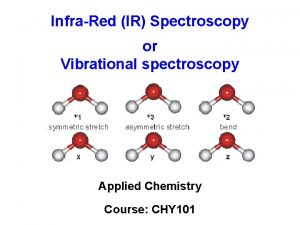
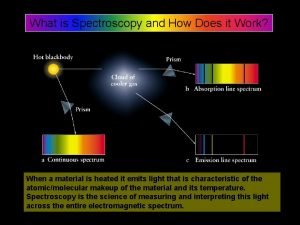
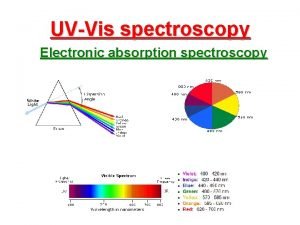
Spectroscopy Infrared Spectroscopy Introduction n Spectroscopy is an











- Slides: 141

Spectroscopy Infrared Spectroscopy

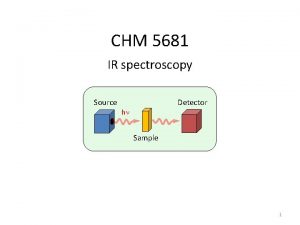
Introduction n Spectroscopy is an analytical technique which helps determine structure n It destroys little or no sample n The amount of light absorbed by the sample is measured as wavelength is varied 2020/11/22 2

Electromagnetic Spectrum n Examples: X rays, microwaves, radio waves, visible light, IR, and UV n Frequency and wavelength are inversely proportional n c = ln, where c is the speed of light n Energy per photon = hn, where h is Planck’s constant 2020/11/22 3

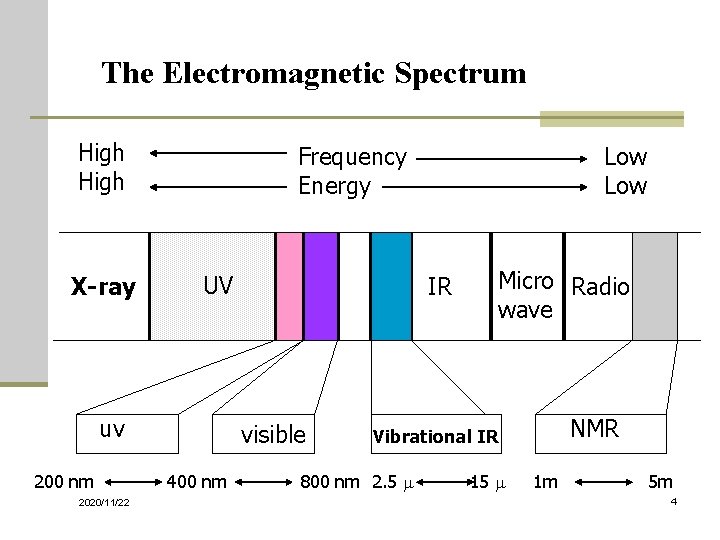
The Electromagnetic Spectrum High X-ray Frequency Energy UV uv 200 nm 2020/11/22 IR visible 400 nm Low Micro Radio wave NMR Vibrational IR 800 nm 2. 5 m 1 m 5 m 4

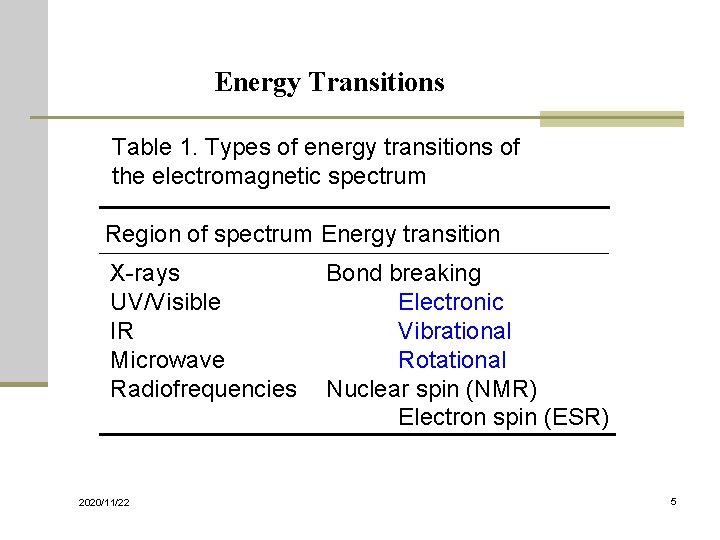
Energy Transitions Table 1. Types of energy transitions of the electromagnetic spectrum Region of spectrum Energy transition X-rays UV/Visible IR Microwave Radiofrequencies 2020/11/22 Bond breaking Electronic Vibrational Rotational Nuclear spin (NMR) Electron spin (ESR) 5

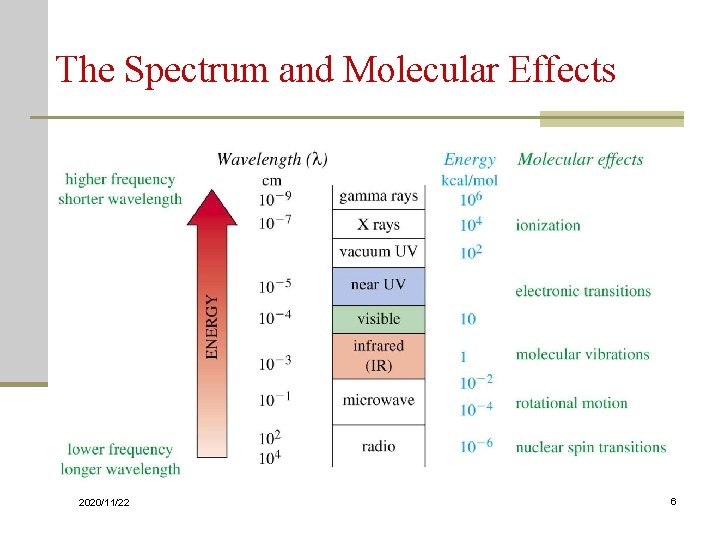
The Spectrum and Molecular Effects => 2020/11/22 6

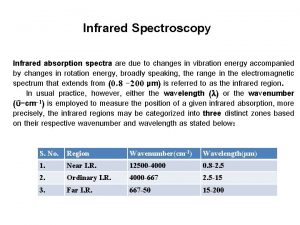
The IR Region n Just below red in the visible region n Wavelengths usually 2. 5 -25 mm n More common units are wavenumbers, or cm-1, the reciprocal of the wavelength in centimeters (4000 - 400 cm-1) n Wavenumbers are proportional to frequency and energy 2020/11/22 7

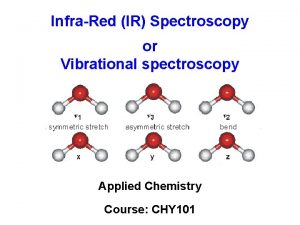
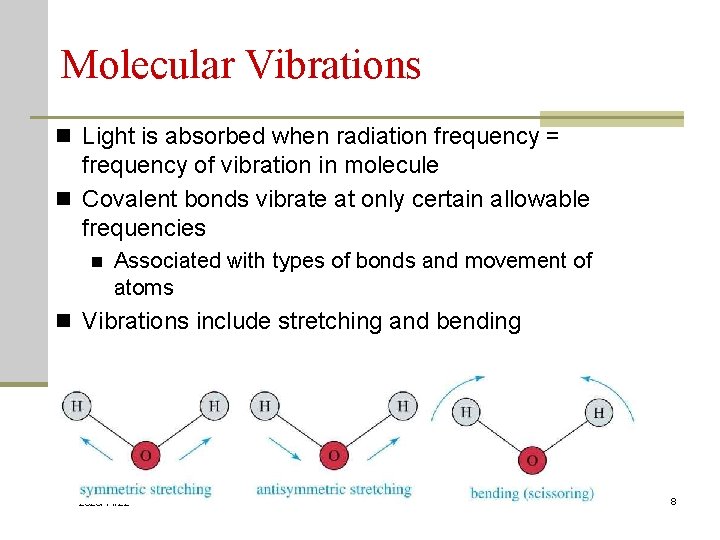
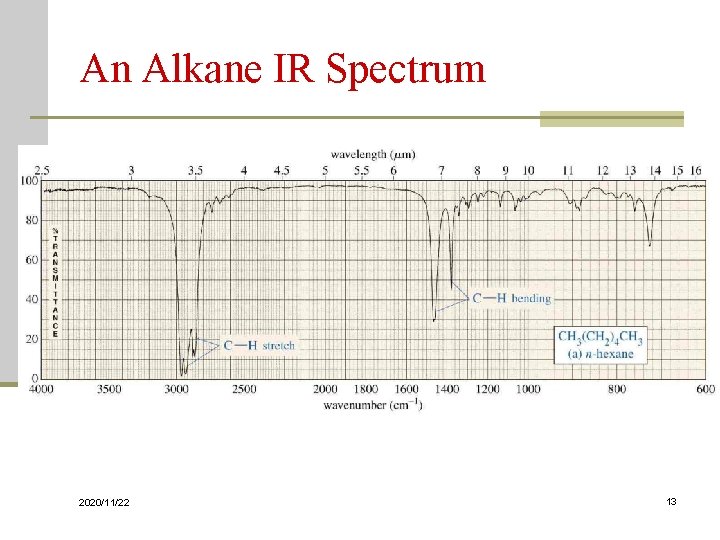
Molecular Vibrations n Light is absorbed when radiation frequency = frequency of vibration in molecule n Covalent bonds vibrate at only certain allowable frequencies n Associated with types of bonds and movement of atoms n Vibrations include stretching and bending 2020/11/22 8

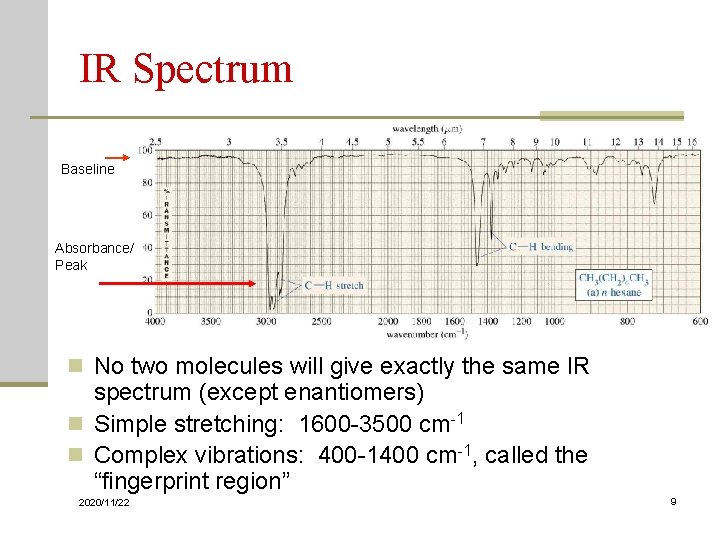
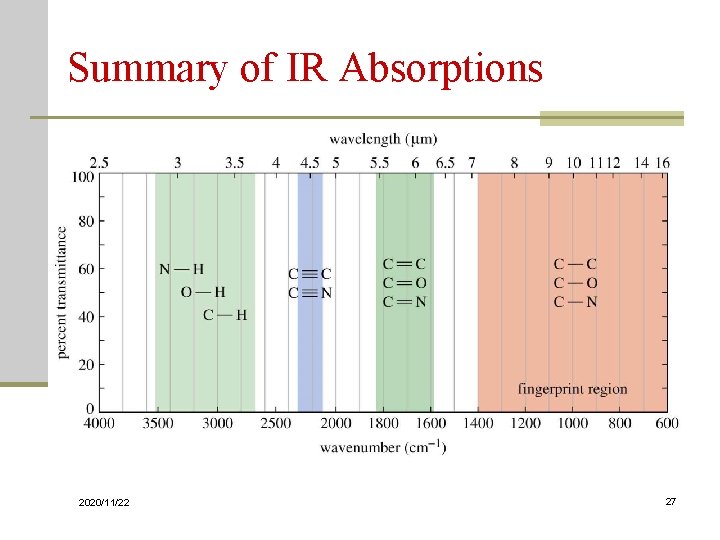
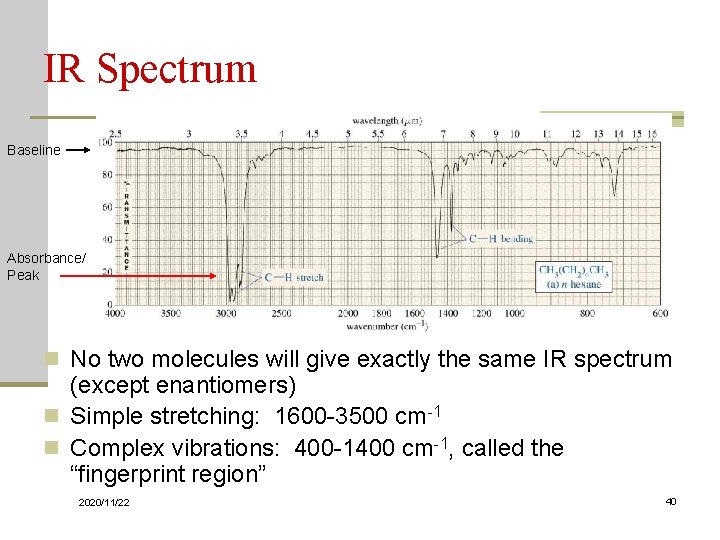
IR Spectrum Baseline Absorbance/ Peak n No two molecules will give exactly the same IR spectrum (except enantiomers) n Simple stretching: 1600 -3500 cm-1 n Complex vibrations: 400 -1400 cm-1, called the “fingerprint region” 2020/11/22 9

Interpretation n Looking for presence/absence of functional groups n Correlation tables n A polar bond is usually IR-active n A nonpolar bond in a symmetrical molecule will absorb weakly or not at all 2020/11/22 10

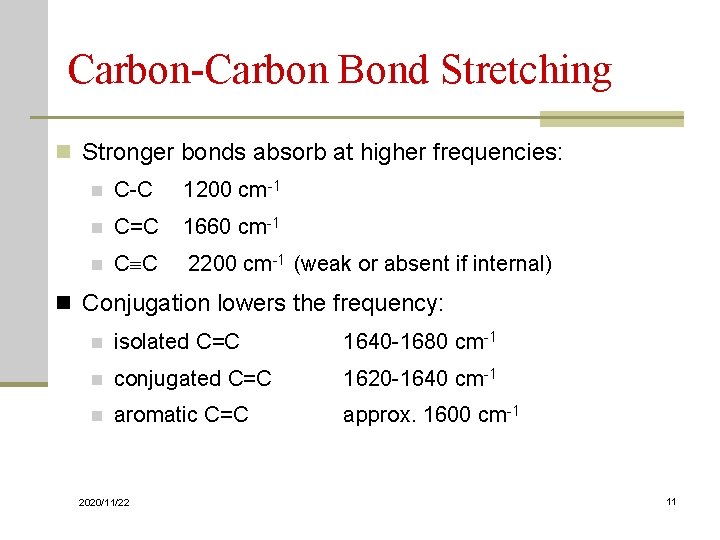
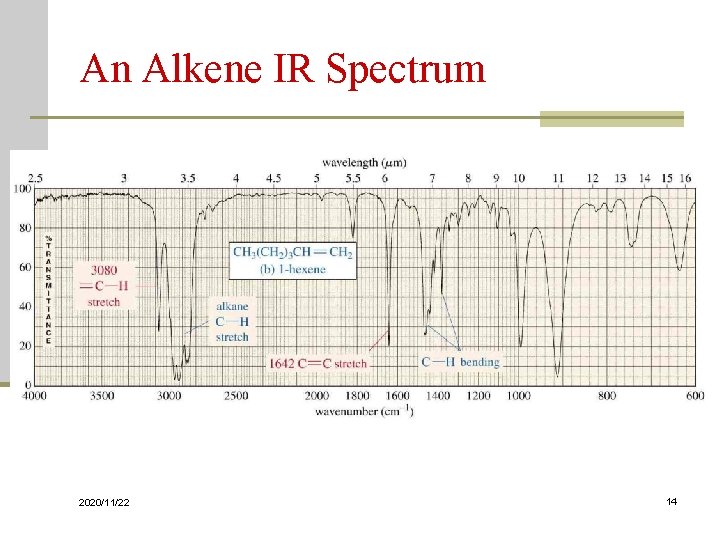
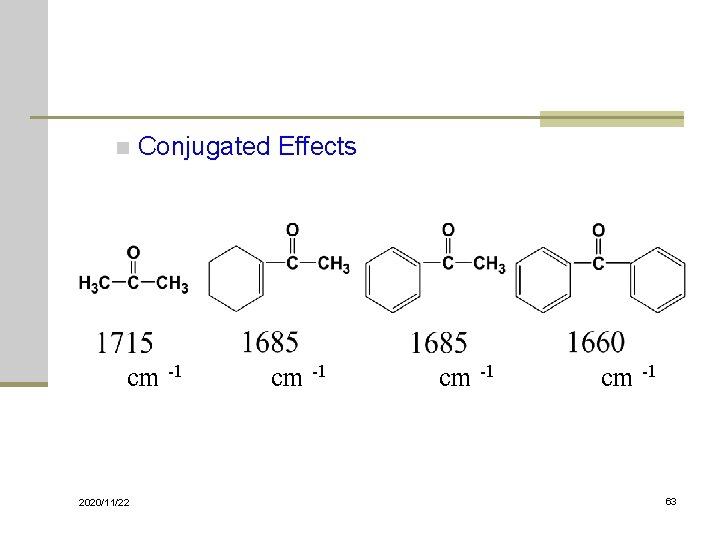
Carbon-Carbon Bond Stretching n Stronger bonds absorb at higher frequencies: n C-C 1200 cm-1 n C=C 1660 cm-1 n C C 2200 cm-1 (weak or absent if internal) n Conjugation lowers the frequency: n isolated C=C 1640 -1680 cm-1 n conjugated C=C 1620 -1640 cm-1 n aromatic C=C approx. 1600 cm-1 2020/11/22 11

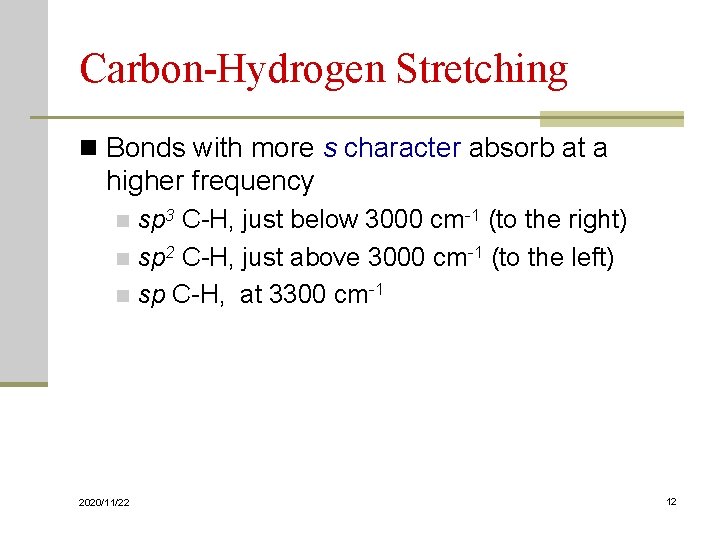
Carbon-Hydrogen Stretching n Bonds with more s character absorb at a higher frequency sp 3 C-H, just below 3000 cm-1 (to the right) n sp 2 C-H, just above 3000 cm-1 (to the left) n sp C-H, at 3300 cm-1 n 2020/11/22 12

An Alkane IR Spectrum 2020/11/22 13

An Alkene IR Spectrum 2020/11/22 14

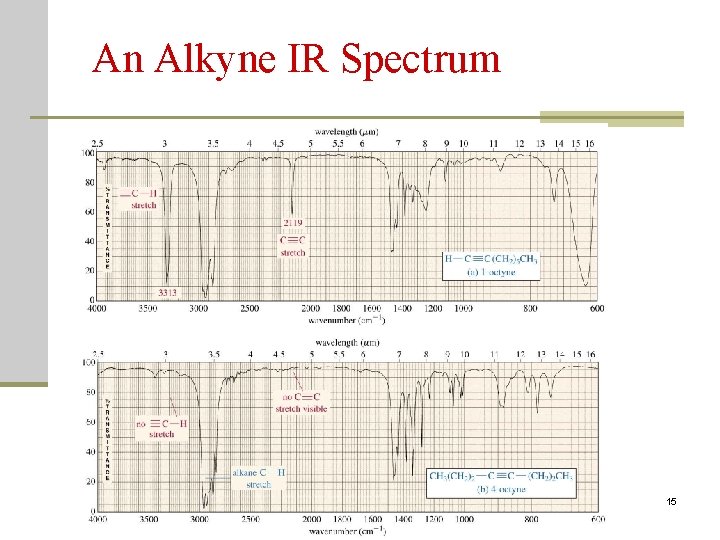
An Alkyne IR Spectrum 2020/11/22 15

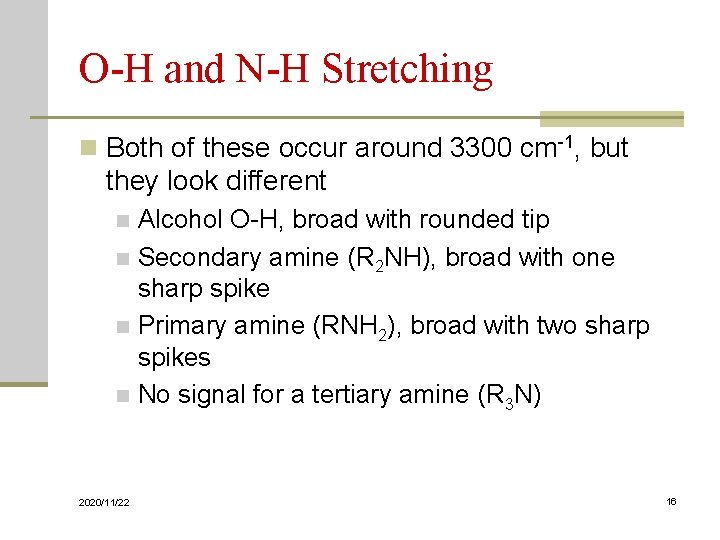
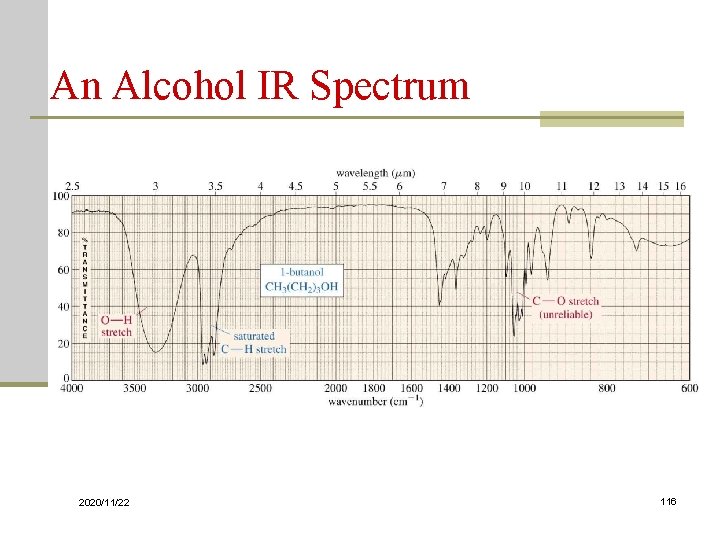
O-H and N-H Stretching n Both of these occur around 3300 cm-1, but they look different Alcohol O-H, broad with rounded tip n Secondary amine (R 2 NH), broad with one sharp spike n Primary amine (RNH 2), broad with two sharp spikes n No signal for a tertiary amine (R 3 N) n 2020/11/22 16

