Chemical Reactions Notes 1 Chemical Reactions A Evidence














- Slides: 35

Chemical Reactions Notes

1. Chemical Reactions

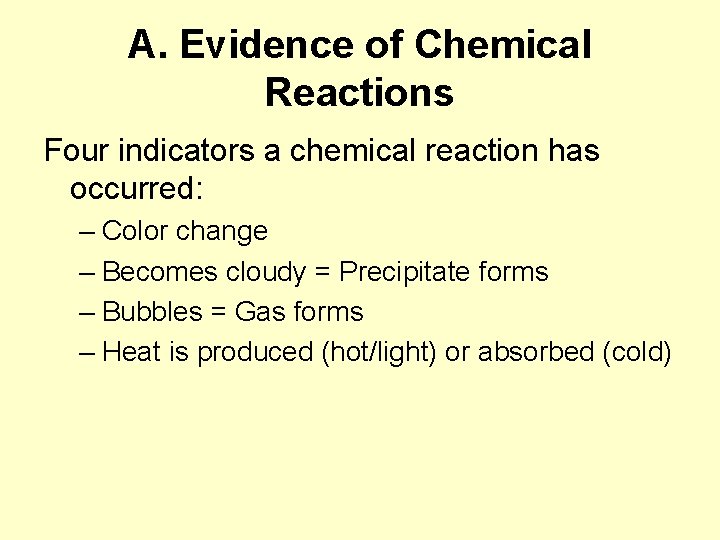
A. Evidence of Chemical Reactions Four indicators a chemical reaction has occurred: – Color change – Becomes cloudy = Precipitate forms – Bubbles = Gas forms – Heat is produced (hot/light) or absorbed (cold)

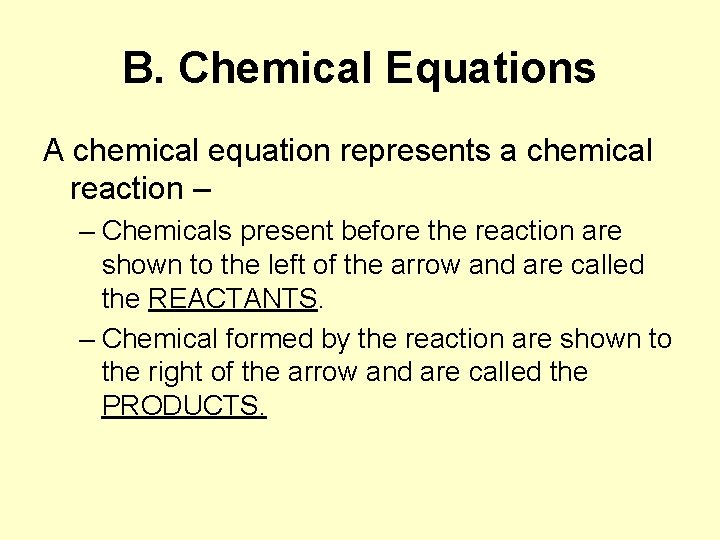
B. Chemical Equations A chemical equation represents a chemical reaction – – Chemicals present before the reaction are shown to the left of the arrow and are called the REACTANTS. – Chemical formed by the reaction are shown to the right of the arrow and are called the PRODUCTS.

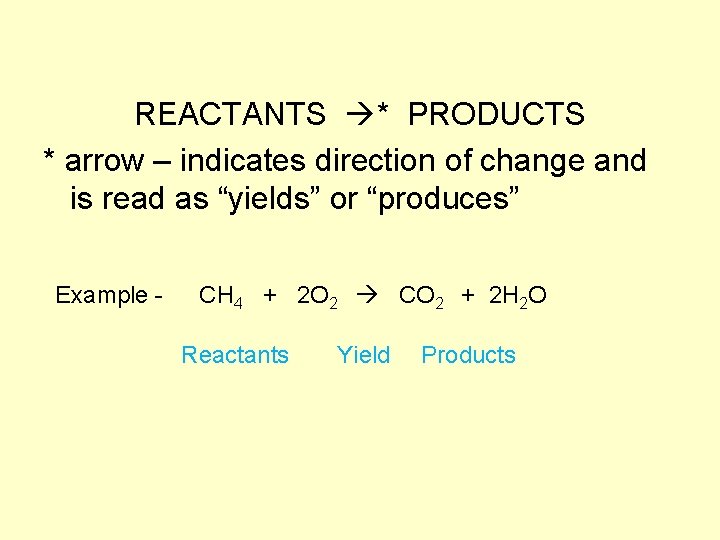
REACTANTS * PRODUCTS * arrow – indicates direction of change and is read as “yields” or “produces” Example - CH 4 + 2 O 2 CO 2 + 2 H 2 O Reactants Yield Products

