply the formulas by an appropriate coefficient until all the atoms are balanced. Keep checking whether the numbers of each type of atom on both sides are balanced. NOTE: Because polyatomic ions generally stay intact, you can count polyatomic ions in the same way you count atoms Step 5: The balanced chemical equation *PRACTICE Page 236 #5, 7

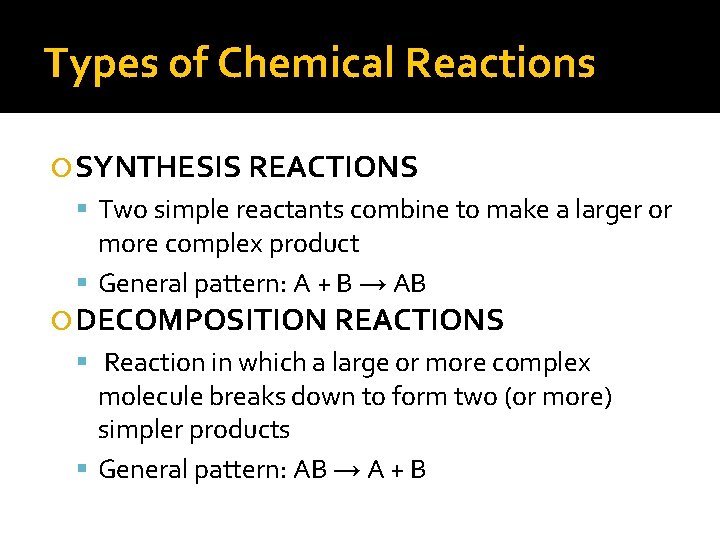
Types of Chemical Reactions SYNTHESIS REACTIONS Two simple reactants combine to make a larger or more complex product General pattern: A + B → AB DECOMPOSITION REACTIONS Reaction in which a large or more complex molecule breaks down to form two (or more) simpler products General pattern: AB → A + B

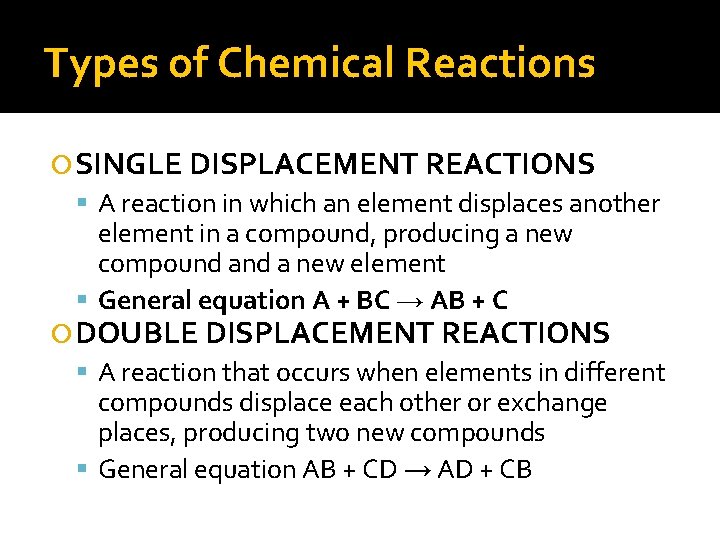
Types of Chemical Reactions SINGLE DISPLACEMENT REACTIONS A reaction in which an element displaces another element in a compound, producing a new compound a new element General equation A + BC → AB + C DOUBLE DISPLACEMENT REACTIONS A reaction that occurs when elements in different compounds displace each other or exchange places, producing two new compounds General equation AB + CD → AD + CB

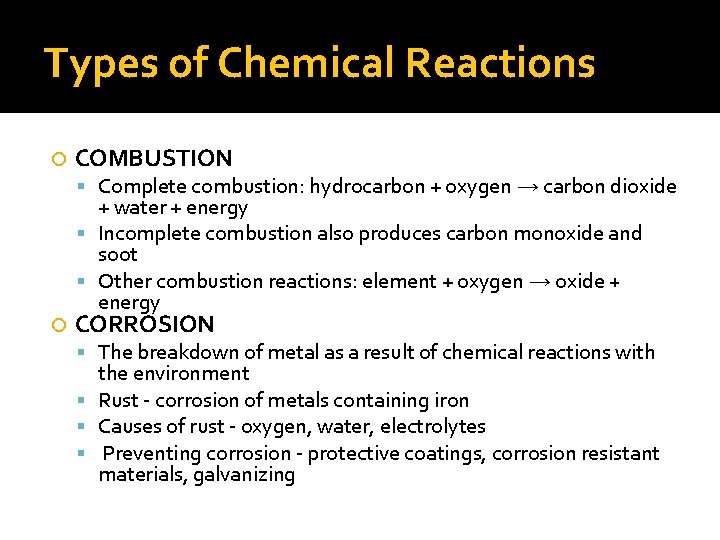
Types of Chemical Reactions COMBUSTION Complete combustion: hydrocarbon + oxygen → carbon dioxide + water + energy Incomplete combustion also produces carbon monoxide and soot Other combustion reactions: element + oxygen → oxide + energy CORROSION The breakdown of metal as a result of chemical reactions with the environment Rust - corrosion of metals containing iron Causes of rust - oxygen, water, electrolytes Preventing corrosion - protective coatings, corrosion resistant materials, galvanizing