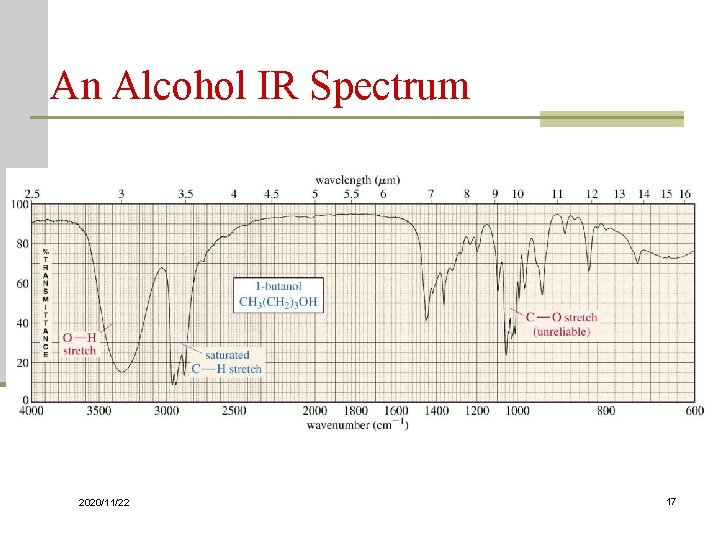
An Alcohol IR Spectrum 2020/11/22 17

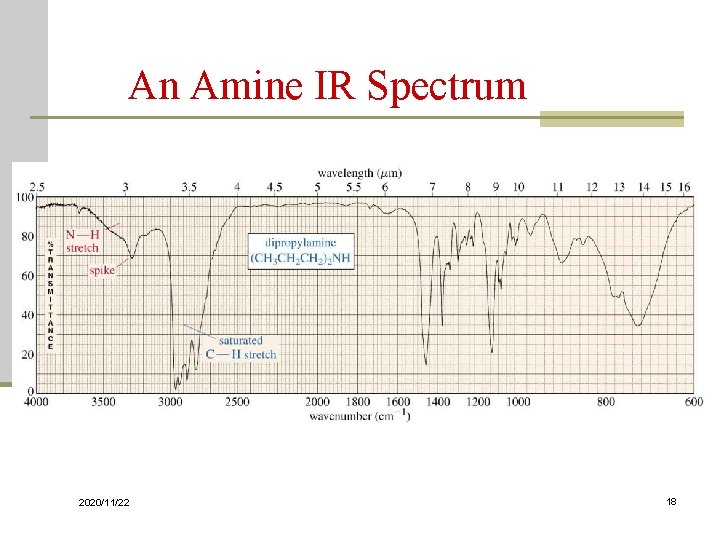
An Amine IR Spectrum 2020/11/22 18

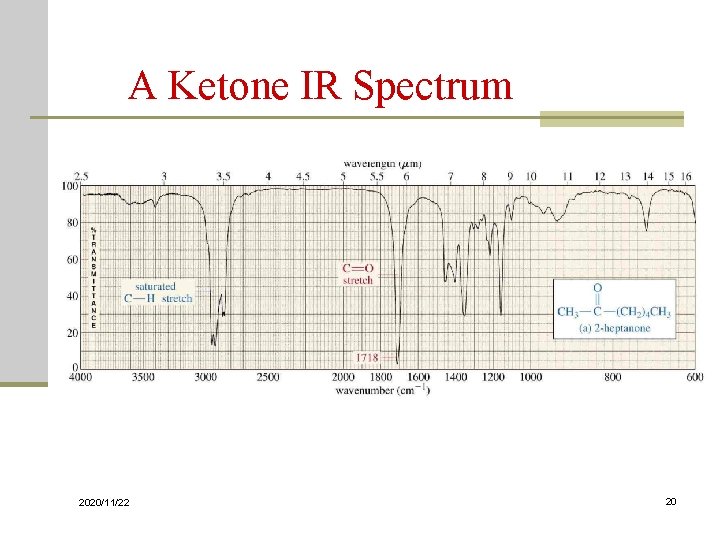
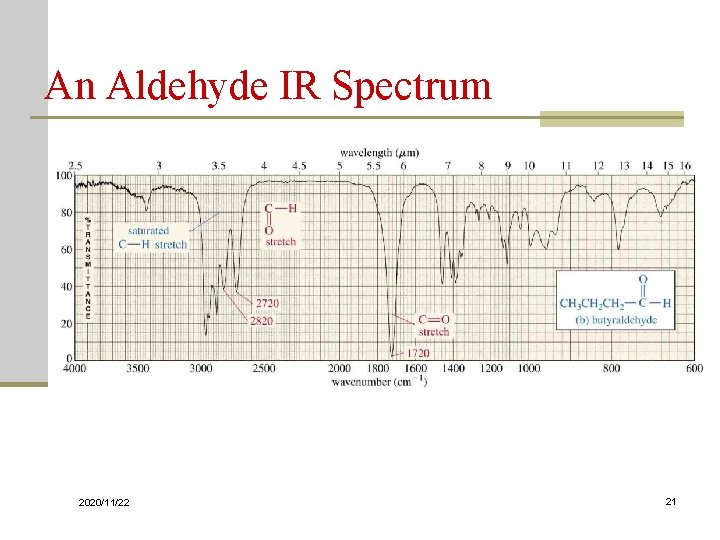
Carbonyl Stretching n The C=O bond of simple ketones, aldehydes, and carboxylic acids absorb around 1710 cm-1 n Usually, it’s the strongest IR signal n Carboxylic acids will have O-H also n Aldehydes have two C-H signals around 2700 and 2800 cm-1 2020/11/22 19

A Ketone IR Spectrum 2020/11/22 20

An Aldehyde IR Spectrum 2020/11/22 21

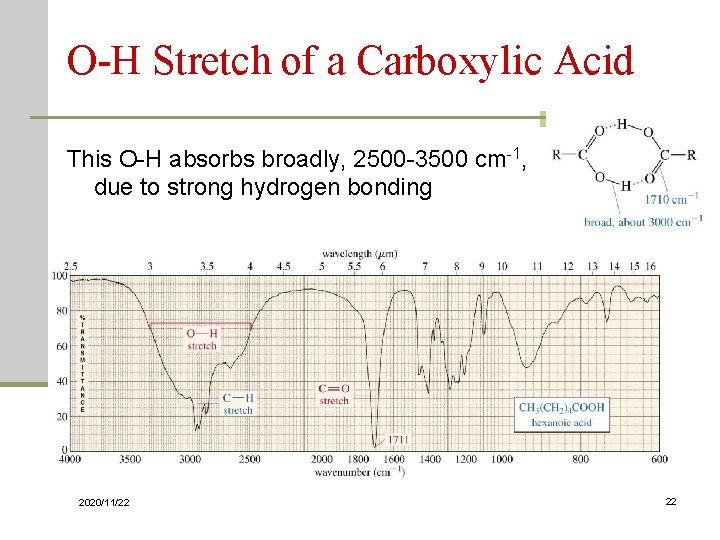
O-H Stretch of a Carboxylic Acid This O-H absorbs broadly, 2500 -3500 cm-1, due to strong hydrogen bonding 2020/11/22 22

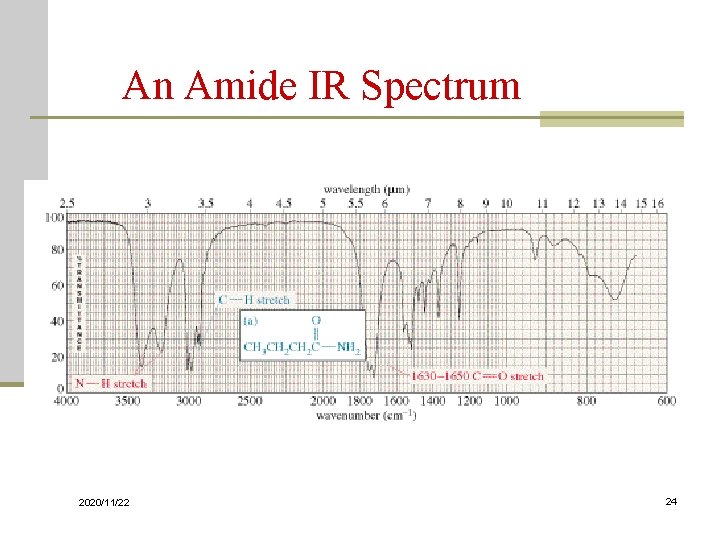
Variations in C=O Absorption n Conjugation of C=O with C=C lowers the stretching frequency to ~1680 cm-1 n The C=O group of an amide absorbs at an even lower frequency, 1640 -1680 cm-1 n The C=O of an ester absorbs at a higher frequency, ~1730 -1740 cm-1 n Carbonyl groups in small rings (5 C’s or less) absorb at an even higher frequency 2020/11/22 23

An Amide IR Spectrum 2020/11/22 24

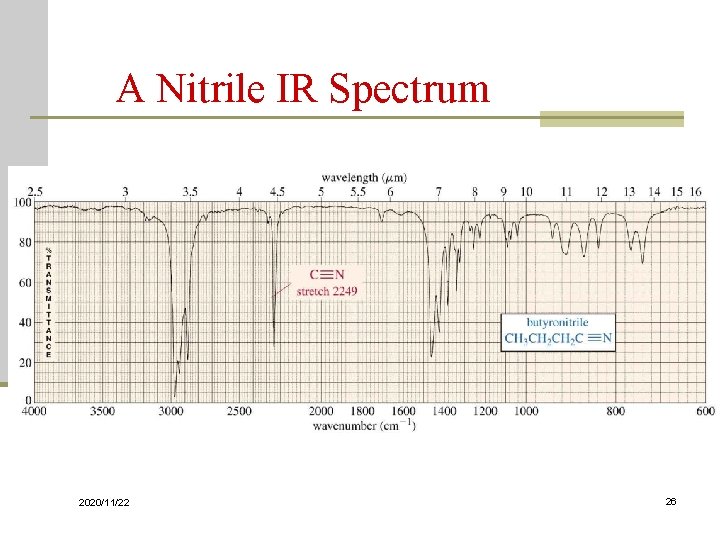
Carbon - Nitrogen Stretching n C - N absorbs around 1200 cm-1 n C = N absorbs around 1660 cm-1 and is much stronger than the C = C absorption in the same region n C N absorbs strongly just above 2200 cm-1. The alkyne C C signal is much weaker and is just below 2200 cm-1 2020/11/22 25

A Nitrile IR Spectrum 2020/11/22 26

Summary of IR Absorptions 2020/11/22 27

Strengths and Limitations n IR alone cannot determine a structure n Some signals may be ambiguous n The functional group is usually indicated n The absence of a signal is definite proof that the functional group is absent n Correspondence with a known sample’s IR spectrum confirms the identity of the compound 2020/11/22 28

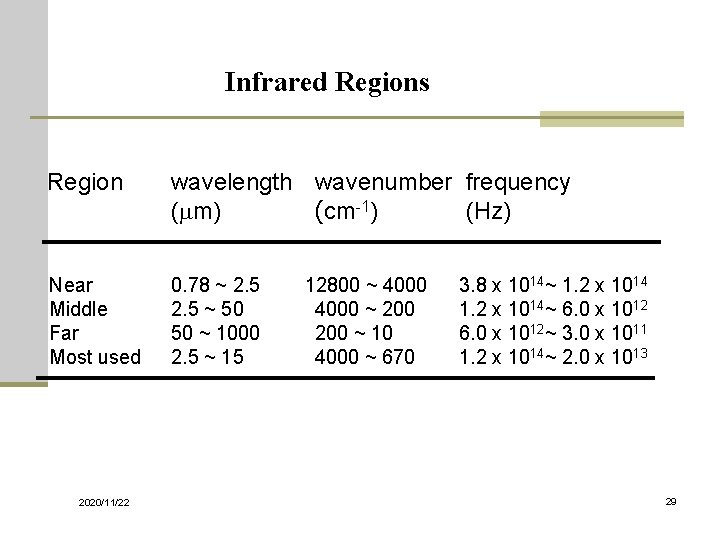
Infrared Regions Region wavelength wavenumber frequency (mm) (cm-1) (Hz) Near Middle Far Most used 0. 78 ~ 2. 5 12800 ~ 4000 2. 5 ~ 50 4000 ~ 200 50 ~ 1000 200 ~ 10 2. 5 ~ 15 4000 ~ 670 2020/11/22 3. 8 x 1014~ 1. 2 x 1014~ 6. 0 x 1012~ 3. 0 x 1011 1. 2 x 1014~ 2. 0 x 1013 29

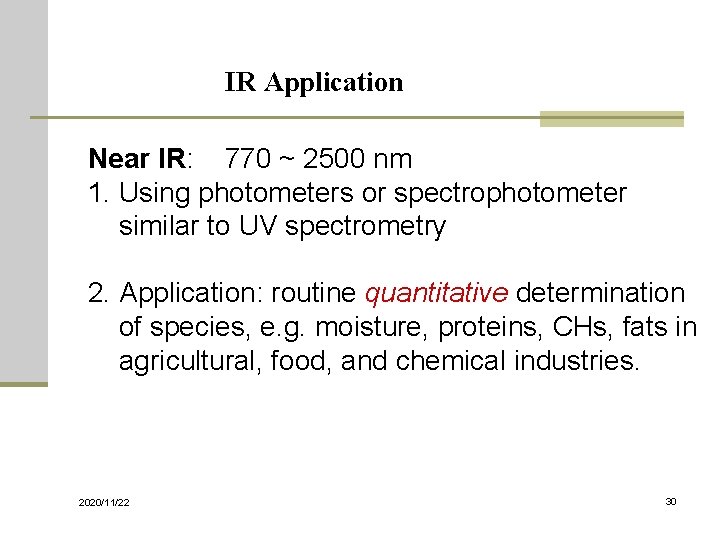
IR Application Near IR: 770 ~ 2500 nm 1. Using photometers or spectrophotometer similar to UV spectrometry 2. Application: routine quantitative determination of species, e. g. moisture, proteins, CHs, fats in agricultural, food, and chemical industries. 2020/11/22 30

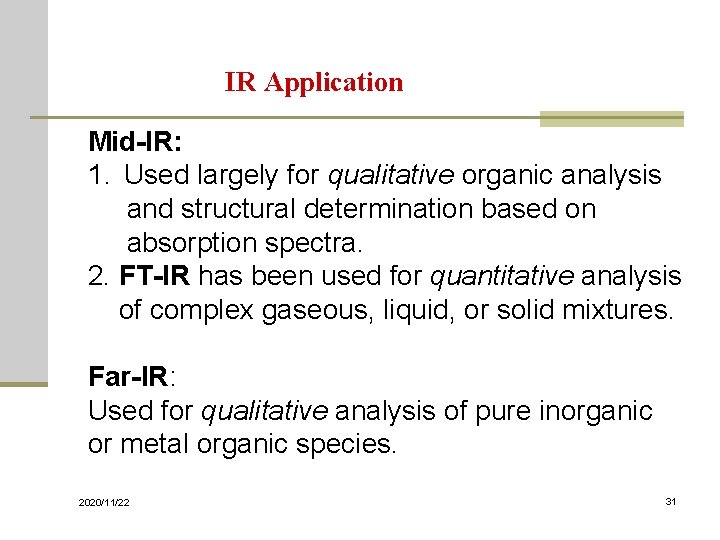
IR Application Mid-IR: 1. Used largely for qualitative organic analysis and structural determination based on absorption spectra. 2. FT-IR has been used for quantitative analysis of complex gaseous, liquid, or solid mixtures. Far-IR: Used for qualitative analysis of pure inorganic or metal organic species. 2020/11/22 31

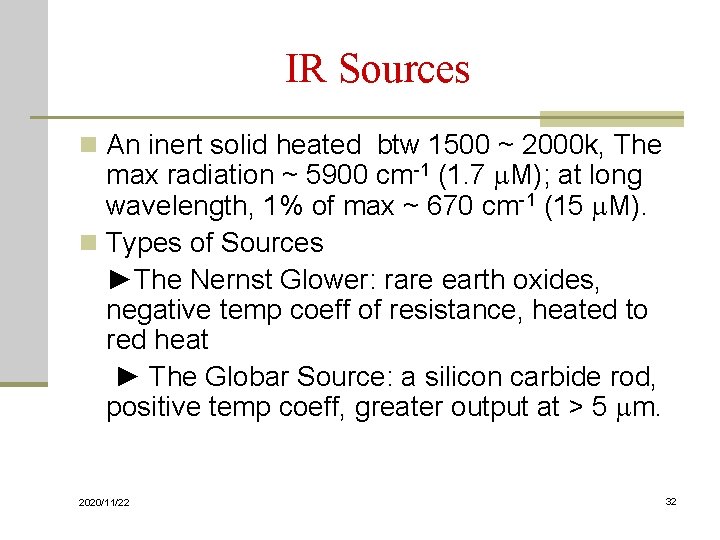
IR Sources n An inert solid heated btw 1500 ~ 2000 k, The max radiation ~ 5900 cm-1 (1. 7 m. M); at long wavelength, 1% of max ~ 670 cm-1 (15 m. M). n Types of Sources ►The Nernst Glower: rare earth oxides, negative temp coeff of resistance, heated to red heat ► The Globar Source: a silicon carbide rod, positive temp coeff, greater output at > 5 mm. 2020/11/22 32

Types of Instruments for IR n Dispersive grating photometers: used for qualitative work n Fourier transform photometers: used for both qualitative and quantitative works; speedy, reliable, and convenient n Nondispersive photometers: for quantitative use 2020/11/22 33

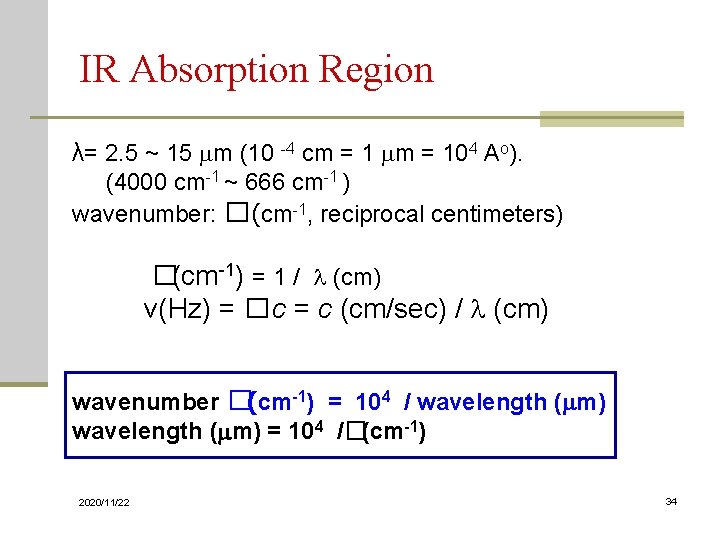
IR Absorption Region λ= 2. 5 ~ 15 mm (10 -4 cm = 1 mm = 104 Ao). (4000 cm-1 ~ 666 cm-1 ) wavenumber: � (cm-1, reciprocal centimeters) �(cm-1) = 1 / (cm) ν(Hz) = � c = c (cm/sec) / (cm) wavenumber �(cm-1) = 104 / wavelength (mm) = 104 /�(cm-1) 2020/11/22 34

Theoretical Introduction ΔE = h. ν = h c /λ = h c � C : velocity of light, 3 x 1010 cm/sec h : Planck’s constant ν : frequency (1/sec) λ : wavelength (mm) � : wavenumber (cm-1) 2020/11/22 35

IR Absorption Process n A Quantized Process: n Only selected frequencies (energies) of infrared n radiation will be absorbed by a molecule n Bonds have dipole moment, Yes ! n Symmetric bonds, No ! (e. g. H 2, Cl 2) 2020/11/22 36

IR Application n Infrared spectrum can be a fingerprint used for identification. n Infrared spectrum gives the structural information about a molecule. 2020/11/22 37

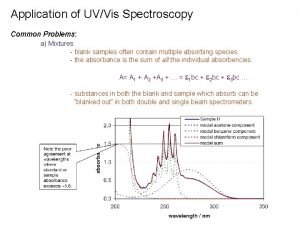
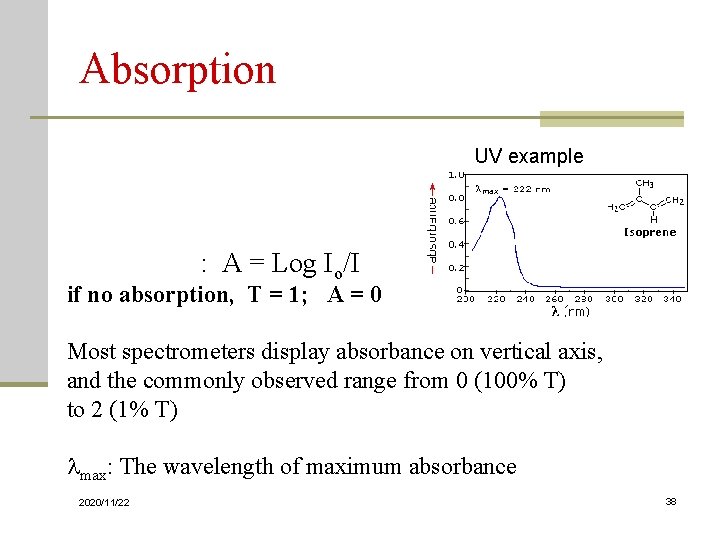
Absorption UV example Transmittance: T = I/Io Absorbance: A = Log Io/I if no absorption, T = 1; A = 0 Most spectrometers display absorbance on vertical axis, and the commonly observed range from 0 (100% T) to 2 (1% T) max: The wavelength of maximum absorbance 2020/11/22 38

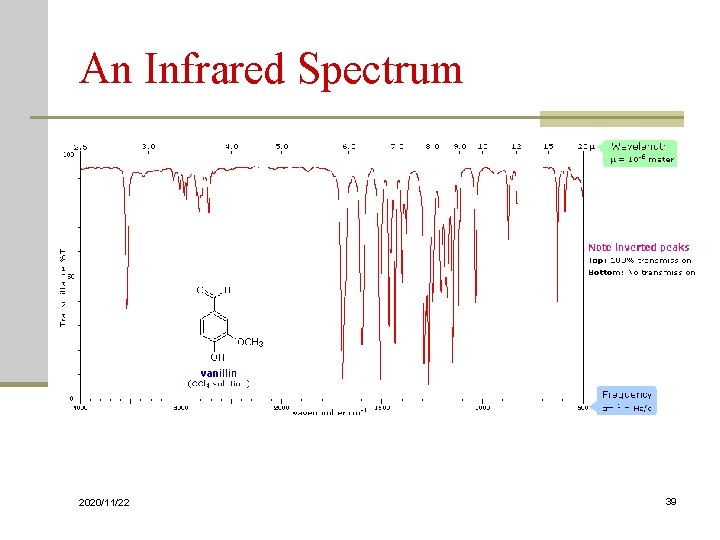
An Infrared Spectrum 2020/11/22 39

IR Spectrum Baseline Absorbance/ Peak n No two molecules will give exactly the same IR spectrum (except enantiomers) n Simple stretching: 1600 -3500 cm-1 n Complex vibrations: 400 -1400 cm-1, called the “fingerprint region” 2020/11/22 40