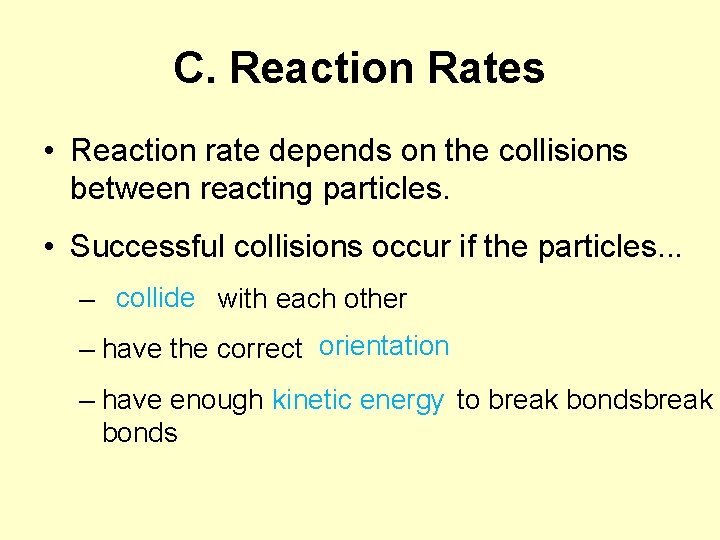
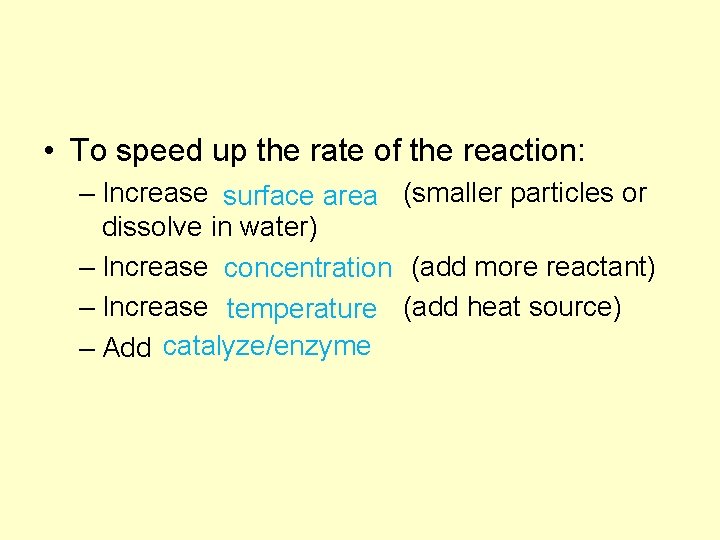
C. Reaction Rates • Reaction rate depends on the collisions between reacting particles. • Successful collisions occur if the particles. . . collide – with each other – have the correct orientation kinetic energy – have enough to break bonds

• To speed up the rate of the reaction: – Increase (smaller particles or surface area dissolve in water) – Increase (add more reactant) concentration – Increase (add heat source) temperature – Add catalyze/enzyme

D. Heat in Reactions • Exothermic reactions release heat – Heat is a product – Feels hot • Endothermic reactions absorb heat – Heat is a reactant – Feels cold

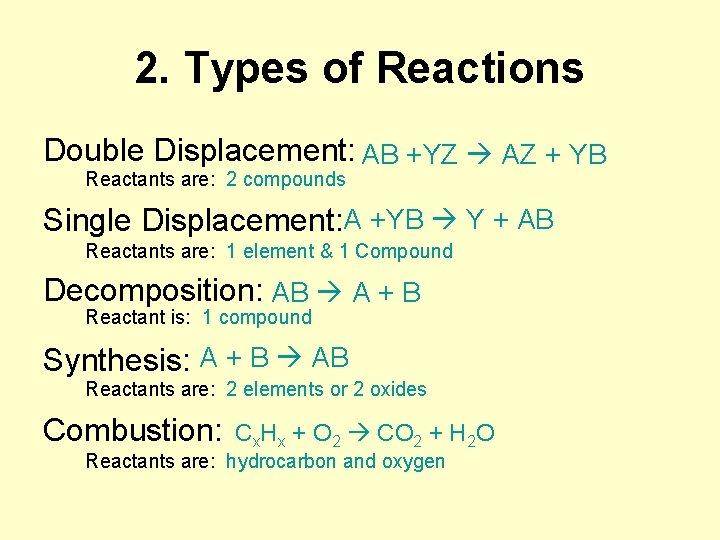
2. Types of Reactions Double Displacement: AB +YZ AZ + YB Reactants are: 2 compounds Single Displacement: A +YB Y + AB Reactants are: 1 element & 1 Compound Decomposition: AB A + B Reactant is: 1 compound Synthesis: A + B AB Reactants are: 2 elements or 2 oxides Combustion: Cx. Hx + O 2 CO 2 + H 2 O Reactants are: hydrocarbon and oxygen