Types of Chemical Reactions *PRACTICE Page 258 # 7

Acids & Bases

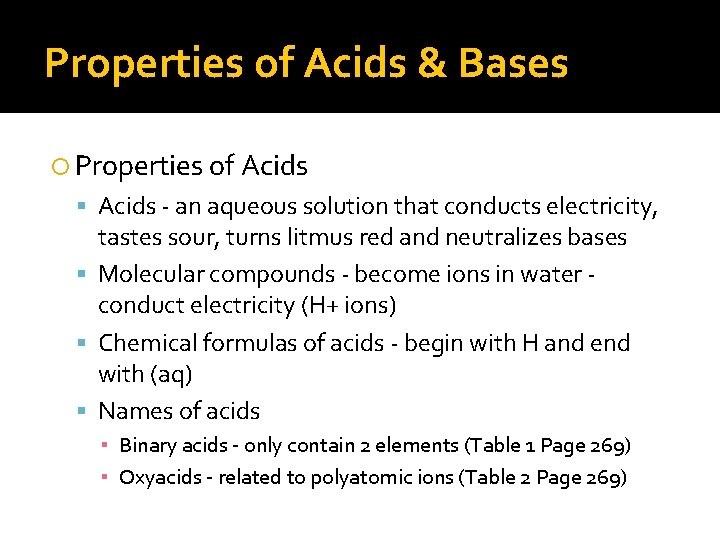
Properties of Acids & Bases Properties of Acids - an aqueous solution that conducts electricity, tastes sour, turns litmus red and neutralizes bases Molecular compounds - become ions in water conduct electricity (H+ ions) Chemical formulas of acids - begin with H and end with (aq) Names of acids ▪ Binary acids - only contain 2 elements (Table 1 Page 269) ▪ Oxyacids - related to polyatomic ions (Table 2 Page 269)

Properties of Acids & Bases Properties of Bases - aqueous solution that conducts electricity, tastes bitter, and turns litmus blue Electrolytes - form ions when dissolved in water Names and Formulas of Bases (Table 4 Page 271) Acid-base indicator A substance that changes color depending on whether it is in an acid or a base ▪ Table 3 Page 270 - Bromo blue, Phenophthalein, Litmus

The p. H Scale p. H is a measure of how acidic or basic a solution is p. H scale is a numerical scale of all the possible numerical values of p. H from 0 -14 used to compare the acidity of solutions Neutral - neither acidic or basic, with p. H of 7 Solution with p. H less than 7 considered acidic Solution with p. H greater than 7 considered basic

Neutralization Reactions Occur when an acid and a base react to form products with p. H closer to 7 than either of the reactants Products are an ionic compound (sometimes called a “salt”) and water Acid + Base → Water + Ionic Compound Applications: Chemical Spills Antacids

Acid Precipitation Acid precipitation - term used to describe any precipitation (rain, snow, fog) that has become acidic from reacting with compounds in atmosphere Pollutants - sulfur oxides and nitrogen oxides combine with water in atmosphere Acid rain has p. H less than 5. 6 (normal rain) Source of pollutants - Combustion of fossil fuels Environmental Impact of Acid Precipitation Aquatic Ecosystems, Soils, Forests Economic Impact Damage to structures, buildings, and monuments
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Unit 6 review questions
Unit 6 review questions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Unit 11 chemical reactions
Unit 11 chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Coles livingston
Coles livingston Coles paradox
Coles paradox Coles y nenas
Coles y nenas Tandaco dry yeast woolworths
Tandaco dry yeast woolworths Springfield coles
Springfield coles Lizzie coles-kemp
Lizzie coles-kemp Coles supplier portal
Coles supplier portal Coles livingston
Coles livingston Henry coles iata
Henry coles iata Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds What is the chemical formula for tetranitrogen heptoxide?
What is the chemical formula for tetranitrogen heptoxide? 5 examples of redox reaction
5 examples of redox reaction Algebra 2 unit 1 test
Algebra 2 unit 1 test Chapter 19 review oxidation-reduction reactions
Chapter 19 review oxidation-reduction reactions Chapter 19 review oxidation reduction reactions answers
Chapter 19 review oxidation reduction reactions answers Facteur g
Facteur g