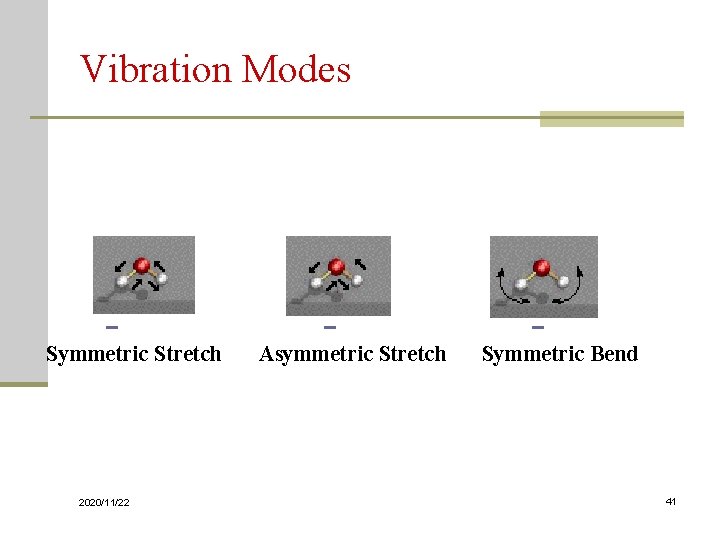
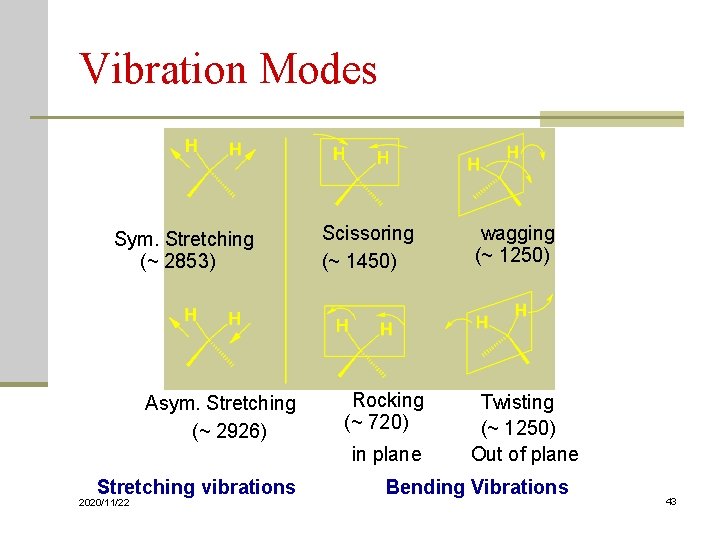
Vibration Modes Symmetric Stretch Asymmetric Stretch Symmetric Bend 2020/11/22 41

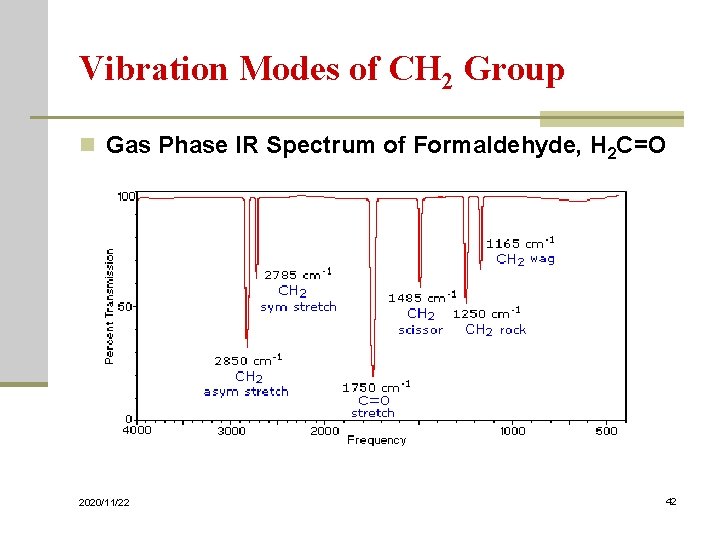
Vibration Modes of CH 2 Group n Gas Phase IR Spectrum of Formaldehyde, H 2 C=O 2020/11/22 42

Vibration Modes Sym. Stretching (~ 2853) Asym. Stretching (~ 2926) Scissoring (~ 1450) Rocking (~ 720) in plane Stretching vibrations 2020/11/22 wagging (~ 1250) Twisting (~ 1250) Out of plane Bending Vibrations 43

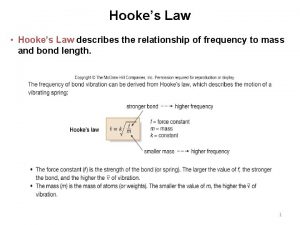
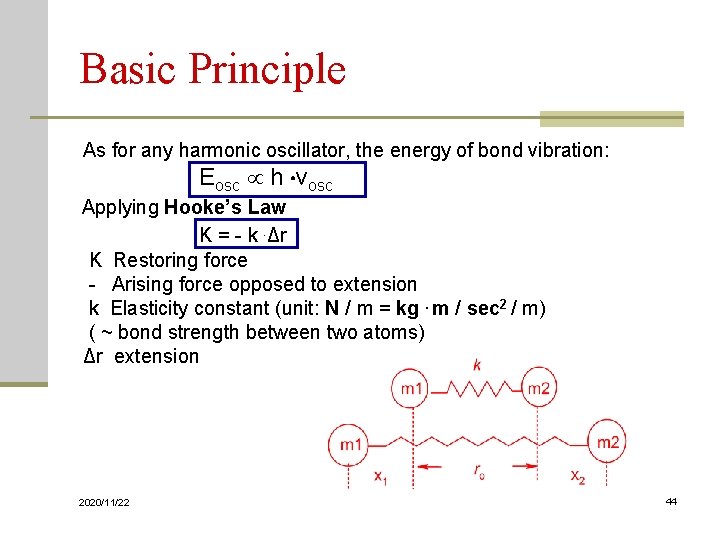
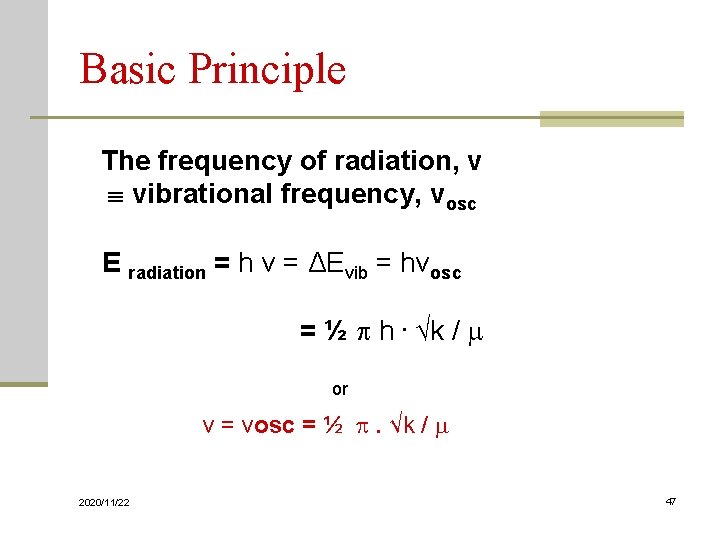
Basic Principle As for any harmonic oscillator, the energy of bond vibration: Eosc h νosc Applying Hooke’s Law K = - k. Δr K Restoring force - Arising force opposed to extension k Elasticity constant (unit: N / m = kg. m / sec 2 / m) ( ~ bond strength between two atoms) Δr extension 2020/11/22 44

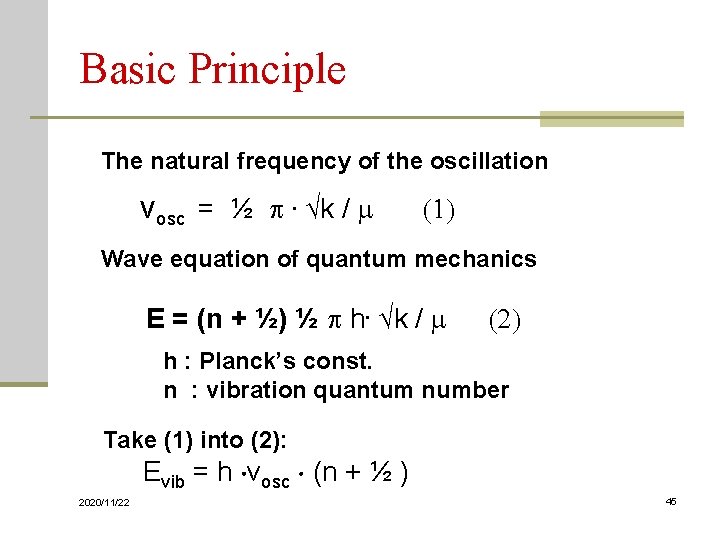
Basic Principle The natural frequency of the oscillation νosc = ½ . √k / m (1) Wave equation of quantum mechanics E = (n + ½) ½ h. √k / m (2) h : Planck’s const. n : vibration quantum number Take (1) into (2): Evib = h νosc (n + ½ ) 2020/11/22 45

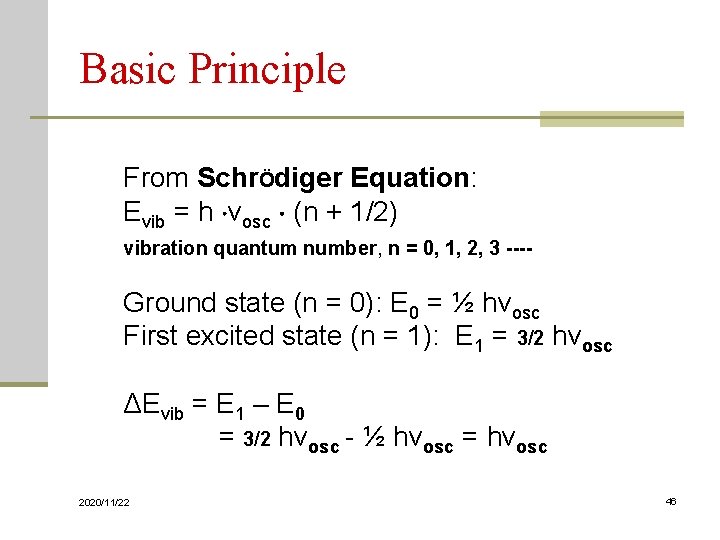
Basic Principle From SchrÖdiger Equation: Evib = h νosc (n + 1/2) vibration quantum number, n = 0, 1, 2, 3 ---- Ground state (n = 0): E 0 = ½ hνosc First excited state (n = 1): E 1 = 3/2 hνosc ΔEvib = E 1 – E 0 = 3/2 hνosc - ½ hνosc = hνosc 2020/11/22 46

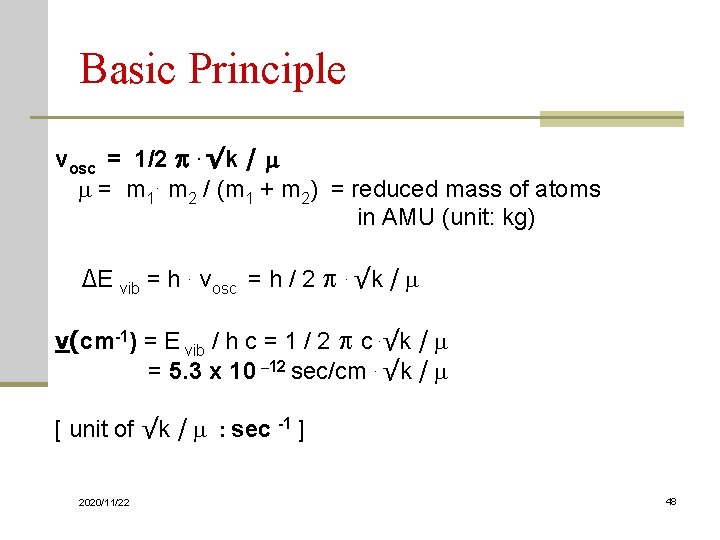
Basic Principle The frequency of radiation, ν vibrational frequency, νosc E radiation = h ν = ΔEvib = hνosc = ½ h. √k / m or ν = νosc = ½ . √k / m 2020/11/22 47

Basic Principle νosc = 1/2 . √k / m m = m 1. m 2 / (m 1 + m 2) = reduced mass of atoms in AMU (unit: kg) ΔE vib = h. νosc = h / 2 . √k / m ν(cm-1) = E vib / h c = 1 / 2 c. √k / m = 5. 3 x 10 – 12 sec/cm. √k / m [ unit of √k / m : sec -1 ] 2020/11/22 48

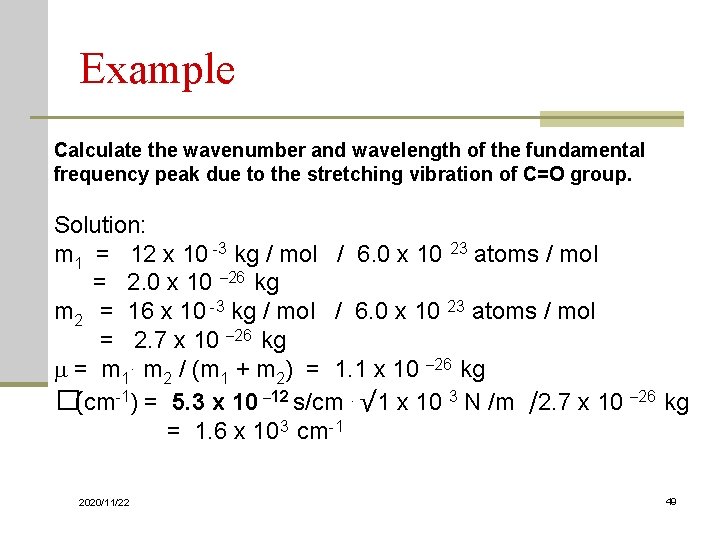
Example Calculate the wavenumber and wavelength of the fundamental frequency peak due to the stretching vibration of C=O group. Solution: m 1 = 12 x 10 -3 kg / mol / 6. 0 x 10 23 atoms / mol = 2. 0 x 10 – 26 kg m 2 = 16 x 10 -3 kg / mol / 6. 0 x 10 23 atoms / mol = 2. 7 x 10 – 26 kg m = m 1. m 2 / (m 1 + m 2) = 1. 1 x 10 – 26 kg �(cm-1) = 5. 3 x 10 – 12 s/cm. √ 1 x 10 3 N /m /2. 7 x 10 – 26 kg = 1. 6 x 103 cm-1 2020/11/22 49

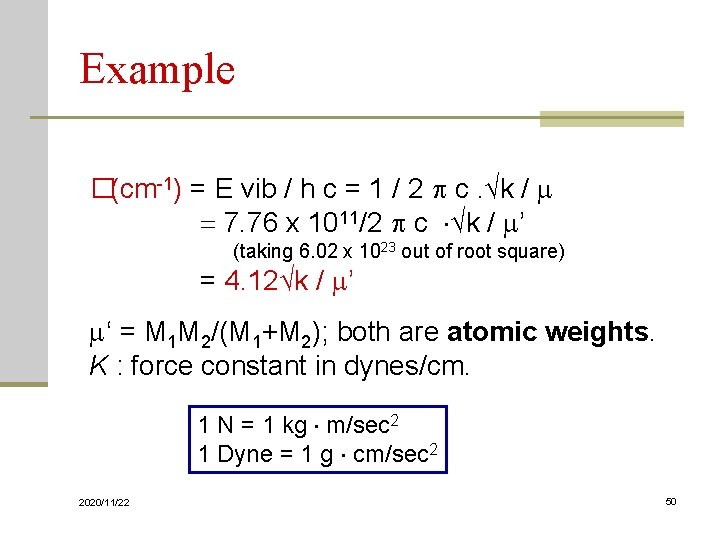
Example �(cm-1) = E vib / h c = 1 / 2 c. √k / m = 7. 76 x 1011/2 c √k / m’ (taking 6. 02 x 1023 out of root square) = 4. 12√k / m’ m‘ = M 1 M 2/(M 1+M 2); both are atomic weights. K : force constant in dynes/cm. 1 N = 1 kg m/sec 2 1 Dyne = 1 g cm/sec 2 2020/11/22 50

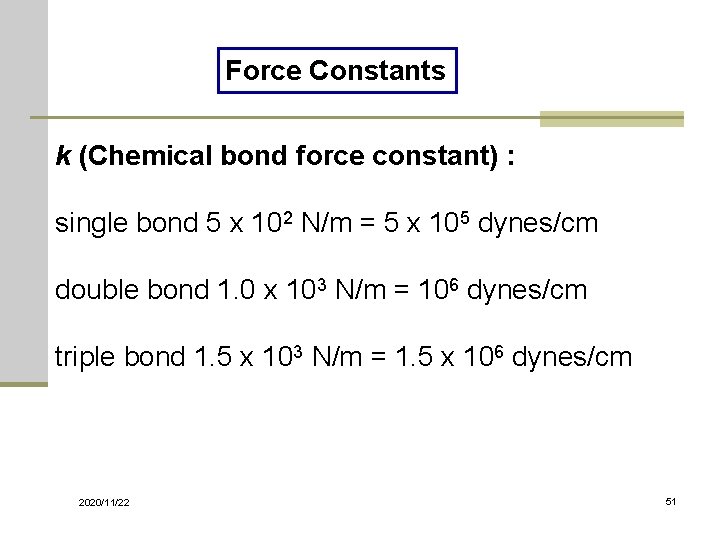
Force Constants k (Chemical bond force constant) : single bond 5 x 102 N/m = 5 x 105 dynes/cm double bond 1. 0 x 103 N/m = 106 dynes/cm triple bond 1. 5 x 103 N/m = 1. 5 x 106 dynes/cm 2020/11/22 51

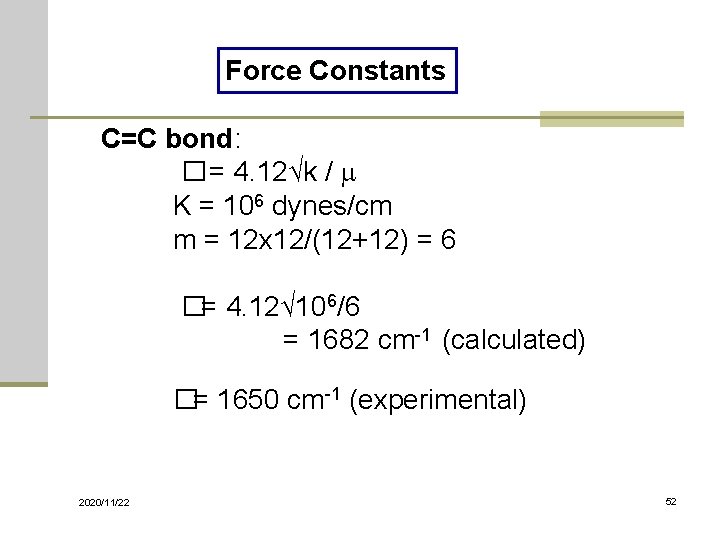
Force Constants C=C bond: � = 4. 12√k / m K = 106 dynes/cm m = 12 x 12/(12+12) = 6 �= 4. 12√ 106/6 = 1682 cm-1 (calculated) �= 1650 cm-1 (experimental) 2020/11/22 52

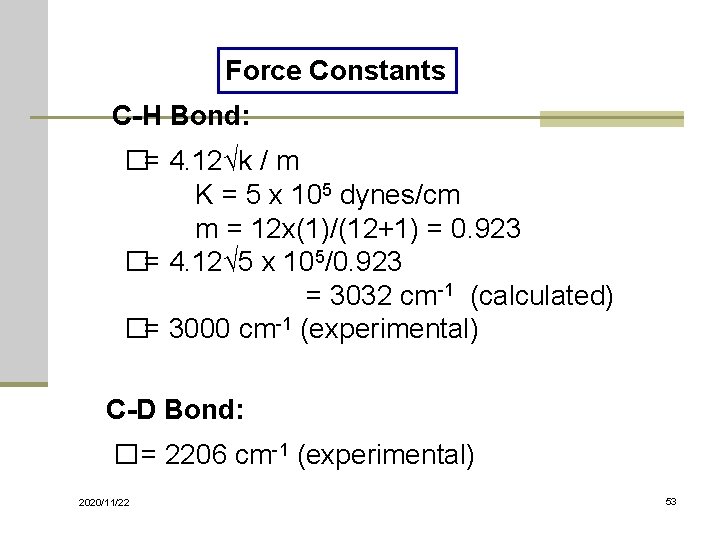
Force Constants C-H Bond: �= 4. 12√k / m K = 5 x 105 dynes/cm m = 12 x(1)/(12+1) = 0. 923 �= 4. 12√ 5 x 105/0. 923 = 3032 cm-1 (calculated) �= 3000 cm-1 (experimental) C-D Bond: � = 2206 cm-1 (experimental) 2020/11/22 53

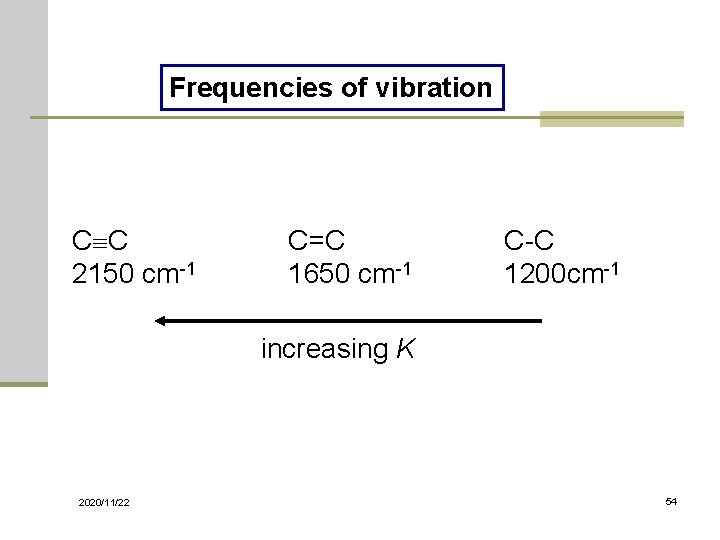
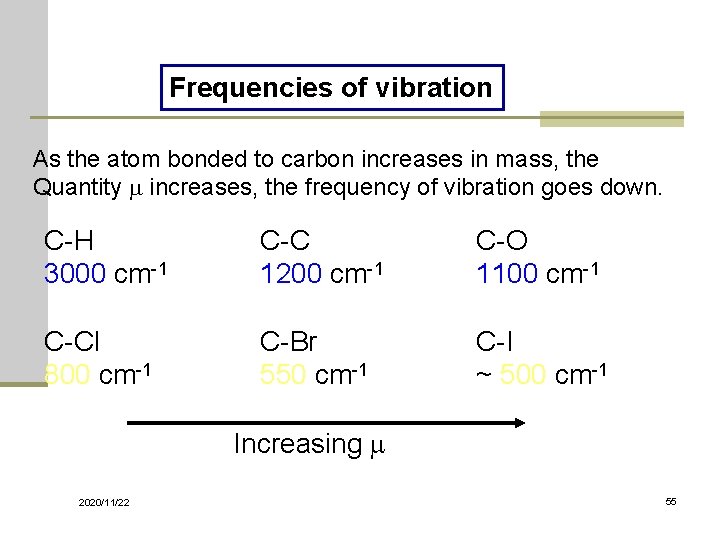
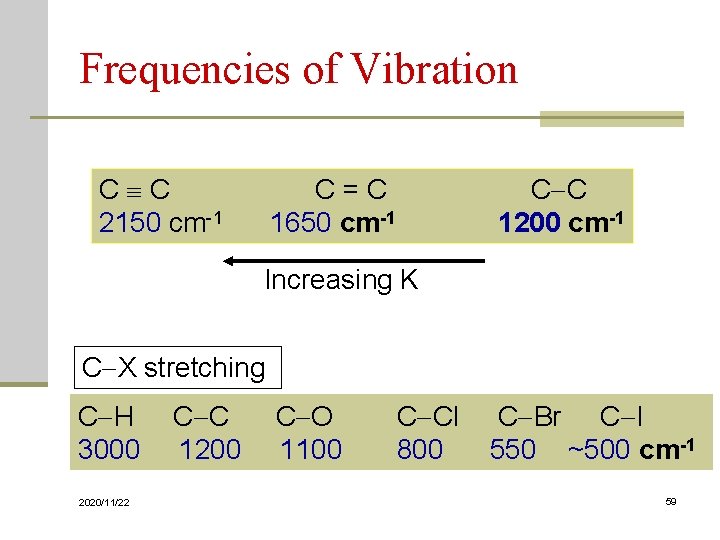
Frequencies of vibration C C 2150 cm-1 C=C 1650 cm-1 C-C 1200 cm-1 increasing K 2020/11/22 54