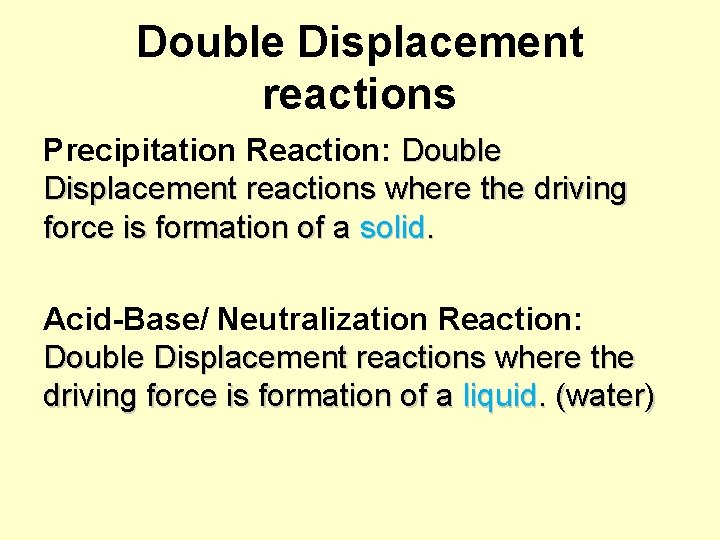
Double Displacement reactions Precipitation Reaction: Double Displacement reactions where the driving force is formation of a solid. Acid-Base/ Neutralization Reaction: Double Displacement reactions where the driving force is formation of a liquid. (water)

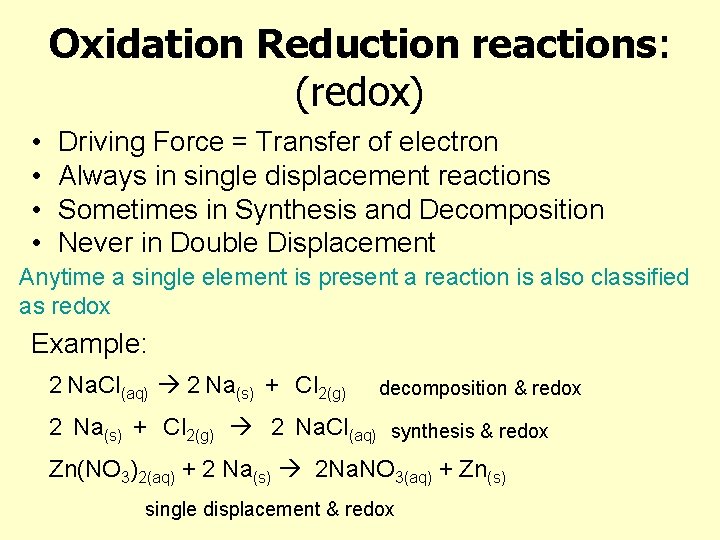
Oxidation Reduction reactions: (redox) • • Driving Force = Transfer of electron Always in single displacement reactions Sometimes in Synthesis and Decomposition Never in Double Displacement Anytime a single element is present a reaction is also classified as redox Example: 2 Na. Cl(aq) 2 Na(s) + Cl 2(g) decomposition & redox 2 Na(s) + Cl 2(g) 2 Na. Cl(aq) synthesis & redox Zn(NO 3)2(aq) + 2 Na(s) 2 Na. NO 3(aq) + Zn(s) single displacement & redox

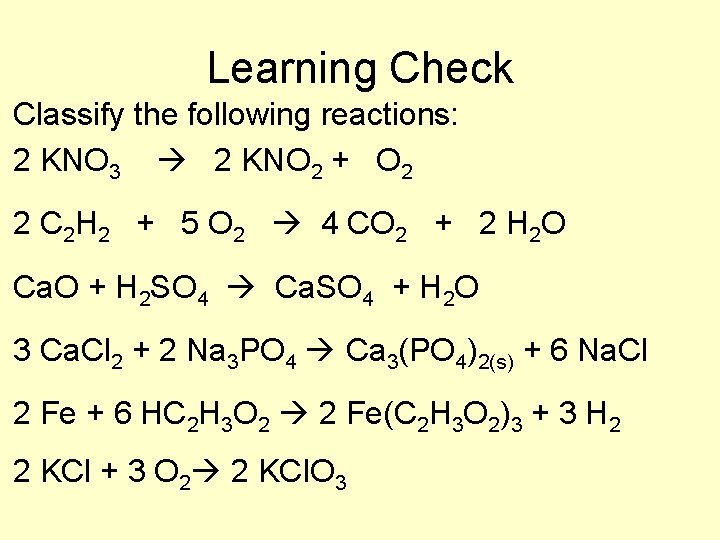
Learning Check Classify the following reactions: 2 KNO 3 2 KNO 2 + O 2 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O Ca. O + H 2 SO 4 Ca. SO 4 + H 2 O 3 Ca. Cl 2 + 2 Na 3 PO 4 Ca 3(PO 4)2(s) + 6 Na. Cl 2 Fe + 6 HC 2 H 3 O 2 2 Fe(C 2 H 3 O 2)3 + 3 H 2 2 KCl + 3 O 2 2 KCl. O 3

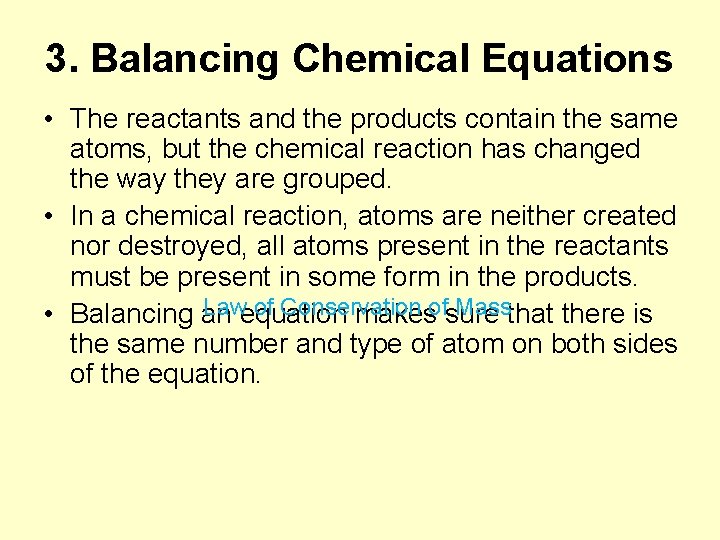
3. Balancing Chemical Equations • The reactants and the products contain the same atoms, but the chemical reaction has changed the way they are grouped. • In a chemical reaction, atoms are neither created nor destroyed, all atoms present in the reactants must be present in some form in the products. Law of Conservation of Mass • Balancing an equation makes sure that there is the same number and type of atom on both sides of the equation.

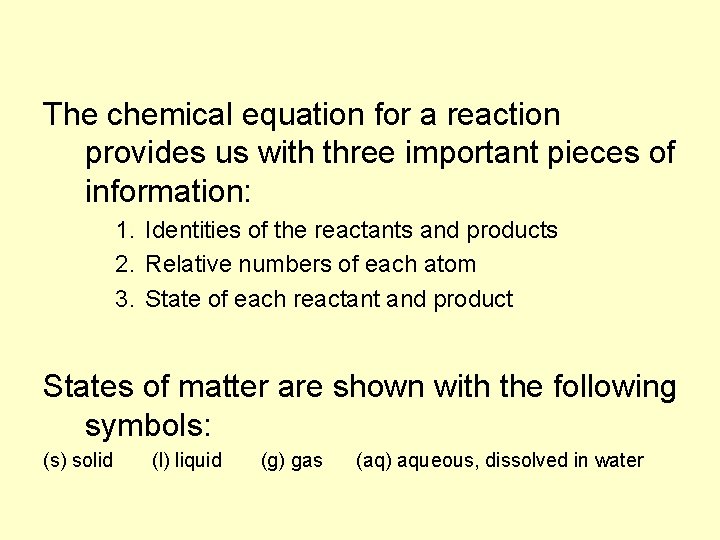
The chemical equation for a reaction provides us with three important pieces of information: 1. Identities of the reactants and products 2. Relative numbers of each atom 3. State of each reactant and product States of matter are shown with the following symbols: (s) solid (l) liquid (g) gas (aq) aqueous, dissolved in water

HINTS : – Don’t forget the diatomics – Br. INCl. HOF if they appear by themselves they must be written as: Br 2, I 2, N 2, Cl 2, H 2, O 2, F 2, – Balance oxygen and hydrogen last. – If you have a split element, make the odd number even – If you have a polyatomic ion that does not change, balance it as the ion

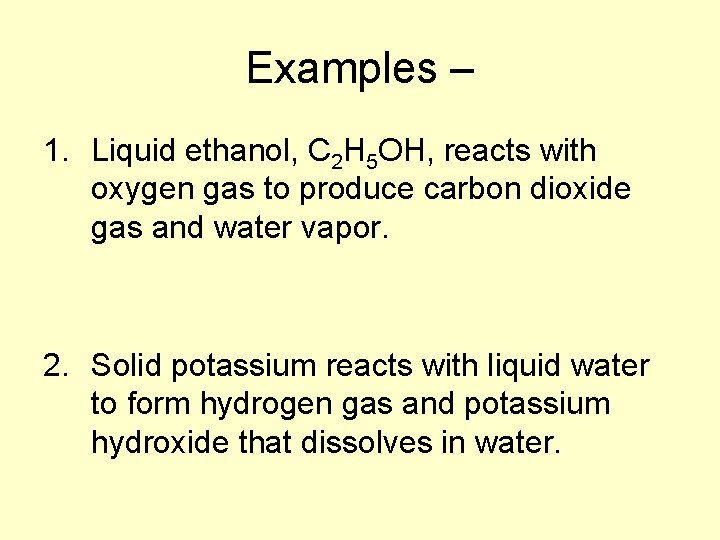
Examples – 1. Liquid ethanol, C 2 H 5 OH, reacts with oxygen gas to produce carbon dioxide gas and water vapor. 2. Solid potassium reacts with liquid water to form hydrogen gas and potassium hydroxide that dissolves in water.