Frequencies of vibration As the atom bonded to carbon increases in mass, the Quantity m increases, the frequency of vibration goes down. C-H 3000 cm-1 C-C 1200 cm-1 C-O 1100 cm-1 C-Cl 800 cm-1 C-Br 550 cm-1 C-I ~ 500 cm-1 Increasing m 2020/11/22 55

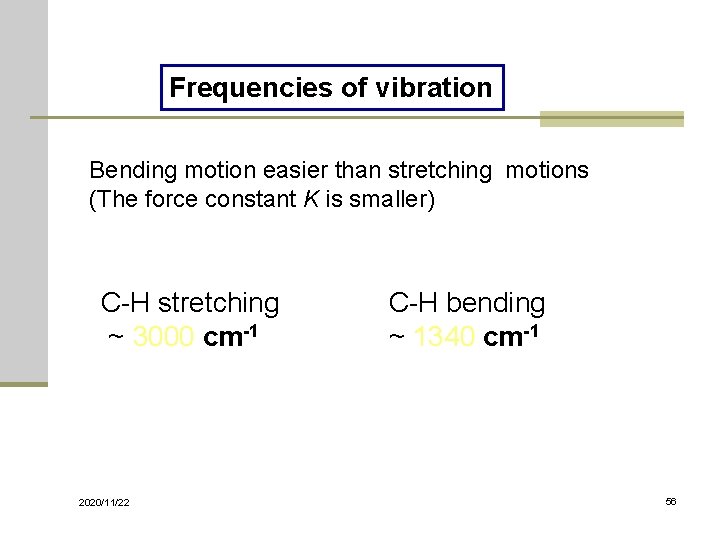
Frequencies of vibration Bending motion easier than stretching motions (The force constant K is smaller) C-H stretching ~ 3000 cm-1 2020/11/22 C-H bending ~ 1340 cm-1 56

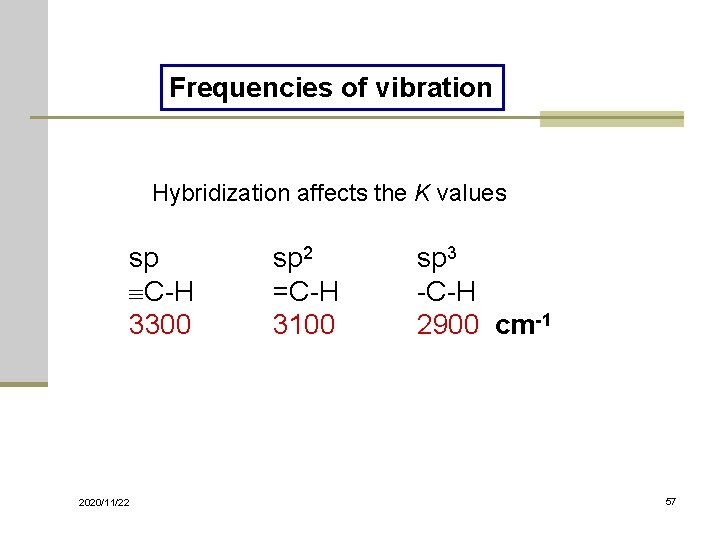
Frequencies of vibration Hybridization affects the K values sp C-H 3300 2020/11/22 sp 2 =C-H 3100 sp 3 -C-H 2900 cm-1 57

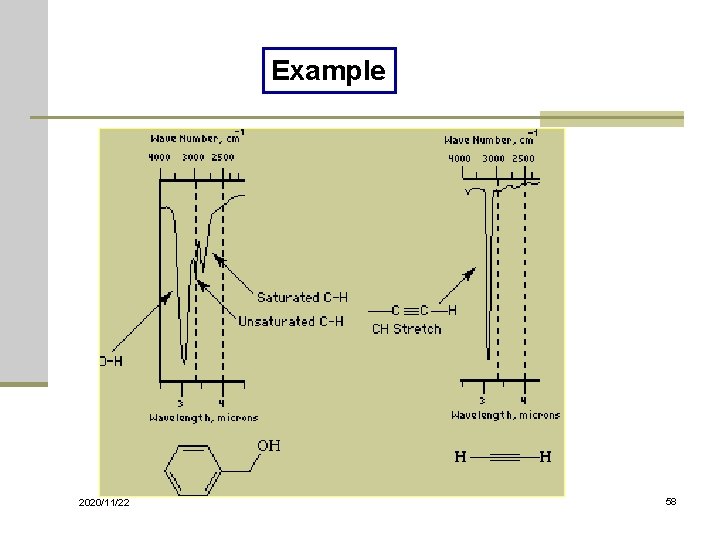
Example 2020/11/22 58

Frequencies of Vibration C C C = C 2150 cm-1 1650 cm-1 C C 1200 cm-1 Increasing K C X stretching C H C C C O C Cl C Br C I 3000 1200 1100 800 550 ~500 cm-1 2020/11/22 59

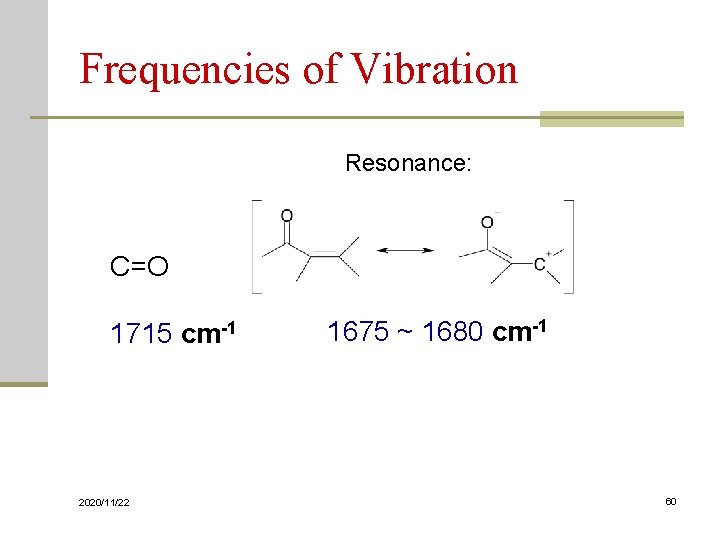
Frequencies of Vibration Resonance: C=O 1715 cm-1 2020/11/22 1675 ~ 1680 cm-1 60

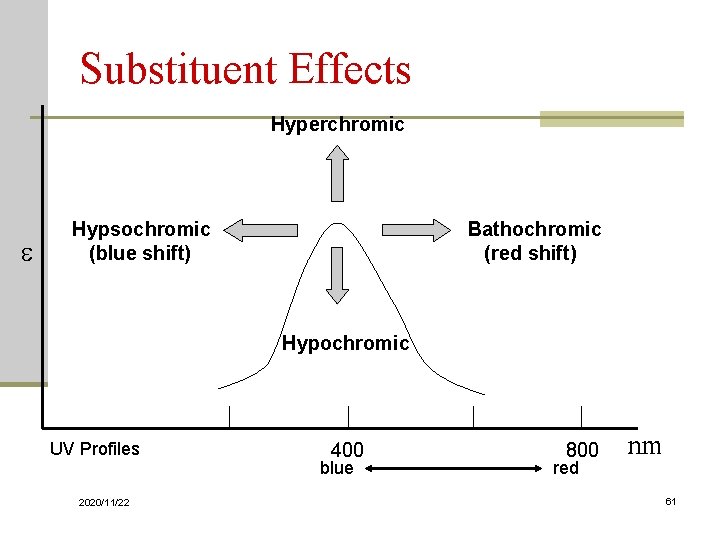
Substituent Effects Hyperchromic e Bathochromic (red shift) Hypsochromic (blue shift) Hypochromic UV Profiles 2020/11/22 400 blue 800 red nm 61

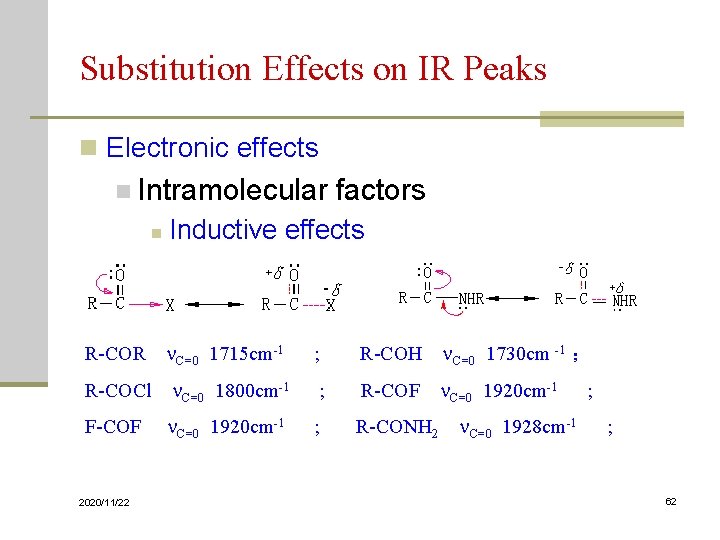
Substitution Effects on IR Peaks n Electronic effects n Intramolecular factors n Inductive effects R-COR C=0 1715 cm-1 ; R-COH C=0 1730 cm -1 ; R-COCl C=0 1800 cm-1 ; R-COF C=0 1920 cm-1 ; F-COF C=0 1920 cm-1 ; R-CONH 2 C=0 1928 cm-1 ; 2020/11/22 62

n Conjugated Effects cm -1 2020/11/22 cm -1 63

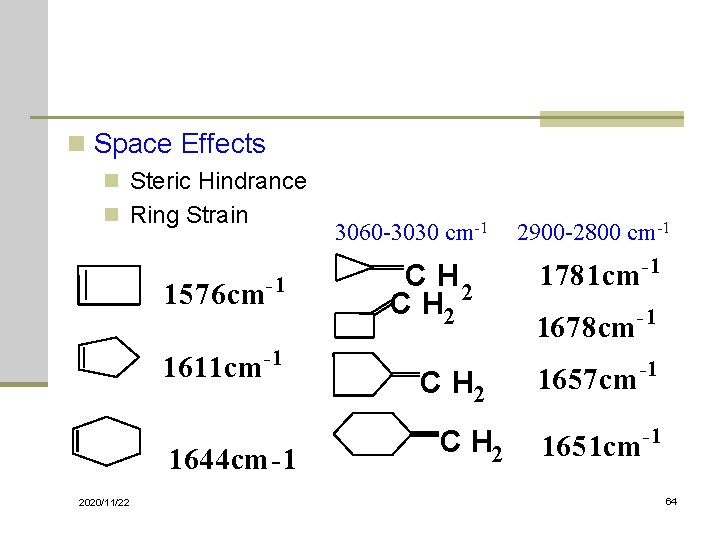
n Space Effects n Steric Hindrance n Ring Strain 1576 cm 3060 -3030 cm-1 -1 1611 cm -1 1644 cm-1 2020/11/22 C H 2 2900 -2800 cm-1 1781 cm -1 1678 cm -1 1657 cm -1 1651 cm -1 64

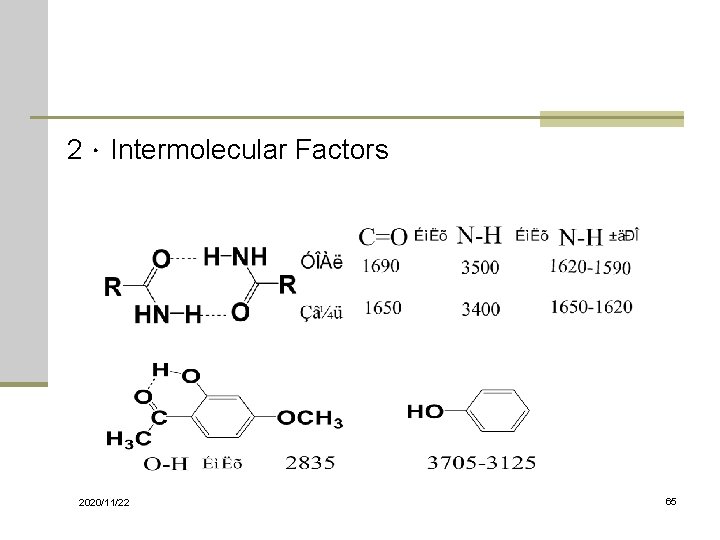
2.Intermolecular Factors 2020/11/22 65

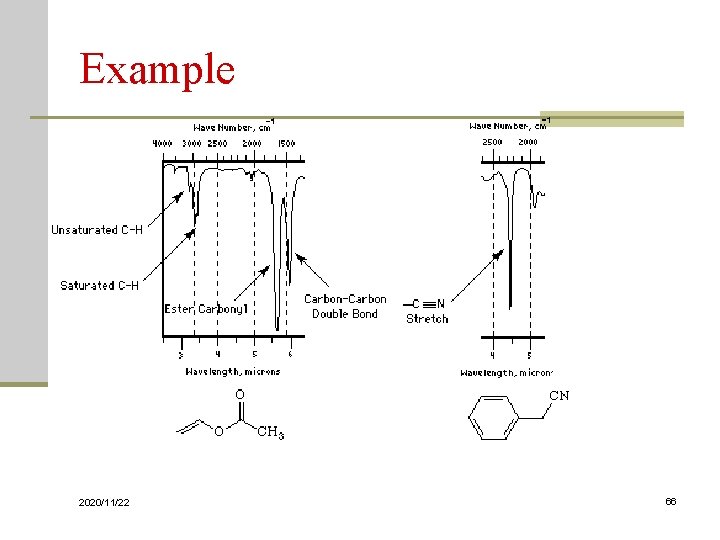
Example 2020/11/22 66

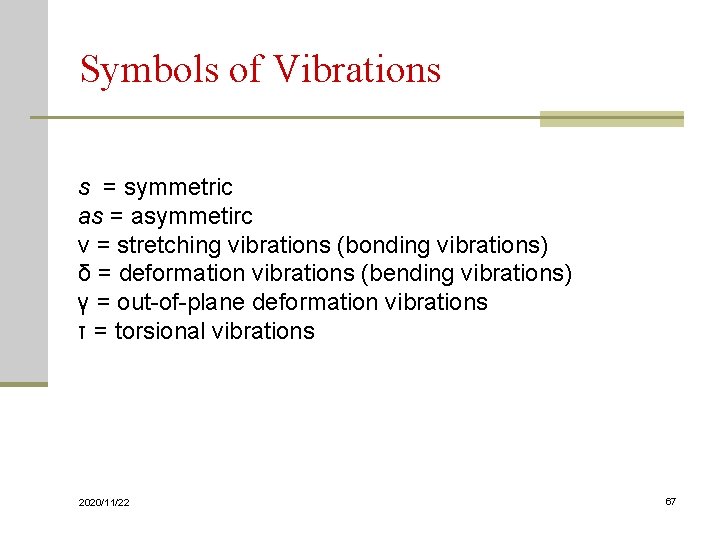
Symbols of Vibrations s = symmetric as = asymmetirc ν = stretching vibrations (bonding vibrations) δ = deformation vibrations (bending vibrations) γ = out-of-plane deformation vibrations τ = torsional vibrations 2020/11/22 67

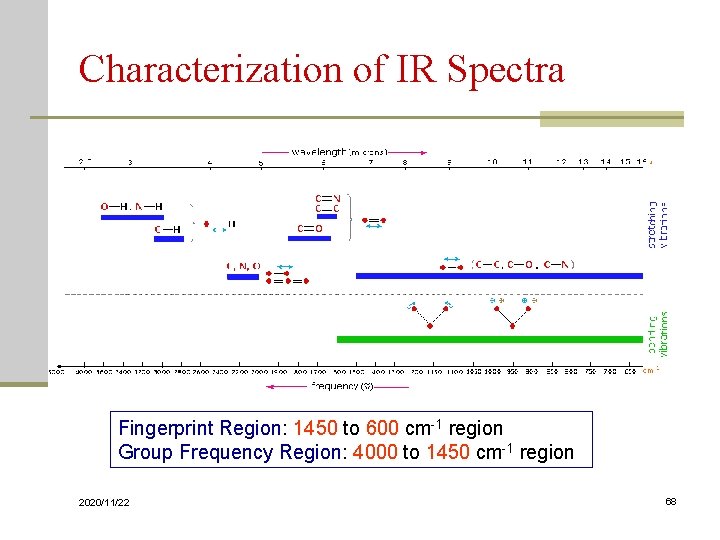
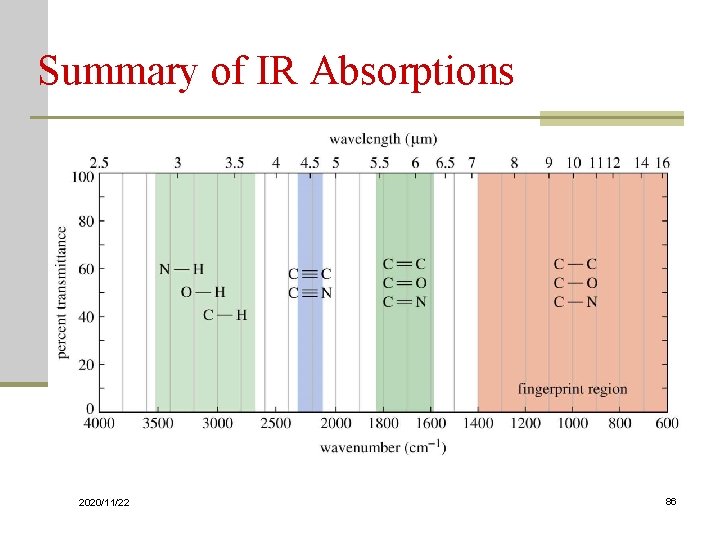
Characterization of IR Spectra Fingerprint Region: 1450 to 600 cm-1 region Group Frequency Region: 4000 to 1450 cm-1 region 2020/11/22 68

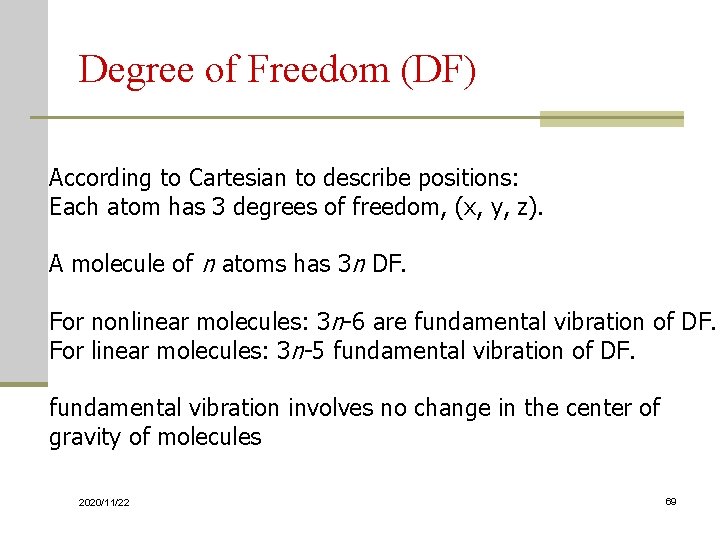
Degree of Freedom (DF) According to Cartesian to describe positions: Each atom has 3 degrees of freedom, (x, y, z). A molecule of n atoms has 3 n DF. For nonlinear molecules: 3 n-6 are fundamental vibration of DF. For linear molecules: 3 n-5 fundamental vibration of DF. fundamental vibration involves no change in the center of gravity of molecules 2020/11/22 69

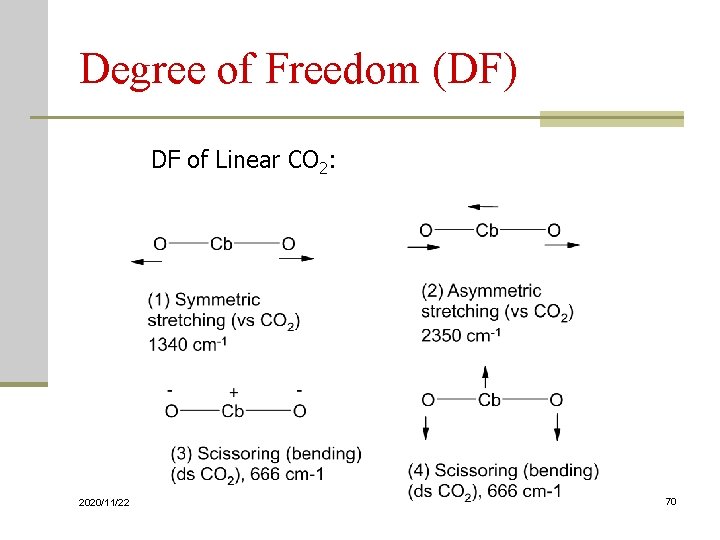
Degree of Freedom (DF) DF of Linear CO 2: 2020/11/22 70

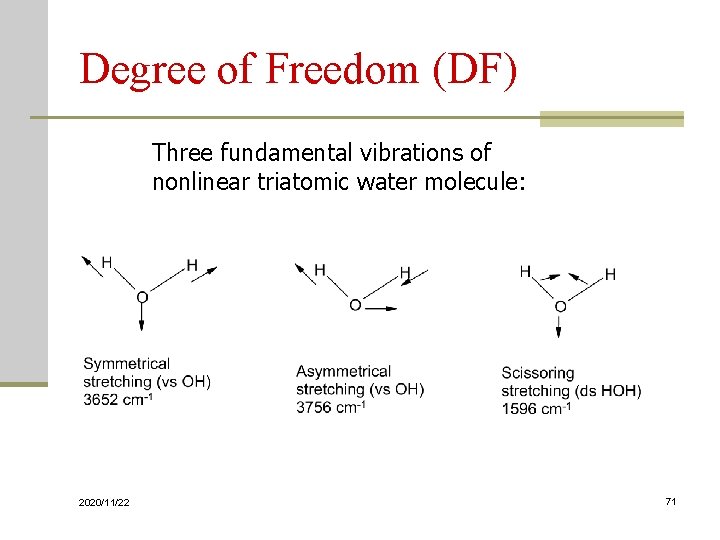
Degree of Freedom (DF) Three fundamental vibrations of nonlinear triatomic water molecule: 2020/11/22 71

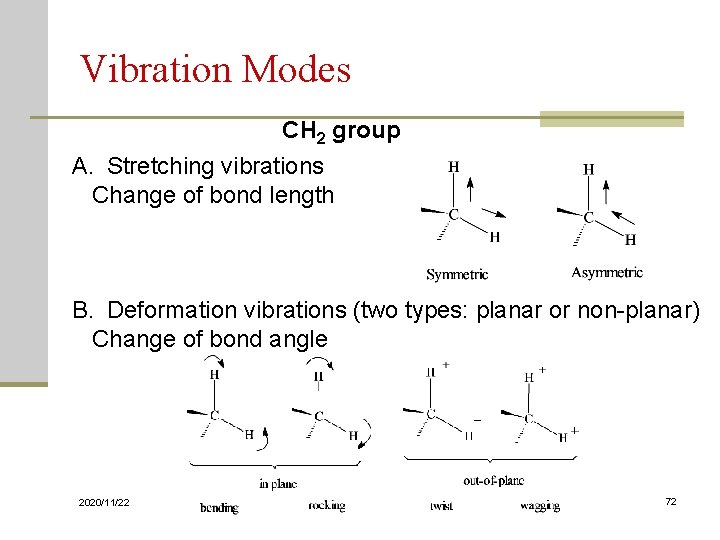
Vibration Modes CH 2 group A. Stretching vibrations Change of bond length B. Deformation vibrations (two types: planar or non-planar) Change of bond angle 2020/11/22 72