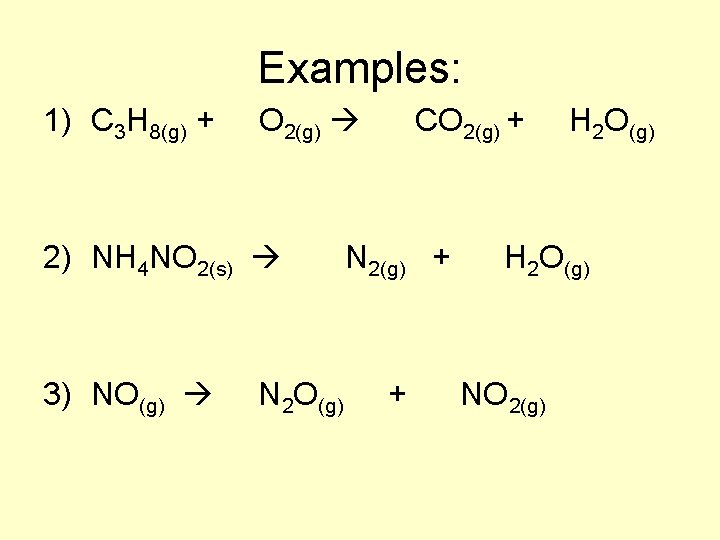
Examples: 1) C 3 H 8(g) + O 2(g) CO 2(g) + H 2 O(g) 2) NH 4 NO 2(s) N 2(g) + H 2 O(g) 3) NO(g) N 2 O(g) + NO 2(g)

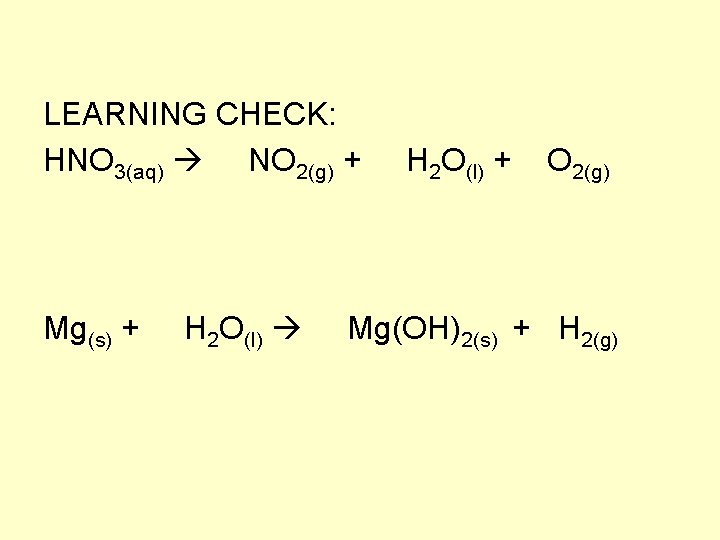
LEARNING CHECK: HNO 3(aq) NO 2(g) + H 2 O(l) + O 2(g) Mg(s) + H 2 O(l) Mg(OH)2(s) + H 2(g)

4. States of Substances 1. Soluble solid – readily dissolves in water State is aqueous - (aq) 2. Insoluble and slightly soluble solid – a solid where such a tiny amount dissolves in water that it is undetectable to the naked eye State is solid - (s) 3. Solubility is temperature dependent States of reactants can be manipulated

Solubility Rules (on snoopy sheet) Ex. Predict whether the following substances are soluble or insoluble. Ag. NO 3 (aq) Al(OH)3 (s) Zn. SO 4 (aq) Li 2 CO 3 (aq) Cu 3 PO 4 (s) Solubility Rules Mainly water soluble (aq) All nitrates are soluble. All acetates are soluble. All chlorides are soluble except Ag. Cl, Hg 2 Cl 2, and Pb. Cl 2 All bromides are soluble except Ag. Br, Hg 2 Br 2, Pb. Br 2, and Hg. Br 2 All iodides are soluble except Ag. I, Hg 2 I 2, Pb. I 2, and Hg. I 2 All sulfates are soluble except Ca. SO 4, Sr. SO 4, Ba. SO 4, Pb. SO 4, Hg 2 SO 4, and Ag 2 SO 4 Mainly water insoluble (s) All sulfides are insoluble except those of 1 A and 2 A elements and (NH 4)2 S All carbonates are insoluble except those of 1 A and (NH 4)2 CO 3 All phosphates are insoluble except those of 1 A and (NH 4)3 PO 4 All hydroxides are insoluble except those of 1 A, Ba(OH)2, Sr(OH)2 and Ca(OH)2

Learning Check • Try these: Determine if the following are AQUEOUS or SOLID 1. lead (II) nitrate 2. potassium sulfide 3. barium hydroxide 4. ammonium carbonate • Turn to your neighbor and compare answers

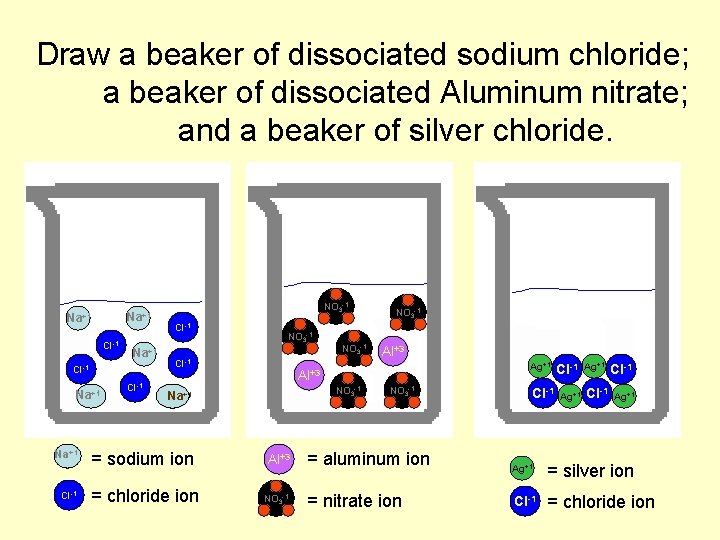
When a soluble ionic salt dissolves in water the ions separate and a hydration shell is formed around each ion (Dissociation) Ex. Ba(NO 3)2 in water – Ba(NO 3)2 (aq) Ba+2(aq) + 2 NO 3 -1(aq) Aluminum carbonate Al 2(CO 3)3 (s) or No RXN

Draw a beaker of dissociated sodium chloride; a beaker of dissociated Aluminum nitrate; and a beaker of silver chloride. Na+1 Cl-1 Na+1 Cl-1 NO 3 -1 Cl-1 = chloride ion Al+3 Ag+1 Al+3 NO 3 -1 Na+1 = sodium ion NO 3 -1 Al+3 NO 3 -1 Cl-1 Ag+1 = aluminum ion Ag+1 = silver ion = nitrate ion Cl-1 = chloride ion

Special Rules 1. Acids are aqueous 2. Most metal oxides are solids 3. Most non-metal oxides are gases

Learning Check • Determine the state: 1. lead(II) phosphate 2. magnesium oxide 3. nickel 4. dinitrogen monoxide 5. chlorine 6. sulfuric acid 7. sodium sulfide

5. Predicting Whether a Reaction Will Occur A. Four Driving Forces 1. 2. 3. 4. Formation of a solid (precipitate) Formation of water Transfer of electrons Formation of a gas B. If a driving force occurs the reaction will take place.

6. Predicting Products Steps for Predicting Products 1. Formulas for reactants 2. Type of Reaction 3. Predict Product formulas (using ions) 4. States (using solubility rules) 5. Balance Equation or NO REACTION (if missing driving force)

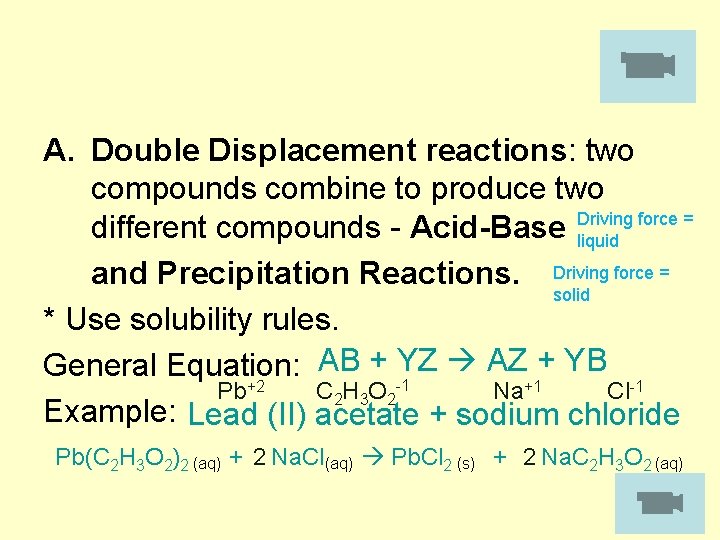
A. Double Displacement reactions: two compounds combine to produce two different compounds - Acid-Base Driving force = liquid and Precipitation Reactions. Driving force = solid * Use solubility rules. AB + YZ AZ + YB General Equation: C 2 H 3 O 2 -1 Pb+2 Na+1 Cl-1 Example: Lead (II) acetate + sodium chloride Pb(C 2 H 3 O 2)2 (aq) + 2 Na. Cl(aq) Pb. Cl 2 (s) + 2 Na. C 2 H 3 O 2 (aq)

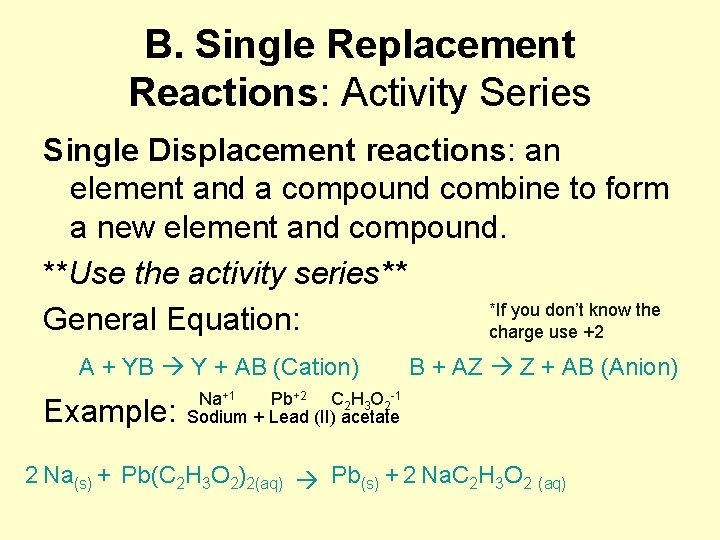
B. Single Replacement Reactions: Activity Series Single Displacement reactions: an element and a compound combine to form a new element and compound. **Use the activity series** *If you don’t know the General Equation: charge use +2 A + YB Y + AB (Cation) B + AZ Z + AB (Anion) Na Pb CHO Example: Sodium + Lead (II) acetate 2 Na + Pb(C H O ) Pb + 2 Na. C H O +1 (s) 2 3 2 2(aq) +2 2 3 (s) 2 -1 2 3 2 (aq)