Fingerprint Regions Localized vibrations: spectra below 1500 cm-1 are difficult to interpretation, which, we call it “fingerprint region”, are characteristic for the molecule as a whole. Most of them are derived from overtone or combination vibrations. 2020/11/22 73

Group Frequency Regions Absorption bands in the 4000 to 1450 cm-1 region are usually due to stretching vibrations of diatomic units 2020/11/22 74

Some General Trends i) Stretching frequencies are higher than corresponding bending frequencies. (It is easier to bend a bond than to stretch or compress it. ) ii) Bonds to hydrogen have higher stretching frequencies than those to heavier atoms. iii) Triple bonds have higher stretching frequencies than corresponding double bonds, which in turn have higher frequencies than single bonds. (Except for bonds to hydrogen). 2020/11/22 75

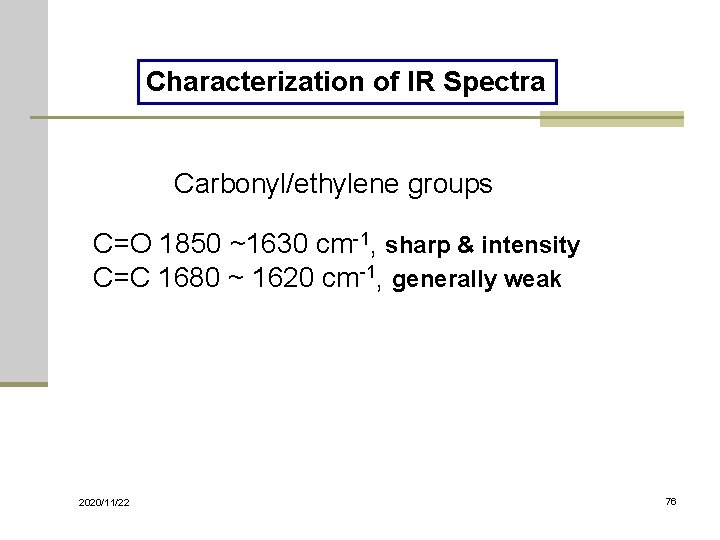
Characterization of IR Spectra Carbonyl/ethylene groups C=O 1850 ~1630 cm-1, sharp & intensity C=C 1680 ~ 1620 cm-1, generally weak 2020/11/22 76

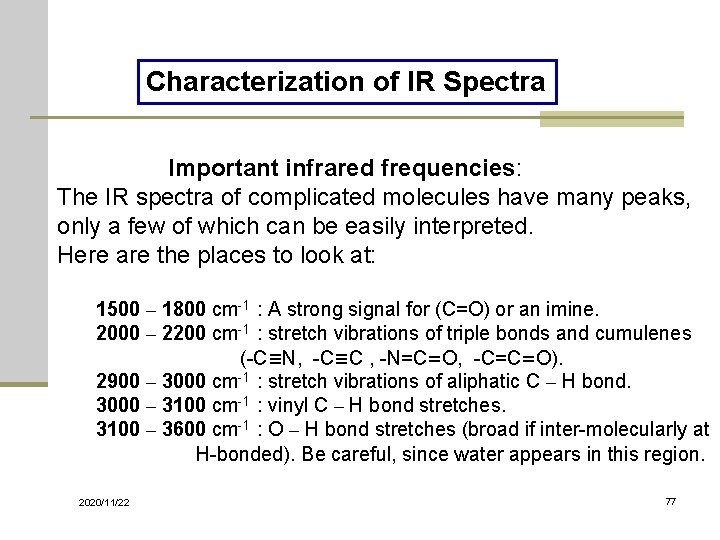
Characterization of IR Spectra Important infrared frequencies: The IR spectra of complicated molecules have many peaks, only a few of which can be easily interpreted. Here are the places to look at: 1500 – 1800 cm-1 : A strong signal for (C=O) or an imine. 2000 – 2200 cm-1 : stretch vibrations of triple bonds and cumulenes (-C≡N, -C≡C , -N=C=O, -C=C=O). 2900 – 3000 cm-1 : stretch vibrations of aliphatic C – H bond. 3000 – 3100 cm-1 : vinyl C – H bond stretches. 3100 – 3600 cm-1 : O – H bond stretches (broad if inter-molecularly at H-bonded). Be careful, since water appears in this region. 2020/11/22 77

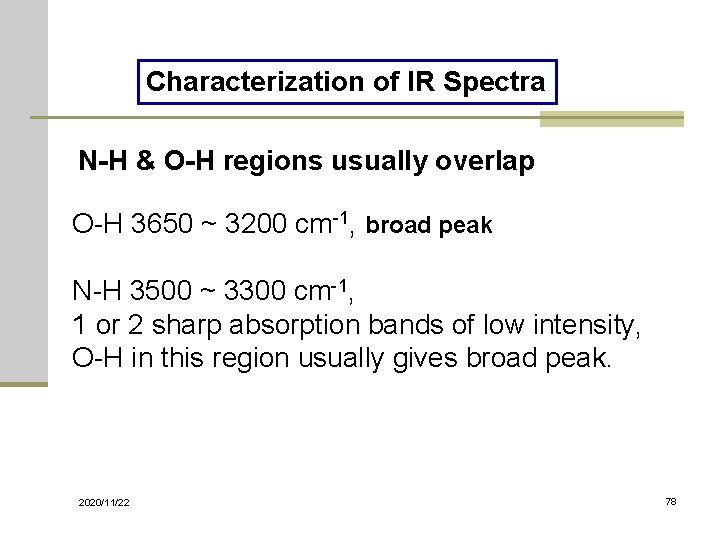
Characterization of IR Spectra N-H & O-H regions usually overlap O-H 3650 ~ 3200 cm-1, broad peak N-H 3500 ~ 3300 cm-1, 1 or 2 sharp absorption bands of low intensity, O-H in this region usually gives broad peak. 2020/11/22 78

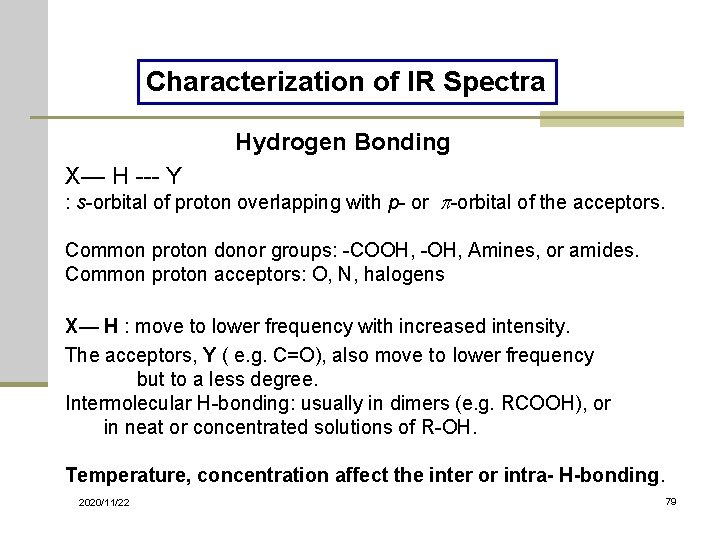
Characterization of IR Spectra Hydrogen Bonding X— H --- Y : s-orbital of proton overlapping with p- or -orbital of the acceptors. Common proton donor groups: -COOH, -OH, Amines, or amides. Common proton acceptors: O, N, halogens X— H : move to lower frequency with increased intensity. The acceptors, Y ( e. g. C=O), also move to lower frequency but to a less degree. Intermolecular H-bonding: usually in dimers (e. g. RCOOH), or in neat or concentrated solutions of R-OH. Temperature, concentration affect the inter or intra- H-bonding. 2020/11/22 79

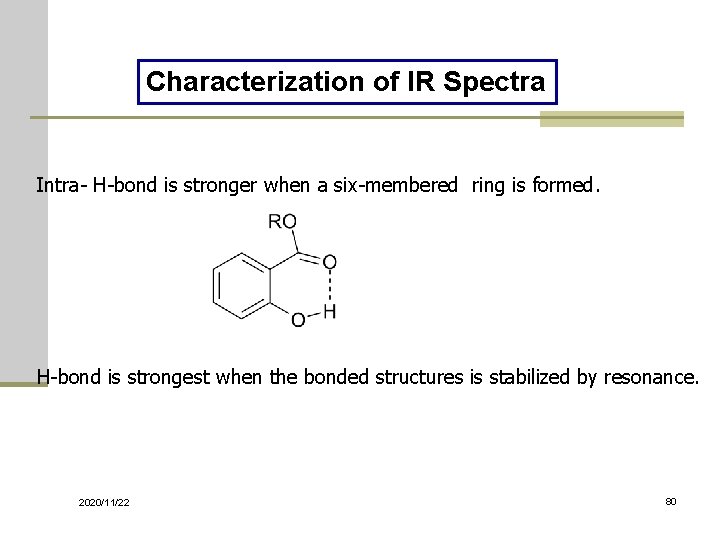
Characterization of IR Spectra Intra- H-bond is stronger when a six-membered ring is formed. H-bond is strongest when the bonded structures is stabilized by resonance. 2020/11/22 80

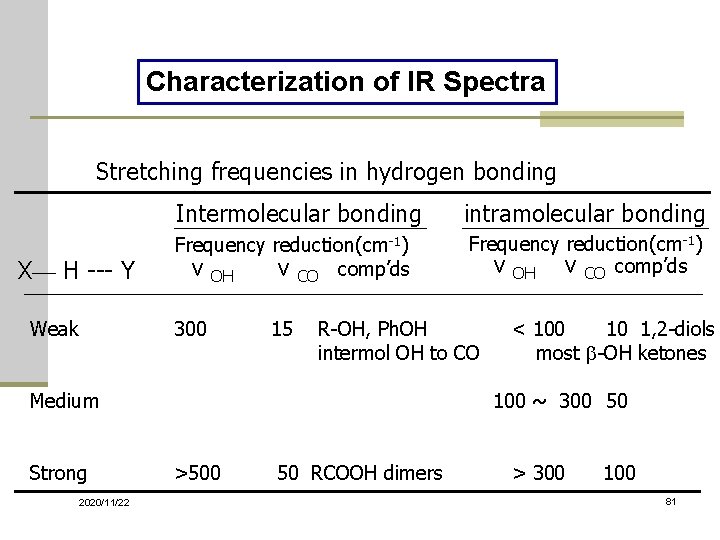
Characterization of IR Spectra Stretching frequencies in hydrogen bonding X— H --- Y Weak Intermolecular bonding intramolecular bonding Frequency reduction(cm-1) ν OH ν CO comp’ds 300 15 R-OH, Ph. OH intermol OH to CO Medium Strong 2020/11/22 < 100 10 1, 2 -diols most b-OH ketones 100 ~ 300 50 >500 50 RCOOH dimers > 300 100 81

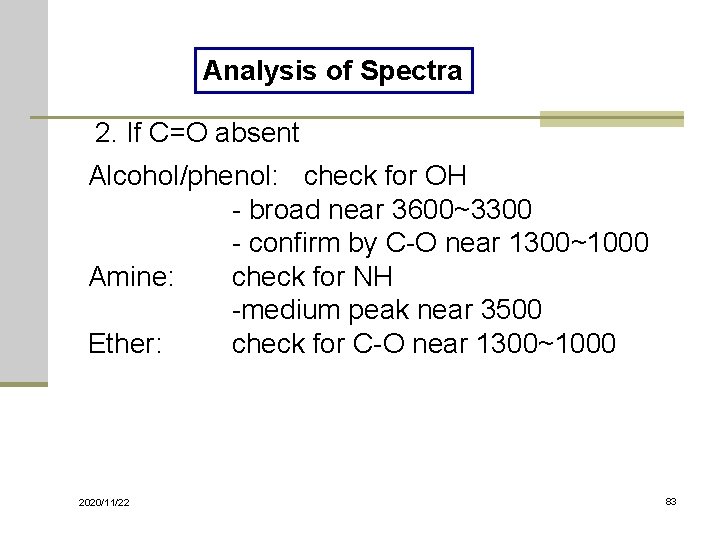
Analysis of Spectra 1. If C=O 1820 ~ 1660 cm-1 : the strongest peak Acid: Amide: Ester: Anhydride: Aldehyde: Ketone: 2020/11/22 is OH present? Broad near 3400~2400 is NH present? Medium peak ~3500 is C-O present? Strong at 1300~1000 two C=O ~ at 1810 & 1760 is C-H present? Two peaks ~ 2850 & 2750 the above 5 choices were eliminated 82

Analysis of Spectra 2. If C=O absent Alcohol/phenol: check for OH - broad near 3600~3300 - confirm by C-O near 1300~1000 Amine: check for NH -medium peak near 3500 Ether: check for C-O near 1300~1000 2020/11/22 83

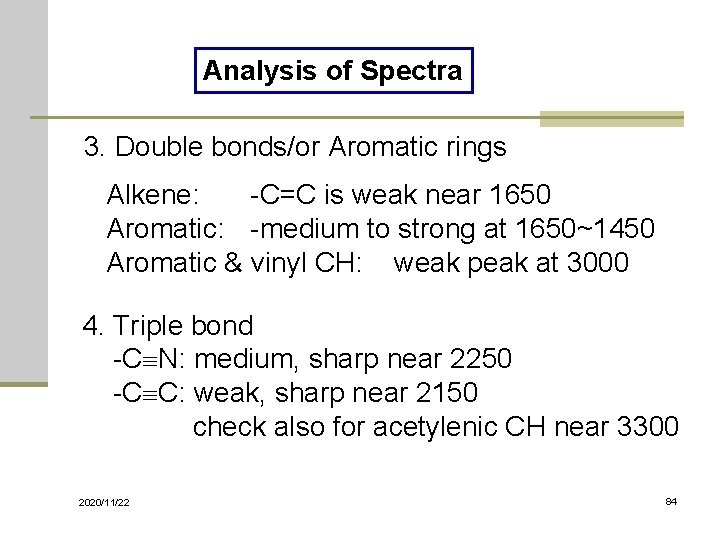
Analysis of Spectra 3. Double bonds/or Aromatic rings Alkene: -C=C is weak near 1650 Aromatic: -medium to strong at 1650~1450 Aromatic & vinyl CH: weak peak at 3000 4. Triple bond -C N: medium, sharp near 2250 -C C: weak, sharp near 2150 check also for acetylenic CH near 3300 2020/11/22 84

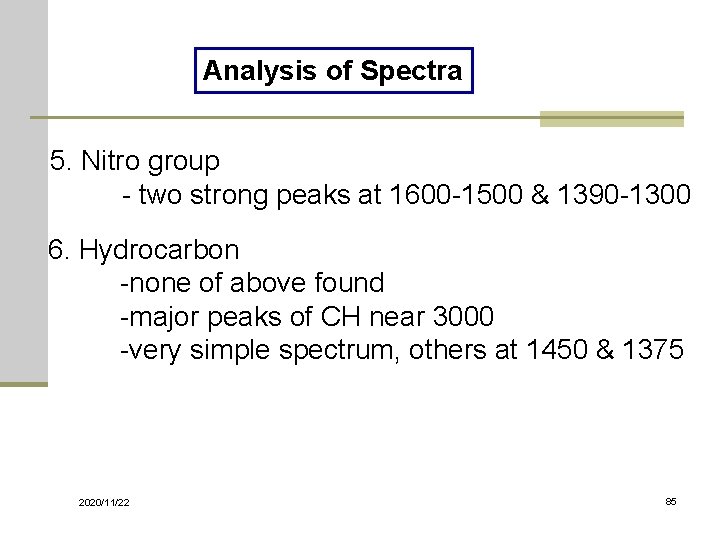
Analysis of Spectra 5. Nitro group - two strong peaks at 1600 -1500 & 1390 -1300 6. Hydrocarbon -none of above found -major peaks of CH near 3000 -very simple spectrum, others at 1450 & 1375 2020/11/22 85

Summary of IR Absorptions 2020/11/22 86

Characterization of IR Spectra 2020/11/22 87

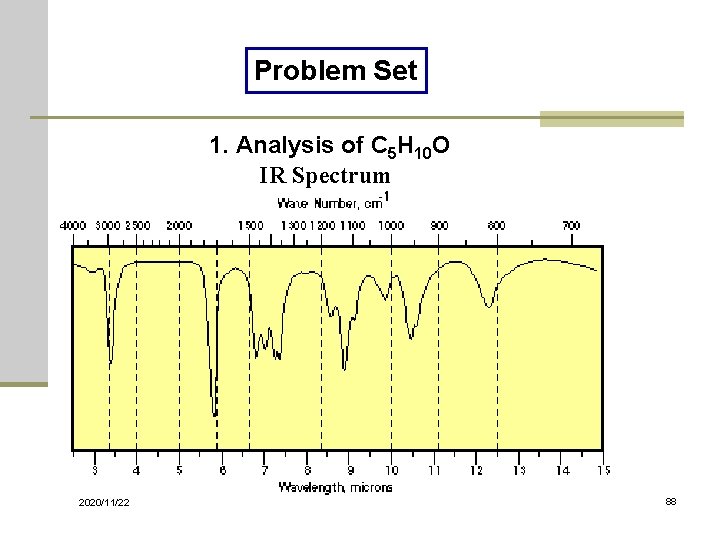
Problem Set 1. Analysis of C 5 H 10 O IR Spectrum 2020/11/22 88

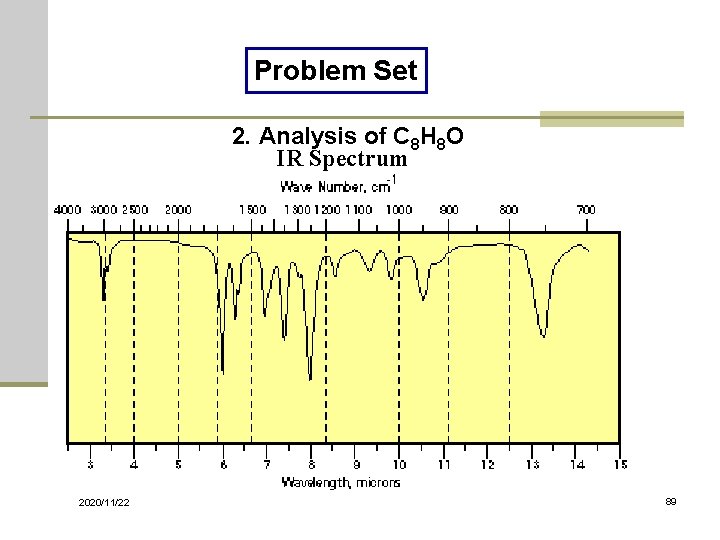
Problem Set 2. Analysis of C 8 H 8 O IR Spectrum 2020/11/22 89

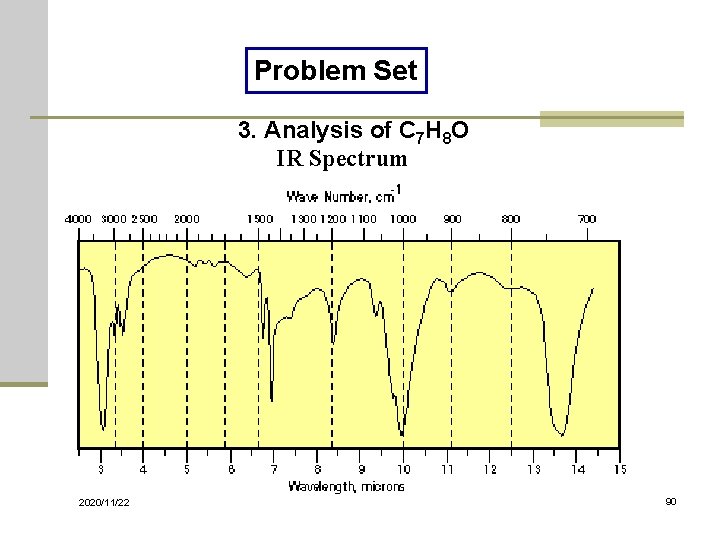
Problem Set 3. Analysis of C 7 H 8 O IR Spectrum 2020/11/22 90

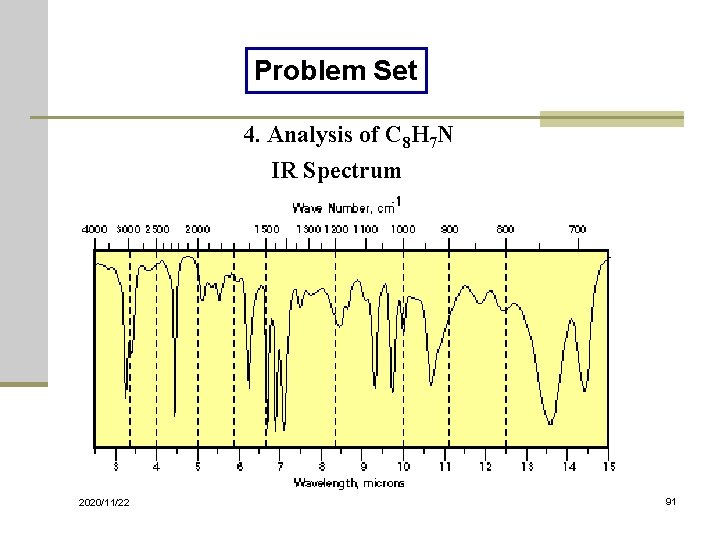
Problem Set 4. Analysis of C 8 H 7 N IR Spectrum 2020/11/22 91

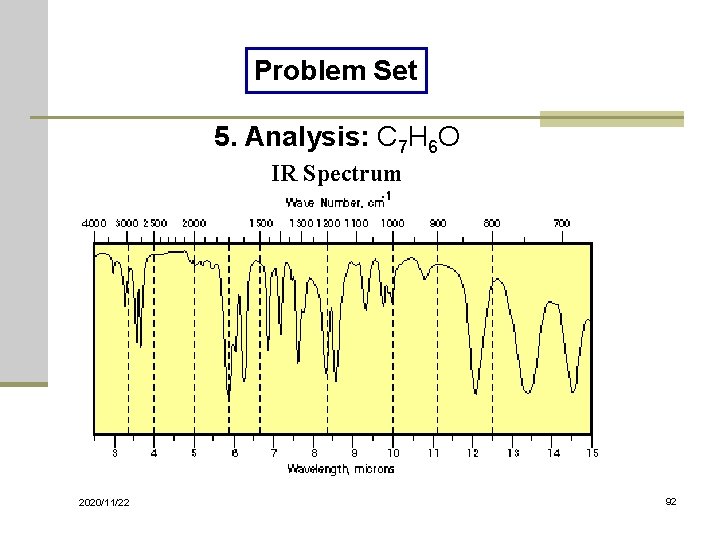
Problem Set 5. Analysis: C 7 H 6 O IR Spectrum 2020/11/22 92

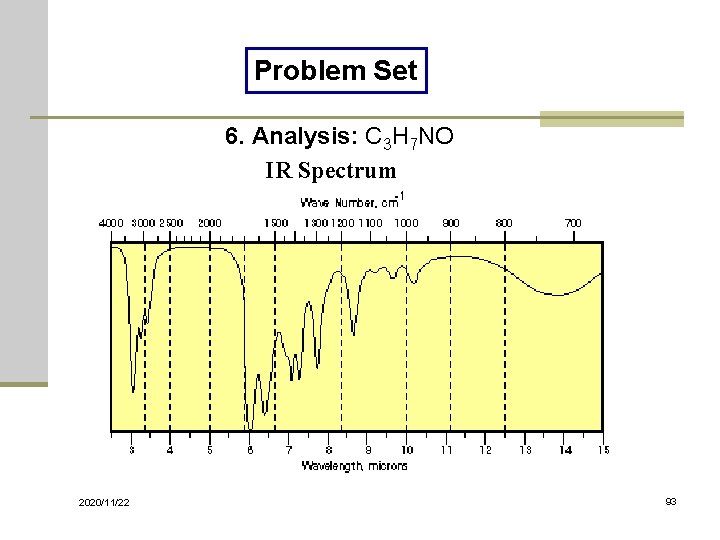
Problem Set 6. Analysis: C 3 H 7 NO IR Spectrum 2020/11/22 93

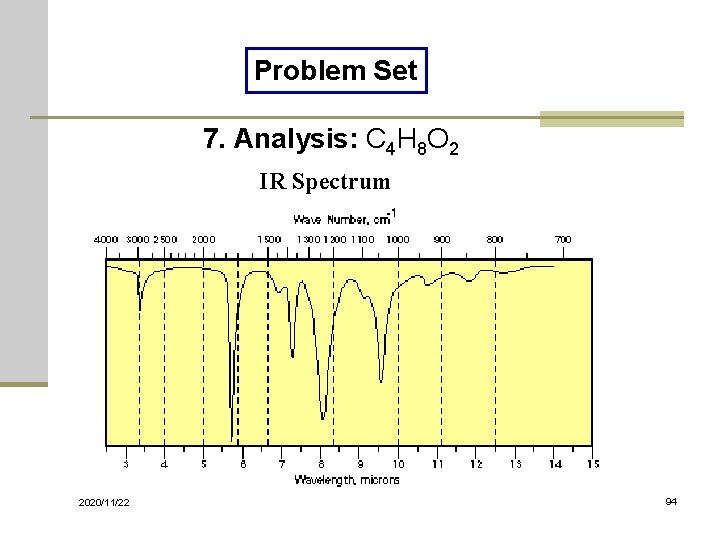
Problem Set 7. Analysis: C 4 H 8 O 2 IR Spectrum 2020/11/22 94

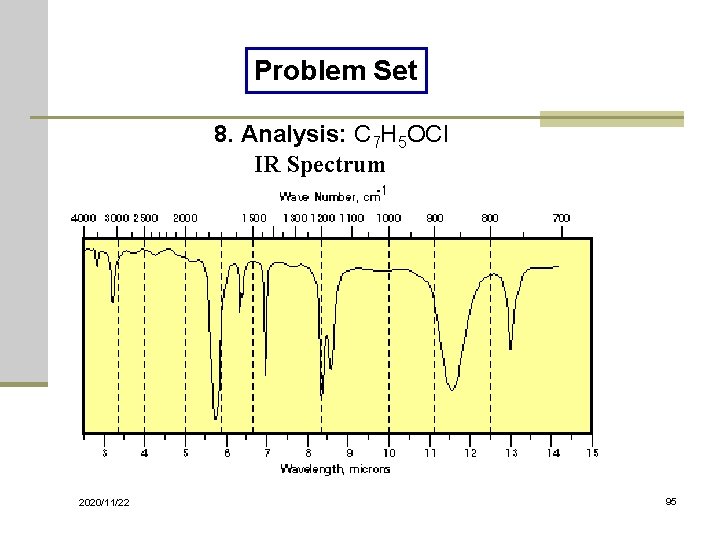
Problem Set 8. Analysis: C 7 H 5 OCl IR Spectrum 2020/11/22 95

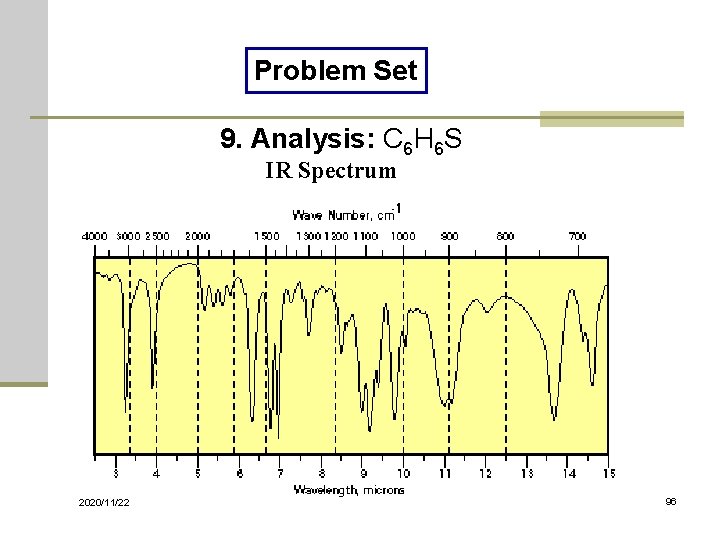
Problem Set 9. Analysis: C 6 H 6 S IR Spectrum 2020/11/22 96

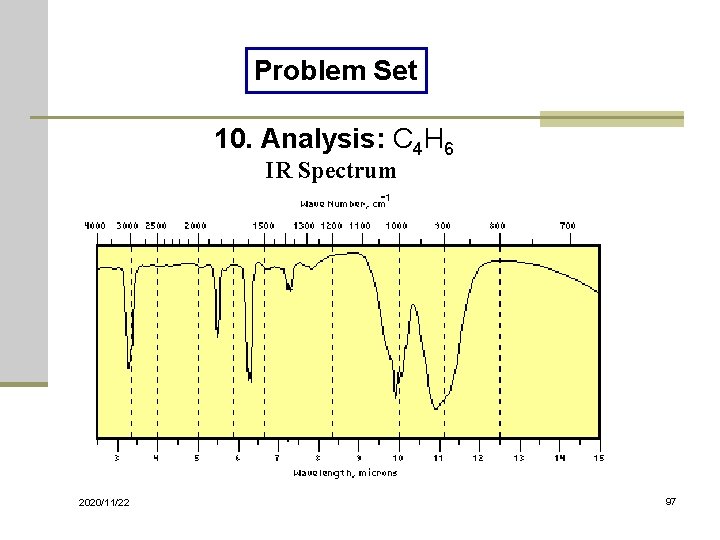
Problem Set 10. Analysis: C 4 H 6 IR Spectrum 2020/11/22 97

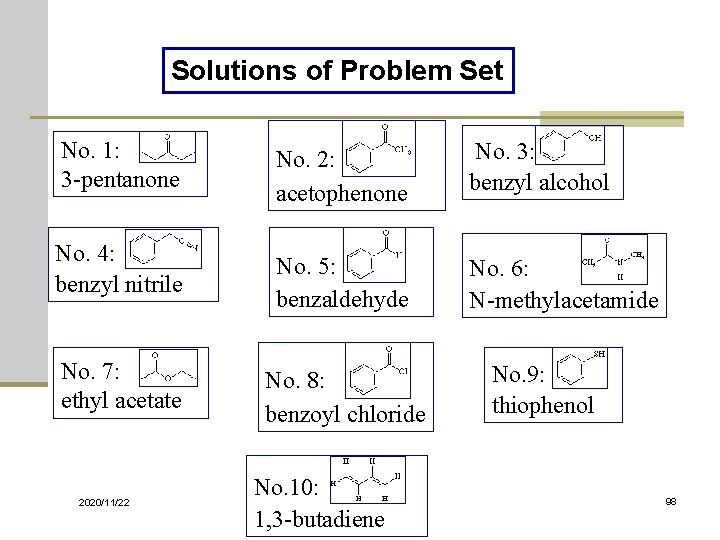
Solutions of Problem Set No. 1: 3 -pentanone No. 4: benzyl nitrile No. 7: ethyl acetate 2020/11/22 No. 2: acetophenone No. 5: benzaldehyde No. 8: benzoyl chloride No. 10: 1, 3 -butadiene No. 3: benzyl alcohol No. 6: N-methylacetamide No. 9: thiophenol 98

A Survey of the Important Functional Groups With Examples 2020/11/22 99

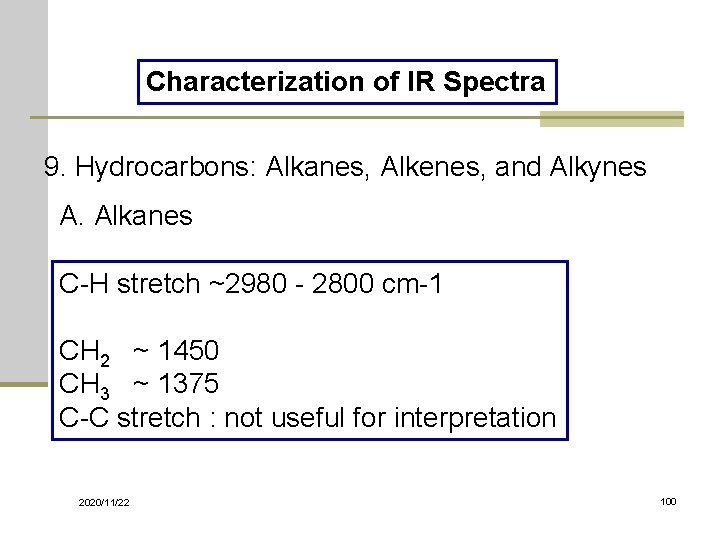
Characterization of IR Spectra 9. Hydrocarbons: Alkanes, Alkenes, and Alkynes A. Alkanes C-H stretch ~2980 - 2800 cm-1 CH 2 ~ 1450 CH 3 ~ 1375 C-C stretch : not useful for interpretation 2020/11/22 100

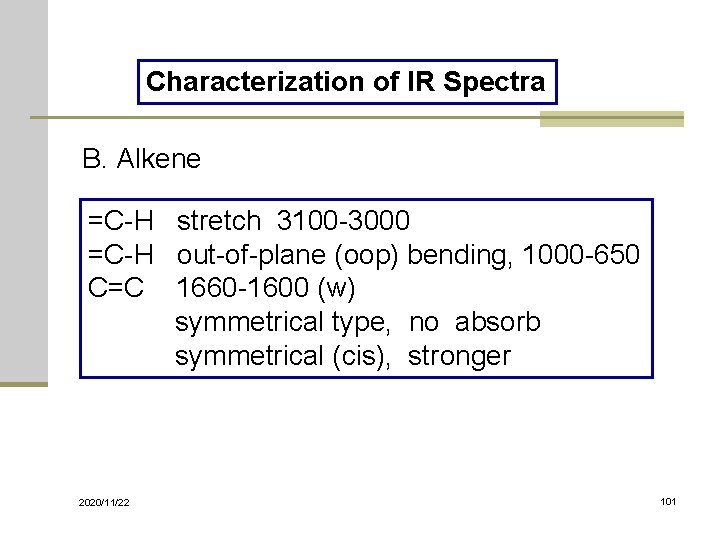
Characterization of IR Spectra B. Alkene =C-H stretch 3100 -3000 =C-H out-of-plane (oop) bending, 1000 -650 C=C 1660 -1600 (w) symmetrical type, no absorb symmetrical (cis), stronger 2020/11/22 101

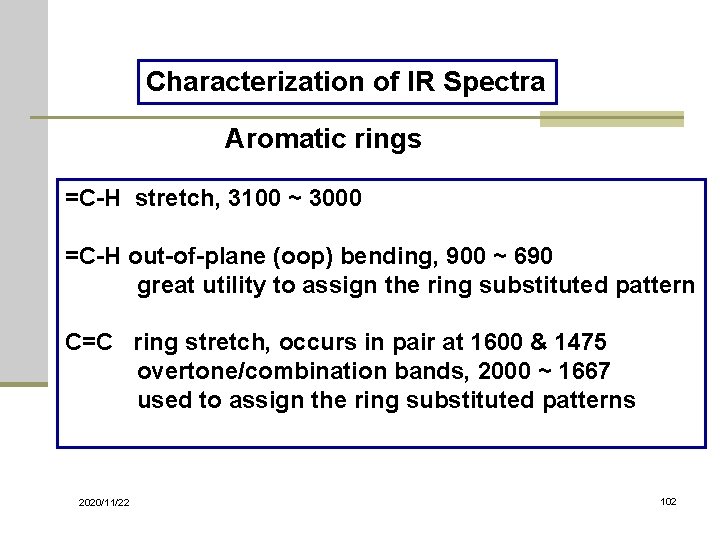
Characterization of IR Spectra Aromatic rings =C-H stretch, 3100 ~ 3000 =C-H out-of-plane (oop) bending, 900 ~ 690 great utility to assign the ring substituted pattern C=C ring stretch, occurs in pair at 1600 & 1475 overtone/combination bands, 2000 ~ 1667 used to assign the ring substituted patterns 2020/11/22 102

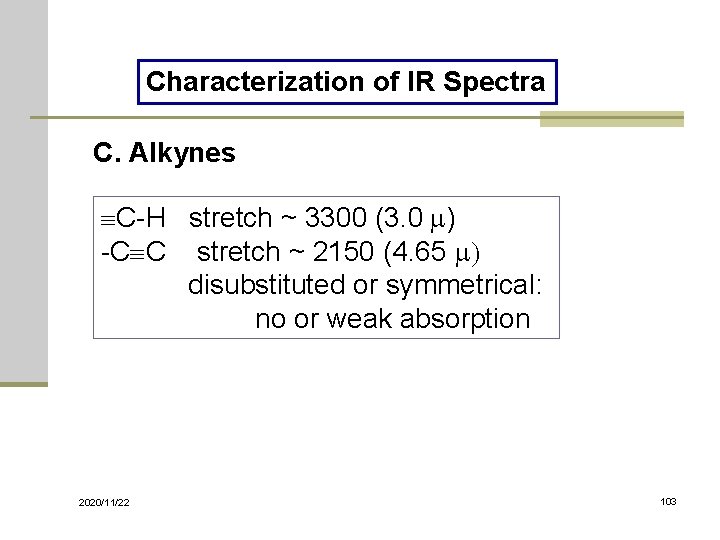
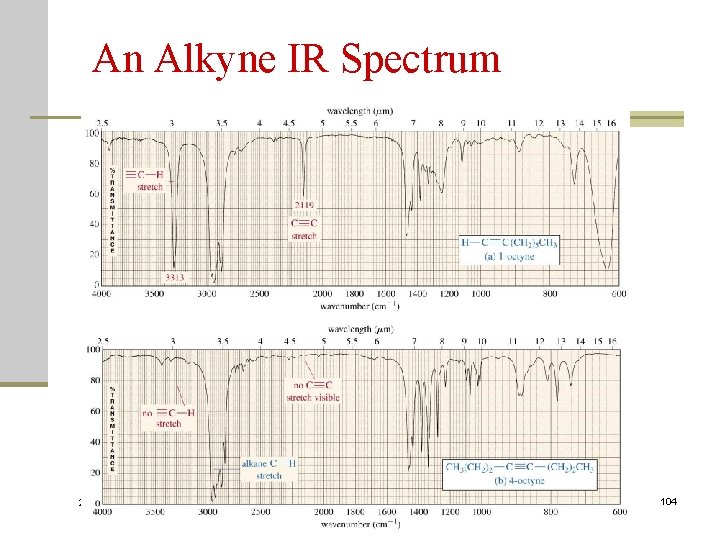
Characterization of IR Spectra C. Alkynes C-H stretch ~ 3300 (3. 0 m) -C C stretch ~ 2150 (4. 65 m) disubstituted or symmetrical: no or weak absorption 2020/11/22 103

An Alkyne IR Spectrum 2020/11/22 104

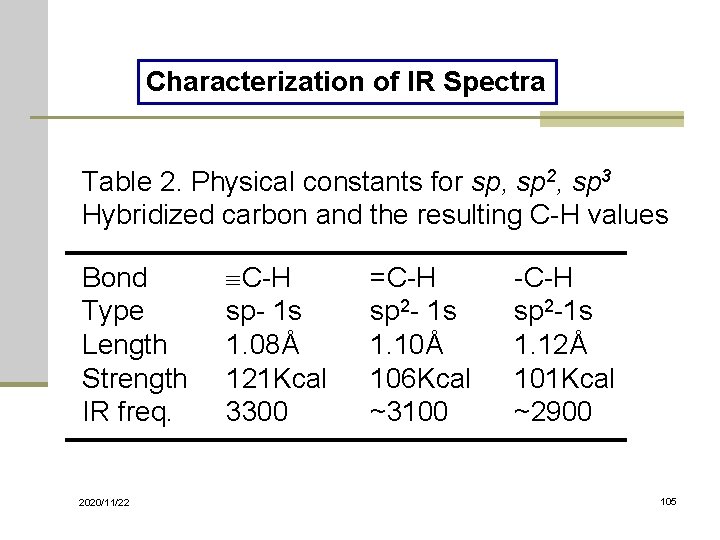
Characterization of IR Spectra Table 2. Physical constants for sp, sp 2, sp 3 Hybridized carbon and the resulting C-H values Bond Type Length Strength IR freq. 2020/11/22 C-H sp- 1 s 1. 08Å 121 Kcal 3300 =C-H sp 2 - 1 s 1. 10Å 106 Kcal ~3100 -C-H sp 2 -1 s 1. 12Å 101 Kcal ~2900 105

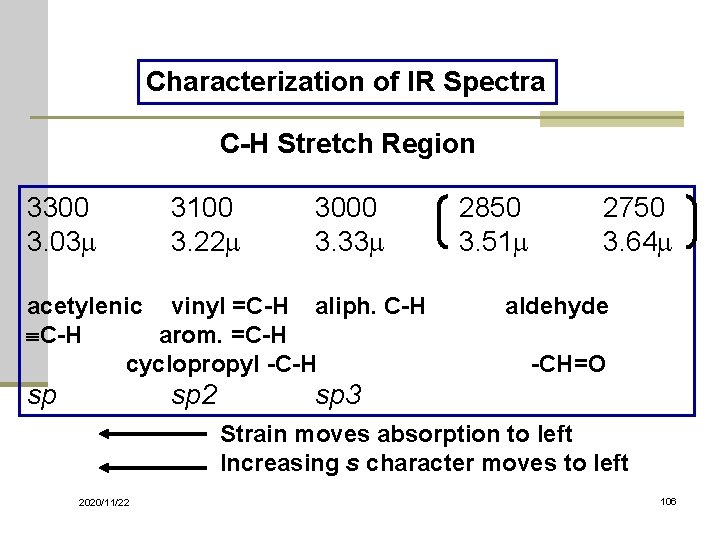
Characterization of IR Spectra C-H Stretch Region 3300 3. 03 m 3100 3. 22 m 3000 3. 33 m acetylenic vinyl =C-H aliph. C-H arom. =C-H cyclopropyl -C-H sp sp 2 2850 3. 51 m 2750 3. 64 m aldehyde -CH=O sp 3 Strain moves absorption to left Increasing s character moves to left 2020/11/22 106

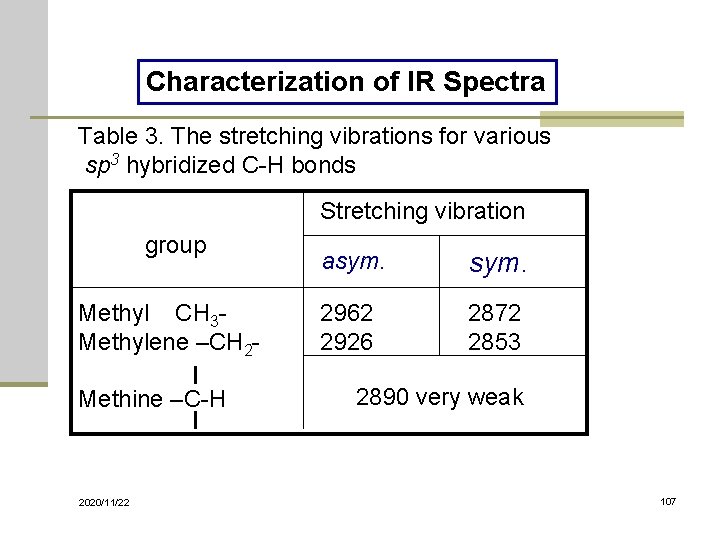
Characterization of IR Spectra Table 3. The stretching vibrations for various sp 3 hybridized C-H bonds Stretching vibration group Methyl CH 3 Methylene –CH 2 Methine –C-H 2020/11/22 asym. 2962 2926 2872 2853 2890 very weak 107

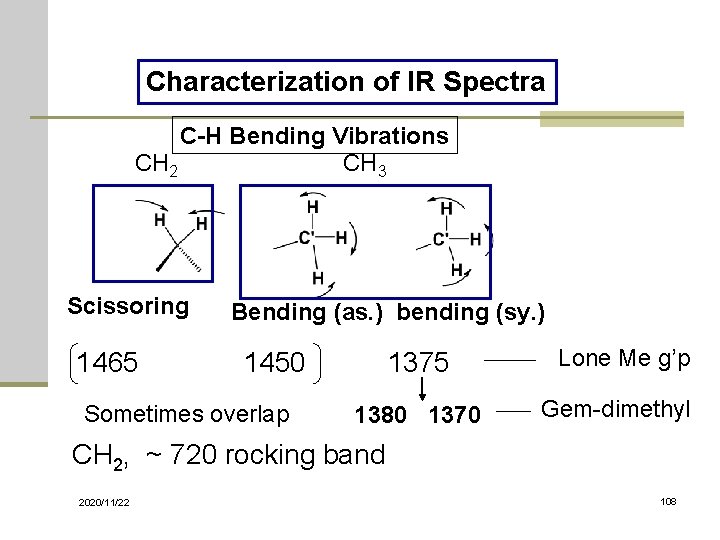
Characterization of IR Spectra C-H Bending Vibrations CH 2 CH 3 Scissoring 1465 Bending (as. ) bending (sy. ) 1450 Sometimes overlap 1375 1380 1370 Lone Me g’p Gem-dimethyl CH 2, ~ 720 rocking band 2020/11/22 108

Characterization of IR Spectra C=C Stretching Vibrations Conjugated effects. 1660 -1640 cm-1 Ring size effects. The frequency of (endo) double bonds in cyclic compounds is sensitive to ring size. 2020/11/22 Higher freq. 109

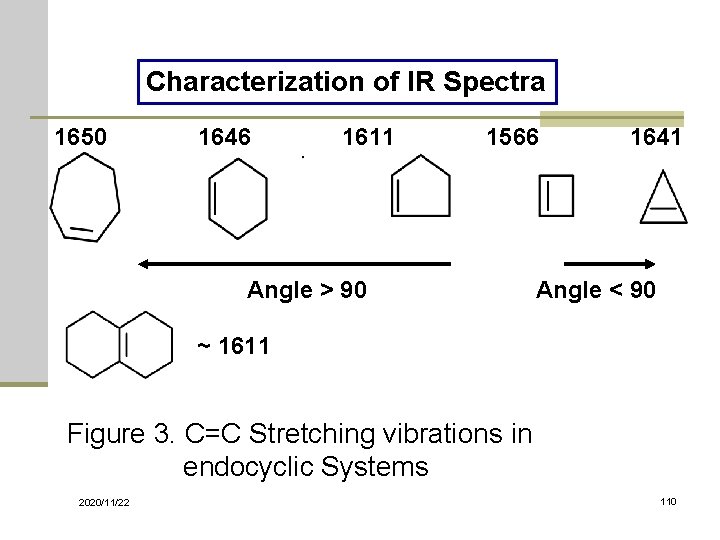
Characterization of IR Spectra 1650 1646 1611 1566 Angle > 90 1641 Angle < 90 ~ 1611 Figure 3. C=C Stretching vibrations in endocyclic Systems 2020/11/22 110