Activity Series • Active metal elements can replace less active metals, active nonmetal elements can replace less active nonmetals. • Use the Activity series (snoopy sheet) to determine whether or not the reaction will occur. • Driving force is the transfer of electrons.

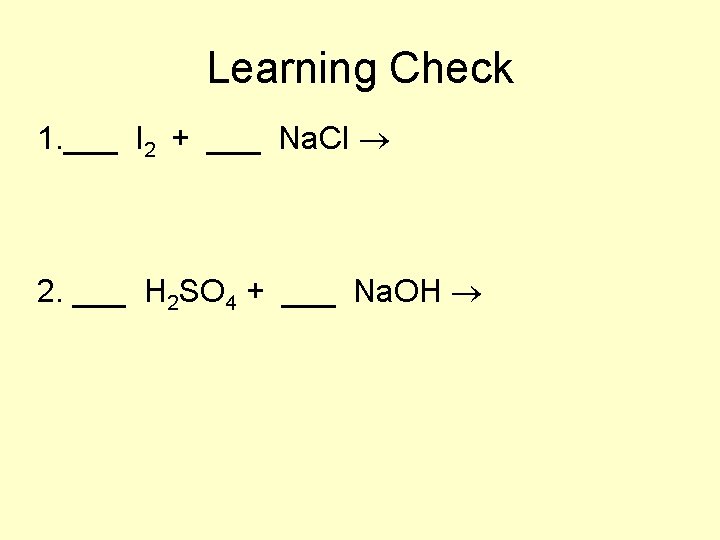
Learning Check 1. ___ I 2 + ___ Na. Cl 2. ___ H 2 SO 4 + ___ Na. OH

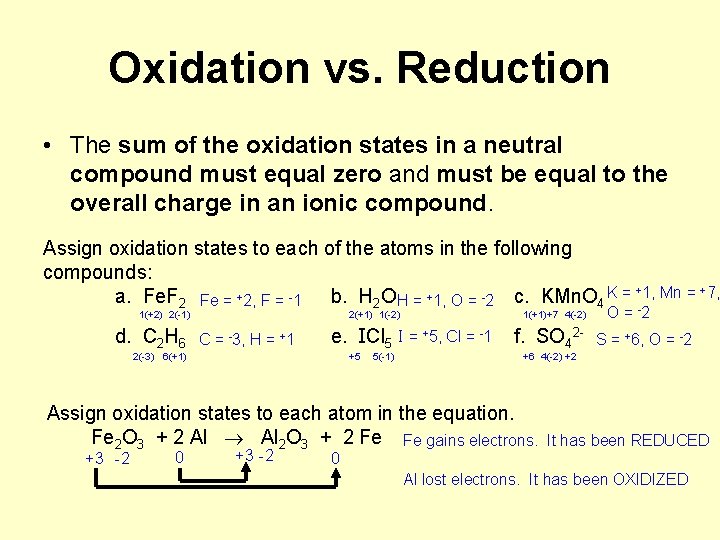
7. Rules for Oxidation Numbers 1. The oxidation number of any uncombined element is 0. 2. The oxidation number of a monatomic ion equals the charge on the ion. 3. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in a compound is always -1. 5. Oxygen has an oxidation number of -2 unless it is combined with F (when it is +2), or it is in a peroxide (such as H 2 O 2 or Na 2 O 2), when it is -1. 6. The oxidation state of hydrogen in most of its compounds is +1 unless it is combined with a metal, in which case it is -1.

Oxidation vs. Reduction • The sum of the oxidation states in a neutral compound must equal zero and must be equal to the overall charge in an ionic compound. Assign oxidation states to each of the atoms in the following compounds: K = +1, Mn = +7, -2 a. Fe. F 2 Fe = +2, F = -1 b. H 2 O H = +1, O = c. KMn. O 4 1(+2) 2(-1) d. C 2 H 6 2(+1) 1(-2) C = -3, H = +1 1(+1)+7 4(-2) -1 2 e. ICl 5 I = +5, Cl = f. SO 4 2(-3) 6(+1) +5 5(-1) O = 2 S = +6, O = -2 +6 4(-2) +2 Assign oxidation states to each atom in the equation. Fe 2 O 3 + 2 Al 2 O 3 + 2 Fe Fe gains electrons. It has been REDUCED +3 -2 0 Al lost electrons. It has been OXIDIZED