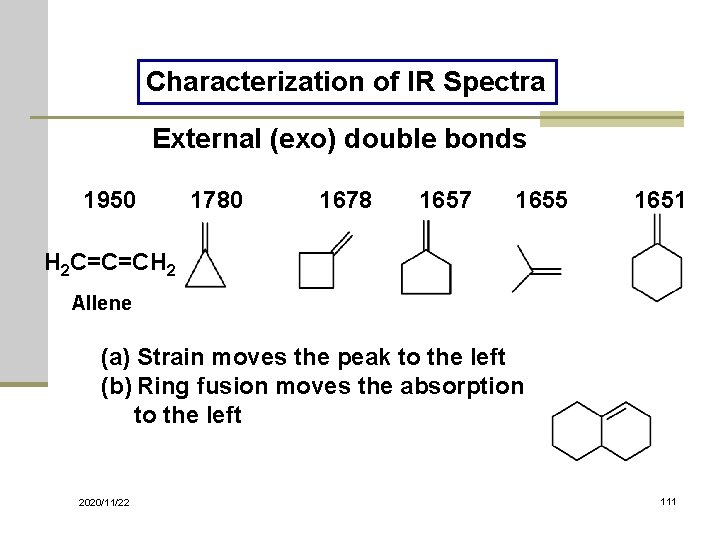
Characterization of IR Spectra External (exo) double bonds 1950 1780 1678 1657 1655 1651 H 2 C=C=CH 2 Allene (a) Strain moves the peak to the left (b) Ring fusion moves the absorption to the left 2020/11/22 111

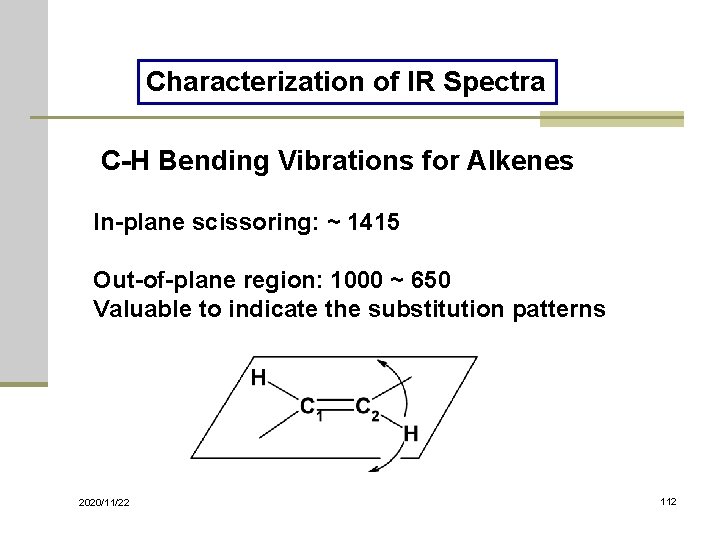
Characterization of IR Spectra C-H Bending Vibrations for Alkenes In-plane scissoring: ~ 1415 Out-of-plane region: 1000 ~ 650 Valuable to indicate the substitution patterns 2020/11/22 112

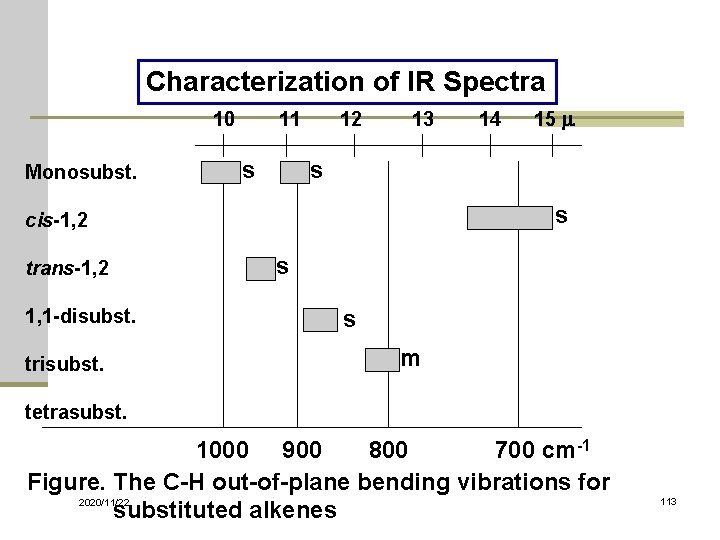
Characterization of IR Spectra 10 11 12 13 14 15 m Monosubst. s s s cis-1, 2 trans-1, 2 1, 1 -disubst. trisubst. s s m tetrasubst. 1000 900 800 700 cm-1 Figure. The C-H out-of-plane bending vibrations for 2020/11/22 substituted alkenes 113

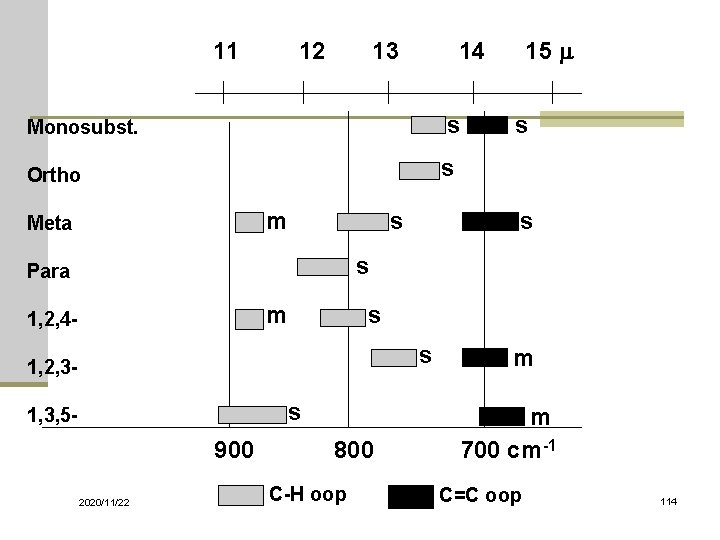
11 12 13 14 15 m Monosubst. s Ortho s m Meta s s Para m 1, 2, 4 - s s 1, 2, 3 - m s m 900 800 700 cm-1 1, 3, 5 - 2020/11/22 C-H oop C=C oop 114

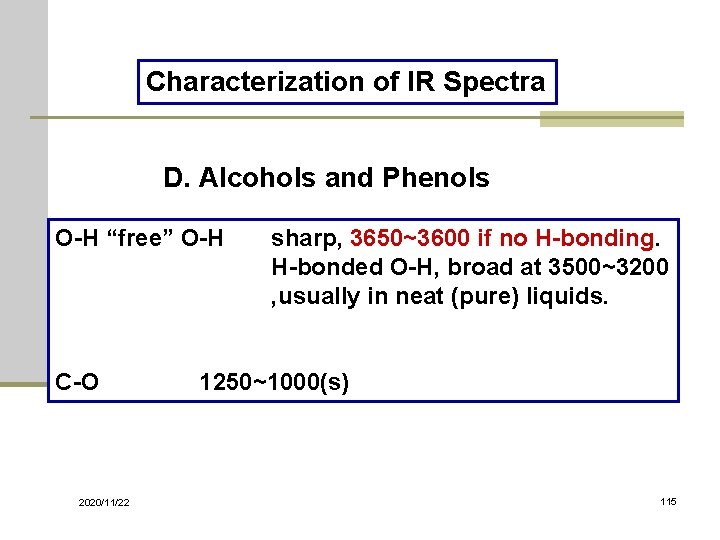
Characterization of IR Spectra D. Alcohols and Phenols O-H “free” O-H C-O 2020/11/22 sharp, 3650~3600 if no H-bonding. H-bonded O-H, broad at 3500~3200 , usually in neat (pure) liquids. 1250~1000(s) 115

An Alcohol IR Spectrum 2020/11/22 116

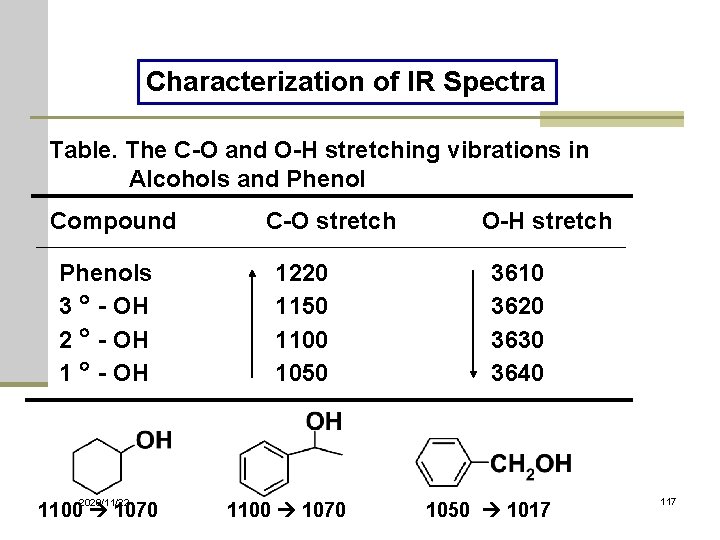
Characterization of IR Spectra Table. The C-O and O-H stretching vibrations in Alcohols and Phenol Compound Phenols 3 - OH 2 - OH 1 - OH 2020/11/22 1100 1070 C-O stretch 1220 1150 1100 1050 1100 1070 O-H stretch 3610 3620 3630 3640 1050 1017 117

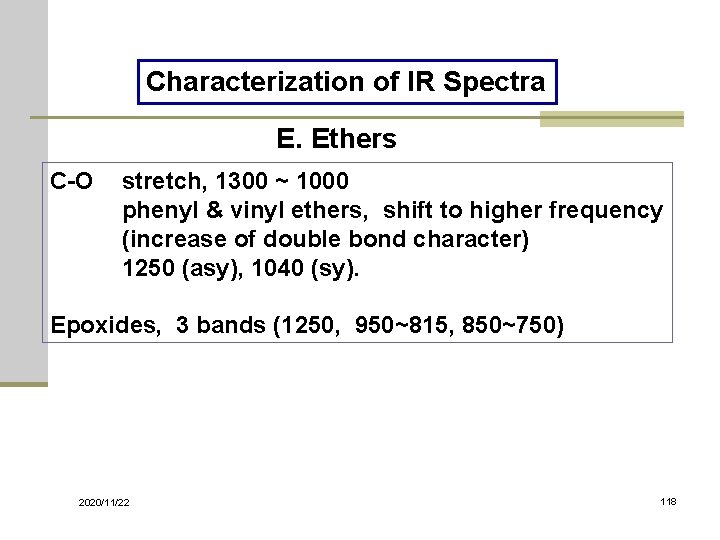
Characterization of IR Spectra E. Ethers C-O stretch, 1300 ~ 1000 phenyl & vinyl ethers, shift to higher frequency (increase of double bond character) 1250 (asy), 1040 (sy). Epoxides, 3 bands (1250, 950~815, 850~750) 2020/11/22 118

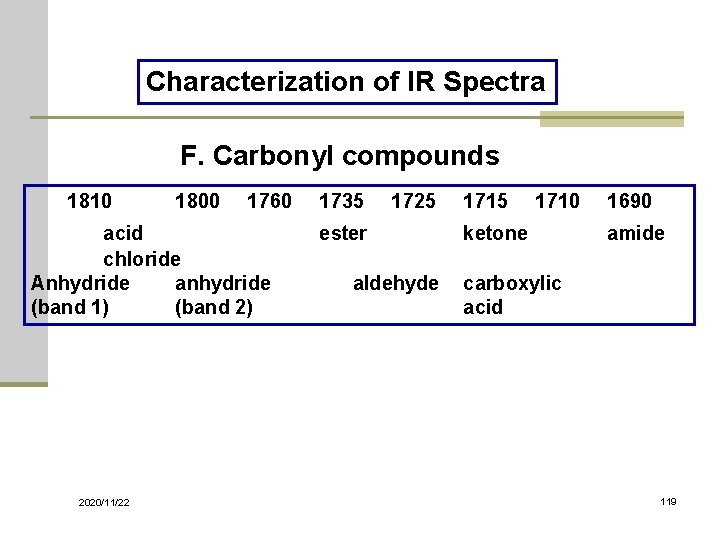
Characterization of IR Spectra F. Carbonyl compounds 1810 1800 1760 acid chloride Anhydride anhydride (band 1) (band 2) 2020/11/22 1735 1725 1710 ester ketone aldehyde carboxylic acid 1690 amide 119

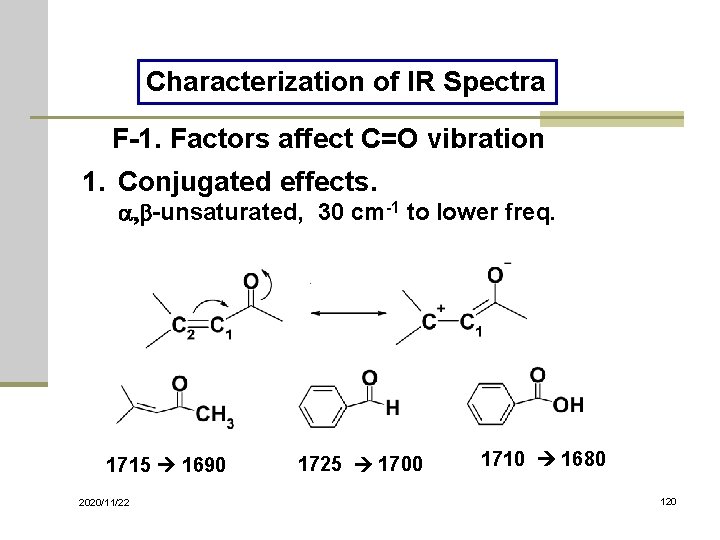
Characterization of IR Spectra F-1. Factors affect C=O vibration 1. Conjugated effects. a, b-unsaturated, 30 cm-1 to lower freq. 1715 1690 2020/11/22 1725 1700 1710 1680 120

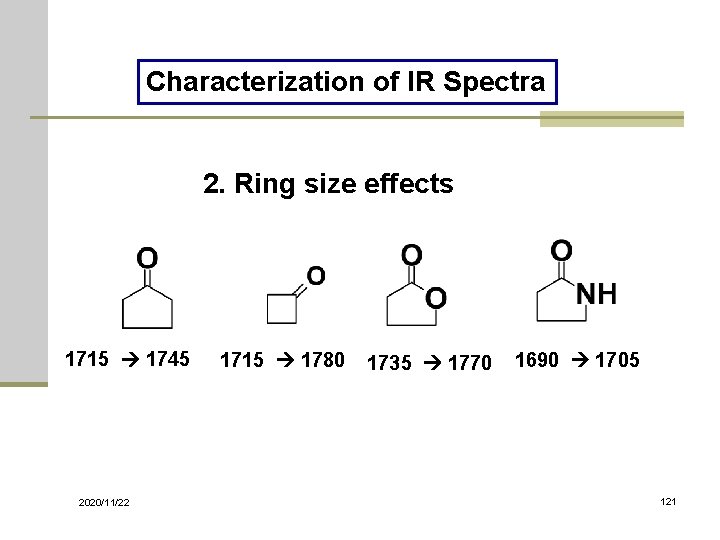
Characterization of IR Spectra 2. Ring size effects 1715 1745 2020/11/22 1715 1780 1735 1770 1690 1705 121

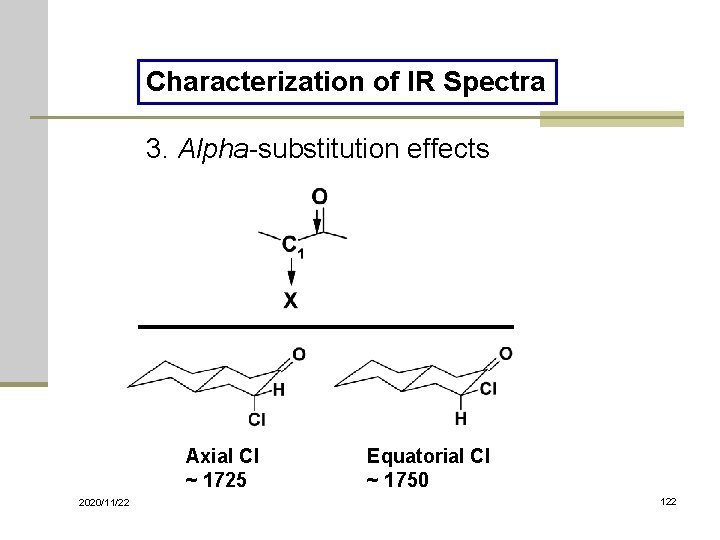
Characterization of IR Spectra 3. Alpha-substitution effects Axial Cl ~ 1725 2020/11/22 Equatorial Cl ~ 1750 122

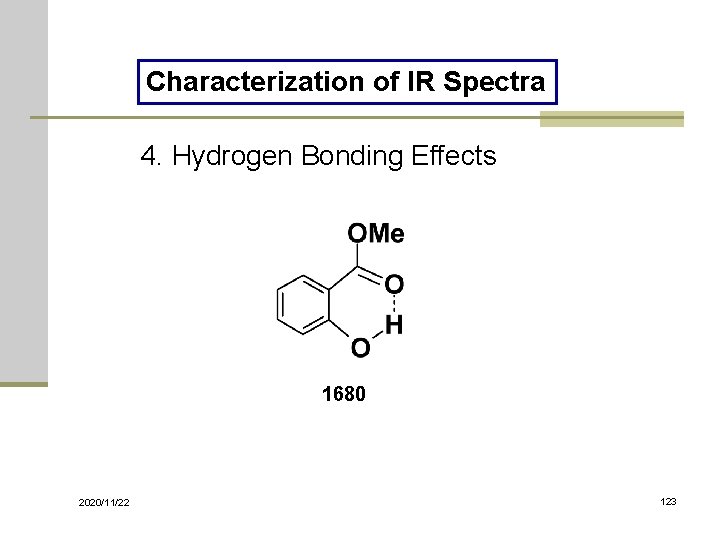
Characterization of IR Spectra 4. Hydrogen Bonding Effects 1680 2020/11/22 123

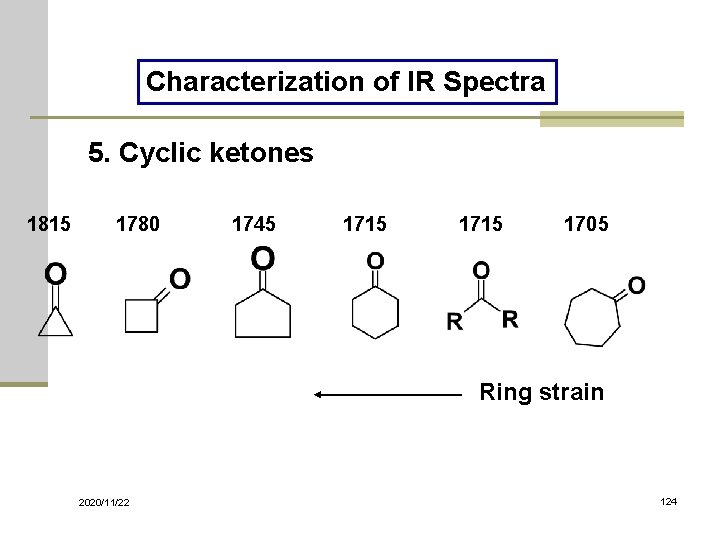
Characterization of IR Spectra 5. Cyclic ketones 1815 1780 1745 1715 1705 Ring strain 2020/11/22 124

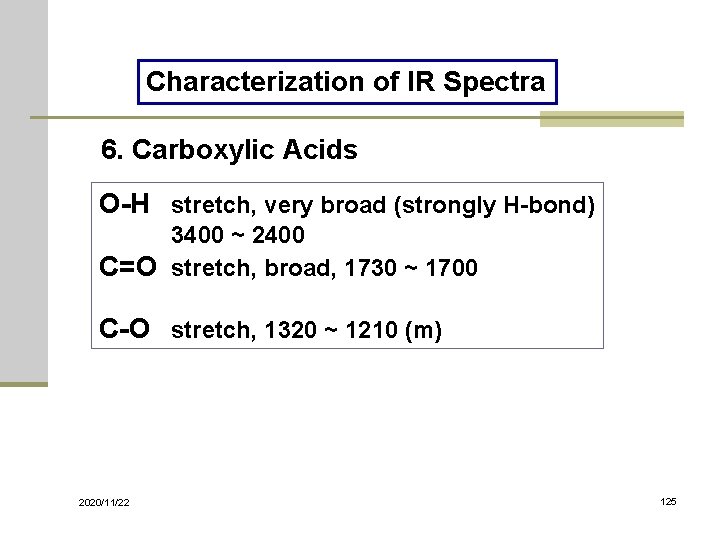
Characterization of IR Spectra 6. Carboxylic Acids O-H stretch, very broad (strongly H-bond) 3400 ~ 2400 C=O stretch, broad, 1730 ~ 1700 C-O stretch, 1320 ~ 1210 (m) 2020/11/22 125

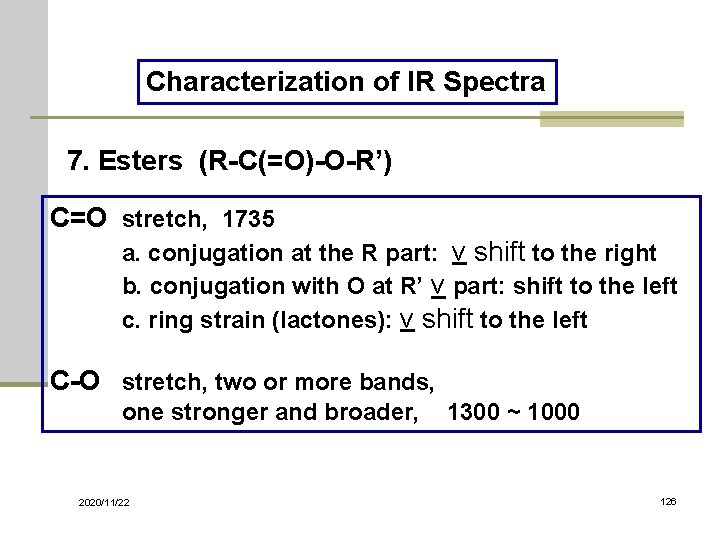
Characterization of IR Spectra 7. Esters (R-C(=O)-O-R’) C=O stretch, 1735 a. conjugation at the R part: ν shift to the right b. conjugation with O at R’ ν part: shift to the left c. ring strain (lactones): ν shift to the left C-O stretch, two or more bands, one stronger and broader, 1300 ~ 1000 2020/11/22 126

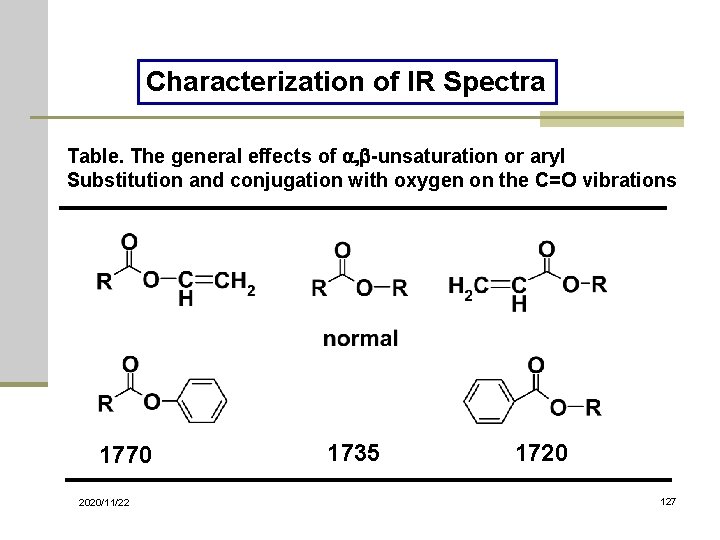
Characterization of IR Spectra Table. The general effects of a, b-unsaturation or aryl Substitution and conjugation with oxygen on the C=O vibrations 1770 2020/11/22 1735 1720 127