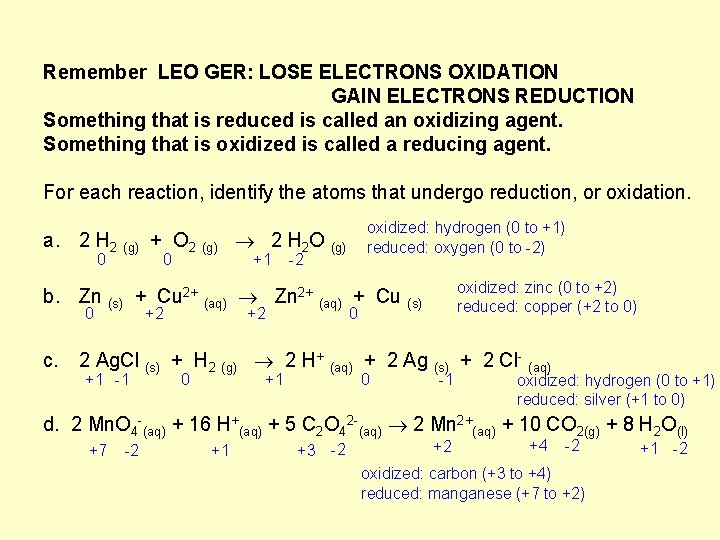
Remember LEO GER: LOSE ELECTRONS OXIDATION GAIN ELECTRONS REDUCTION Something that is reduced is called an oxidizing agent. Something that is oxidized is called a reducing agent. For each reaction, identify the atoms that undergo reduction, or oxidation. oxidized: hydrogen (0 to +1) reduced: oxygen (0 to -2) a. 2 H 2 (g) + O 2 (g) 2 H 2 O (g) 0 0 +1 -2 oxidized: zinc (0 to +2) reduced: copper (+2 to 0) b. Zn (s) + Cu 2+ (aq) Zn 2+ (aq) + Cu (s) 0 +2 +2 0 c. 2 Ag. Cl (s) + H 2 (g) 2 H+ (aq) + 2 Ag (s) + 2 Cl- (aq) +1 -1 0 +1 0 -1 oxidized: hydrogen (0 to +1) reduced: silver (+1 to 0) d. 2 Mn. O 4 -(aq) + 16 H+(aq) + 5 C 2 O 42 -(aq) 2 Mn 2+(aq) + 10 CO 2(g) + 8 H 2 O(l) +7 -2 +1 +3 -2 +2 +4 -2 oxidized: carbon (+3 to +4) reduced: manganese (+7 to +2) +1 -2

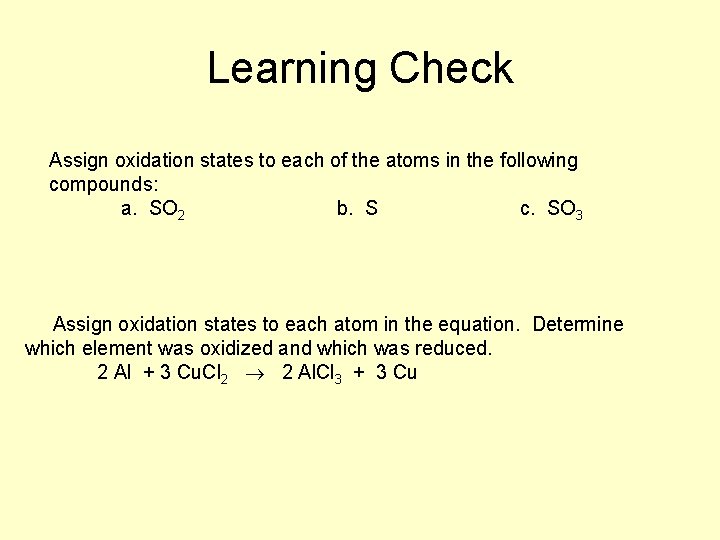
Learning Check Assign oxidation states to each of the atoms in the following compounds: a. SO 2 b. S c. SO 3 Assign oxidation states to each atom in the equation. Determine which element was oxidized and which was reduced. 2 Al + 3 Cu. Cl 2 2 Al. Cl 3 + 3 Cu
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal 5 examples of redox reaction
5 examples of redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Lesson 90 solid evidence precipitation reactions answer key
Lesson 90 solid evidence precipitation reactions answer key Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Are fibers class evidence ?
Are fibers class evidence ? Class vs individual evidence
Class vs individual evidence Individual evidence can have probative value
Individual evidence can have probative value Class vs individual evidence
Class vs individual evidence The absence of evidence is not the evidence of absence
The absence of evidence is not the evidence of absence Evidence for evolution doodle notes
Evidence for evolution doodle notes Weave patterns of fibers forensics
Weave patterns of fibers forensics Facteur g
Facteur g Stoichiometry mole island diagram
Stoichiometry mole island diagram Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Different types of redox reactions
Different types of redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry Reaction type
Reaction type Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products synthesis
Predicting products synthesis Section 3 predicting the products of chemical reactions
Section 3 predicting the products of chemical reactions