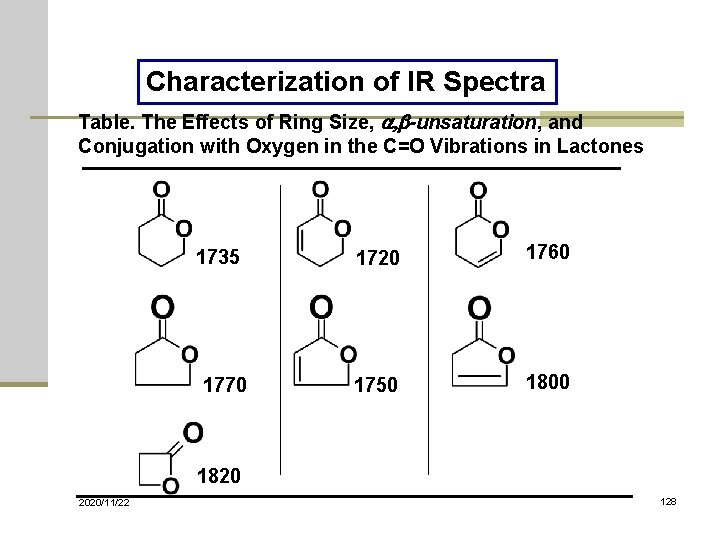
Characterization of IR Spectra Table. The Effects of Ring Size, a, b-unsaturation, and Conjugation with Oxygen in the C=O Vibrations in Lactones 1735 1770 1720 1760 1750 1800 1820 2020/11/22 128

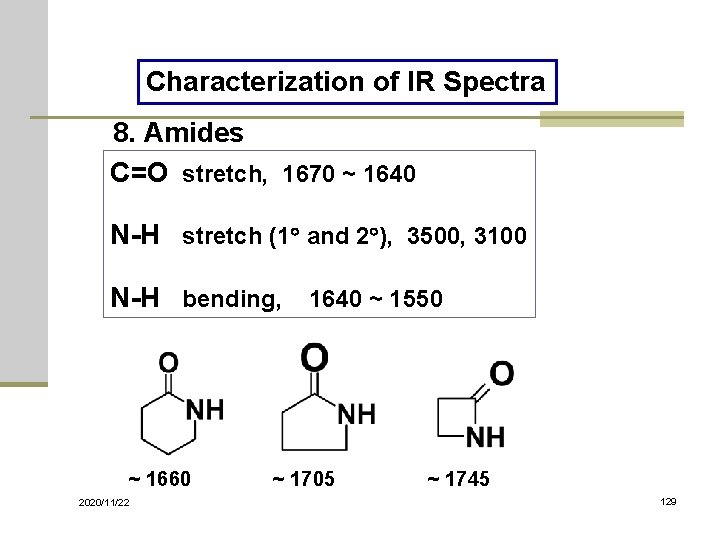
Characterization of IR Spectra 8. Amides C=O stretch, 1670 ~ 1640 N-H stretch (1 and 2 ), 3500, 3100 N-H bending, 1640 ~ 1550 ~ 1660 2020/11/22 ~ 1705 ~ 1745 129

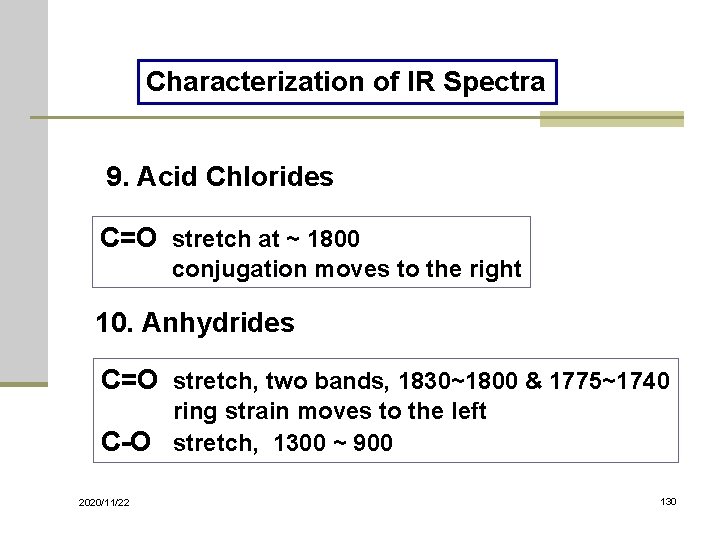
Characterization of IR Spectra 9. Acid Chlorides C=O stretch at ~ 1800 conjugation moves to the right 10. Anhydrides C=O stretch, two bands, 1830~1800 & 1775~1740 ring strain moves to the left C-O stretch, 1300 ~ 900 2020/11/22 130

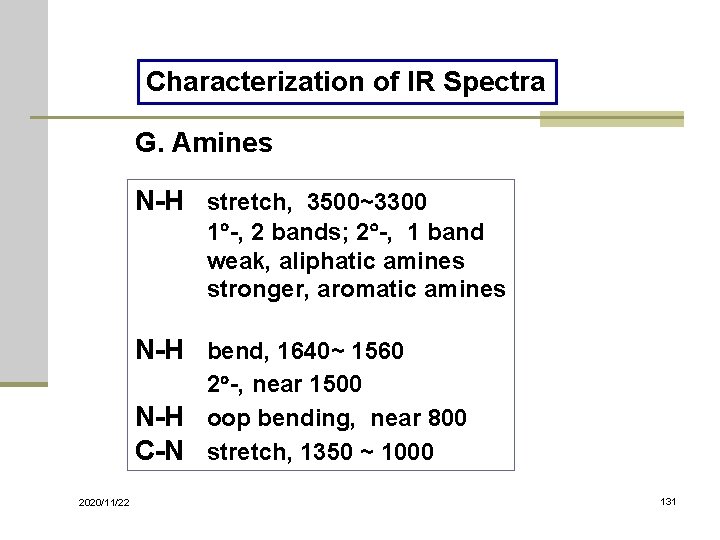
Characterization of IR Spectra G. Amines N-H stretch, 3500~3300 1 -, 2 bands; 2 -, 1 band weak, aliphatic amines stronger, aromatic amines N-H bend, 1640~ 1560 2 -, near 1500 N-H oop bending, near 800 C-N stretch, 1350 ~ 1000 2020/11/22 131

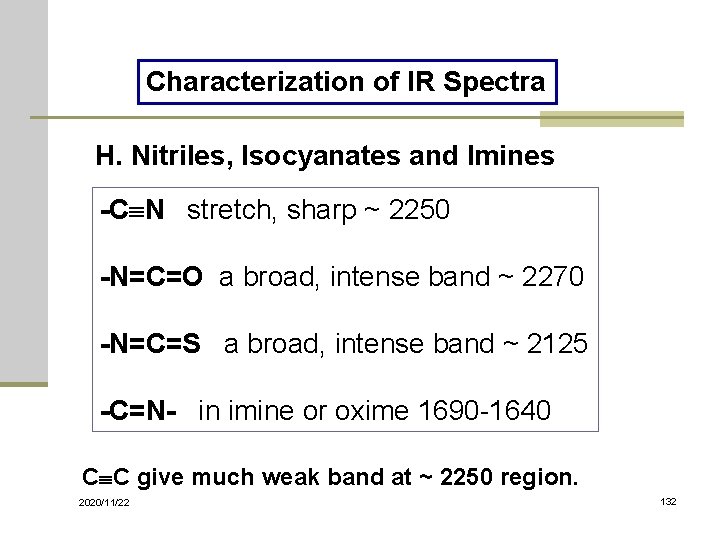
Characterization of IR Spectra H. Nitriles, Isocyanates and Imines -C N stretch, sharp ~ 2250 -N=C=O a broad, intense band ~ 2270 -N=C=S a broad, intense band ~ 2125 -C=N- in imine or oxime 1690 -1640 C C give much weak band at ~ 2250 region. 2020/11/22 132

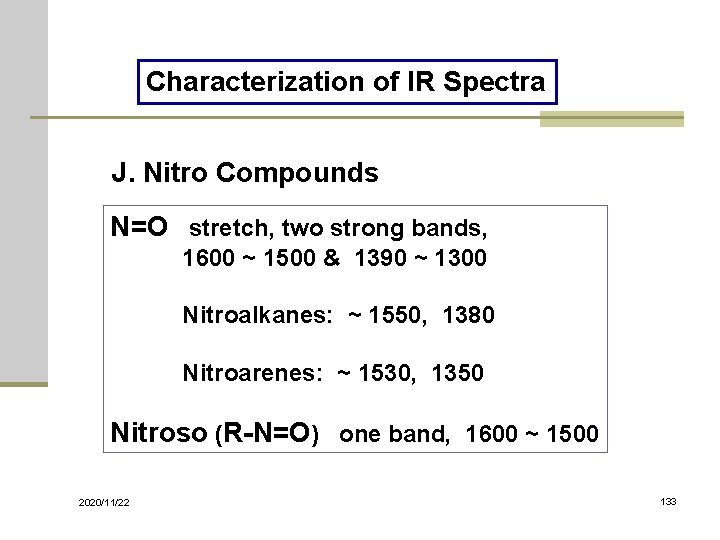
Characterization of IR Spectra J. Nitro Compounds N=O stretch, two strong bands, 1600 ~ 1500 & 1390 ~ 1300 Nitroalkanes: ~ 1550, 1380 Nitroarenes: ~ 1530, 1350 Nitroso (R-N=O) one band, 1600 ~ 1500 2020/11/22 133

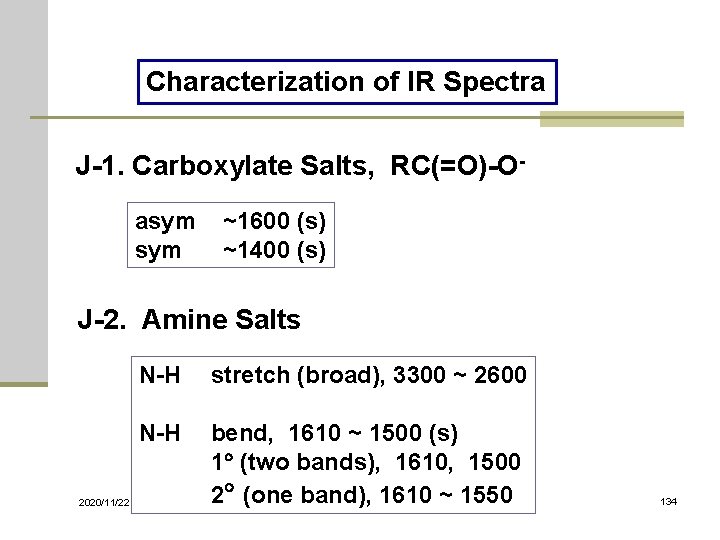
Characterization of IR Spectra J-1. Carboxylate Salts, RC(=O)-Oasym ~1600 (s) sym ~1400 (s) J-2. Amine Salts 2020/11/22 N-H stretch (broad), 3300 ~ 2600 N-H bend, 1610 ~ 1500 (s) 1 (two bands), 1610, 1500 2 (one band), 1610 ~ 1550 134

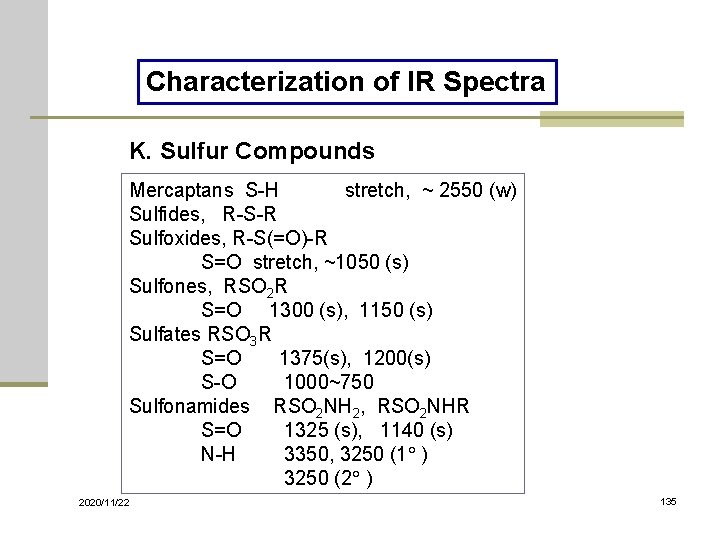
Characterization of IR Spectra K. Sulfur Compounds Mercaptans S-H stretch, ~ 2550 (w) Sulfides, R-S-R Sulfoxides, R-S(=O)-R S=O stretch, ~1050 (s) Sulfones, RSO 2 R S=O 1300 (s), 1150 (s) Sulfates RSO 3 R S=O 1375(s), 1200(s) S-O 1000~750 Sulfonamides RSO 2 NH 2, RSO 2 NHR S=O 1325 (s), 1140 (s) N-H 3350, 3250 (1 ) 3250 (2 ) 2020/11/22 135

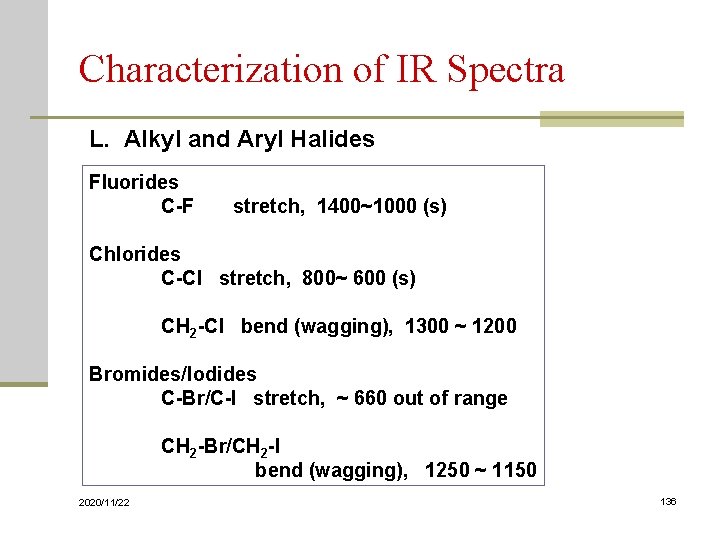
Characterization of IR Spectra L. Alkyl and Aryl Halides Fluorides C-F stretch, 1400~1000 (s) Chlorides C-Cl stretch, 800~ 600 (s) CH 2 -Cl bend (wagging), 1300 ~ 1200 Bromides/Iodides C-Br/C-I stretch, ~ 660 out of range CH 2 -Br/CH 2 -I bend (wagging), 1250 ~ 1150 2020/11/22 136

Infrared Sources and Transducers Infrared sources: An inert solid with continuous radiation at heated temperature: 1500 ~ 2000 K. Range of radiation intensity: 5000 ~ 670 cm-1 (2 ~ 15 m. M) 2020/11/22 137

1. The Nernst Glower temperature: 1200 ~ 2200 K 2. The Globar Source a silicon carbide rod electrically heated (1300 ~ 1500 K) 2020/11/22 138

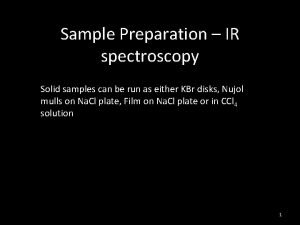
Sample Handling Techniques n Neat liquids: 一滴樣品夾於二鹽片(rock-salt plate)間(厚 度約為 ≦ 0. 01 mm), a neat spectrum obtained. 一般 僅適合用於定性的分析. n Solids: n n KBr pellet: 2 ~ 5 mg sample, mixing with 100~150 mg of powdered KBr, pressing with pressure of 5 ~ 10 Ton to give a transparent disc. n KBr is hygroscopic, weak OH band at 3450 cm-1 appeared Nujol mull: grinding the sample with mineral oil (Nujol) to create a suspension and placed between salt plates n Solutions: compound dissolved in common solvents, ex. CS 2, CCl 4, CHCl 3, dioxane, cyclohexane, benzene. 2020/11/22 139

2020/11/22 140

2020/11/22 141
 Near infrared spectroscopy instrumentation
Near infrared spectroscopy instrumentation Nitro group ir peak
Nitro group ir peak Infrared spectroscopy theory
Infrared spectroscopy theory Infrared spectroscopy ppt
Infrared spectroscopy ppt Spectroscopy infrared
Spectroscopy infrared Infrared vs bluetooth
Infrared vs bluetooth Infrared vs bluetooth
Infrared vs bluetooth Pengertian infrared
Pengertian infrared Bill nye light and optics
Bill nye light and optics Characteristic of infrared waves
Characteristic of infrared waves Infrared spektroskopisi
Infrared spektroskopisi What is an infrared thermometer best used for servsafe
What is an infrared thermometer best used for servsafe Shortwave infrared camera
Shortwave infrared camera Bluetooth vs infrared
Bluetooth vs infrared Manufacturer infrared heating
Manufacturer infrared heating Herschel infrared discovery
Herschel infrared discovery Thermal infrared
Thermal infrared Drainage network
Drainage network Advantage and disadvantage of touch screen
Advantage and disadvantage of touch screen Nirops
Nirops Wide field infrared survey explorer
Wide field infrared survey explorer Electro magnetic scale
Electro magnetic scale Infrared radiation hazards
Infrared radiation hazards Infrared sensor principle
Infrared sensor principle Infrared building envelope
Infrared building envelope Made up school
Made up school Catalytic heater oil and gas
Catalytic heater oil and gas Introduction to molecular spectroscopy
Introduction to molecular spectroscopy Charge d'un électron
Charge d'un électron Body paragraph
Body paragraph What is spectroscopy
What is spectroscopy Woodward fieser rules examples
Woodward fieser rules examples Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Definition of ir spectroscopy
Definition of ir spectroscopy Absorbance chemistry
Absorbance chemistry Sample holder in ir spectroscopy
Sample holder in ir spectroscopy Pengertian ssa
Pengertian ssa What is the difference between ftir and raman spectroscopy
What is the difference between ftir and raman spectroscopy Difference between ir and raman spectroscopy
Difference between ir and raman spectroscopy Nmr associates
Nmr associates Objectives of spectroscopy
Objectives of spectroscopy Pes spectroscopy
Pes spectroscopy Stretching and bending vibrations in ir spectroscopy
Stretching and bending vibrations in ir spectroscopy Mass spectroscopy principle
Mass spectroscopy principle Advantages of nmr spectroscopy
Advantages of nmr spectroscopy Gross and specific selection rules
Gross and specific selection rules Introduction to spectrophotometry
Introduction to spectrophotometry Pes spectrum for magnesium
Pes spectrum for magnesium Dept nmr spectroscopy
Dept nmr spectroscopy Low na
Low na Ortec renaissance
Ortec renaissance Periklis papadopoulos
Periklis papadopoulos Application for ir spectroscopy
Application for ir spectroscopy Stretching and bending vibrations in ir spectroscopy
Stretching and bending vibrations in ir spectroscopy Interferogram
Interferogram Components of flame photometer
Components of flame photometer Dispersive ir spectroscopy
Dispersive ir spectroscopy C triple bond n ir spectra
C triple bond n ir spectra Theory of atomic absorption spectroscopy
Theory of atomic absorption spectroscopy Principle of fluorescence spectroscopy
Principle of fluorescence spectroscopy Atomic emission spectroscopy ppt
Atomic emission spectroscopy ppt Atomic emission spectroscopy principle
Atomic emission spectroscopy principle Principle of atomic absorption
Principle of atomic absorption Nebulizer in aas
Nebulizer in aas Applications of uv visible spectroscopy
Applications of uv visible spectroscopy Spectroscopy ap chem
Spectroscopy ap chem Photoelectron spectroscopy ap chemistry
Photoelectron spectroscopy ap chemistry How is beer-lambert law used in spectroscopy
How is beer-lambert law used in spectroscopy Spectroscopy definition
Spectroscopy definition Lambert beer law and its limitations
Lambert beer law and its limitations Auxochrome in uv spectroscopy
Auxochrome in uv spectroscopy Beer lambert law definition
Beer lambert law definition Application of woodward fieser rule
Application of woodward fieser rule Selection rules for raman spectroscopy
Selection rules for raman spectroscopy Props wikipedia
Props wikipedia Uv vis spectroscopy in pharmaceutical analysis
Uv vis spectroscopy in pharmaceutical analysis Laser diffraction spectroscopy
Laser diffraction spectroscopy Principle of mossbauer spectroscopy
Principle of mossbauer spectroscopy Application of rotational spectroscopy
Application of rotational spectroscopy Rotational spectroscopy
Rotational spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Gas separation
Gas separation Hook's law describes the relationship between:
Hook's law describes the relationship between: Chirped pulse fourier transform microwave spectroscopy
Chirped pulse fourier transform microwave spectroscopy Erzeng
Erzeng Jpl molecular spectroscopy
Jpl molecular spectroscopy Impedance definition
Impedance definition Applications of atomic emission spectroscopy
Applications of atomic emission spectroscopy Interference of atomic absorption spectroscopy
Interference of atomic absorption spectroscopy Flame aas
Flame aas Spectroscopy jokes
Spectroscopy jokes Vernier spectral analysis
Vernier spectral analysis Spectroscopy problem set
Spectroscopy problem set Use of raman spectroscopy
Use of raman spectroscopy Ir spectroscopy provides information about
Ir spectroscopy provides information about Conjugation in spectroscopy
Conjugation in spectroscopy Fes spectroscopy
Fes spectroscopy Atomic fluorescence spectroscopy principle
Atomic fluorescence spectroscopy principle Atomic emission spectroscopy lecture notes
Atomic emission spectroscopy lecture notes Application of atomic absorption spectroscopy
Application of atomic absorption spectroscopy Kaiser raman probe
Kaiser raman probe Absorbtion spectroscopy
Absorbtion spectroscopy Spectroscopy
Spectroscopy Slit spectroscopy
Slit spectroscopy Slit spectroscopy
Slit spectroscopy Mossbauer spectroscopy
Mossbauer spectroscopy Mossbauer spectroscopy
Mossbauer spectroscopy Spectroscopy
Spectroscopy Mossbauer spectroscopy
Mossbauer spectroscopy Mossbauer spectroscopy
Mossbauer spectroscopy Photoluminescence spectroscopy
Photoluminescence spectroscopy Spectroscopy
Spectroscopy Mossbauer spectroscopy
Mossbauer spectroscopy Bu organic chemistry
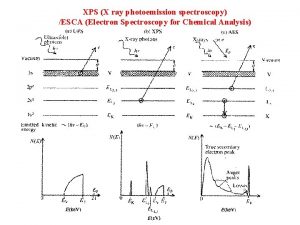
Bu organic chemistry Xray photoelectron spectroscopy
Xray photoelectron spectroscopy Spectroscopy
Spectroscopy Raman spectroscopy disadvantages
Raman spectroscopy disadvantages Ir spectroscopy
Ir spectroscopy P-diethylbenzene nmr
P-diethylbenzene nmr Non rigid rotator
Non rigid rotator Rotational spectral lines
Rotational spectral lines Benzene and acetone deviation
Benzene and acetone deviation Principle of absorption
Principle of absorption Absorption equation
Absorption equation Nmr spectroscopy
Nmr spectroscopy Factors affecting chemical shift
Factors affecting chemical shift Larmor frequency
Larmor frequency Microwave spectroscopy definition
Microwave spectroscopy definition Spectroscopy types
Spectroscopy types Isms2017
Isms2017 Ir spectroscopy
Ir spectroscopy Carboxylic acid ir
Carboxylic acid ir Spectroscopy
Spectroscopy Ir spectra chart
Ir spectra chart Rotational spectroscopy notes
Rotational spectroscopy notes Explain quantum yield
Explain quantum yield Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy Reflectance spectroscopy
Reflectance spectroscopy Machine learning in astronomy
Machine learning in astronomy Spatially resolved acoustic spectroscopy
Spatially resolved acoustic spectroscopy International symposium on molecular spectroscopy
International symposium on molecular spectroscopy Principles of fluorescence spectroscopy
Principles of fluorescence spectroscopy