Lecture 4 Intro to Spectroscopy Ch 12 Spectral

- Slides: 13

Lecture 4 • • Intro to Spectroscopy Ch 12: Spectral Unknown HDI (Hydrogen Deficiency Index) Lecture Problem 1 Due This week in lab: Ch 4: Recrystallization & Melting Point Procedures 1 & 2 Ch 4 Pre. Lab Due Quiz 1 Next week in lab: • Ch 4: Procedures 3 & 5 • Ch 5: Extraction, Procedure 1 • Ch 5 Pre. Lab Due (Common Shelf Chemical Data Table!) & Quiz 2

Organic Chemistry: From Yesterday to Today Late 1700’s: Atomic Theory 1800’s: Organic Structural Theory - Combustion Analysis - Functional Group Tests 1900’s: Synthesis and Analysis Today: Automated Synthesis & Spectroscopic Analysis

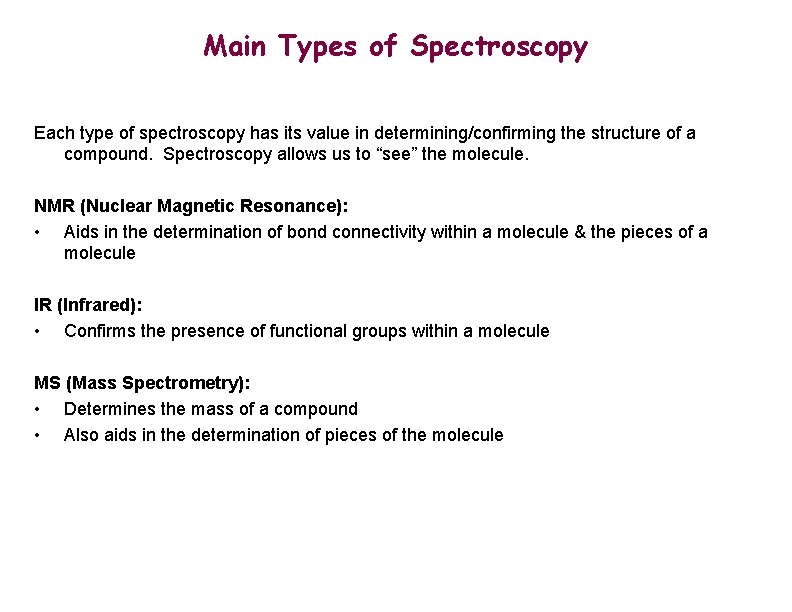
Main Types of Spectroscopy Each type of spectroscopy has its value in determining/confirming the structure of a compound. Spectroscopy allows us to “see” the molecule. NMR (Nuclear Magnetic Resonance): • Aids in the determination of bond connectivity within a molecule & the pieces of a molecule IR (Infrared): • Confirms the presence of functional groups within a molecule MS (Mass Spectrometry): • Determines the mass of a compound • Also aids in the determination of pieces of the molecule

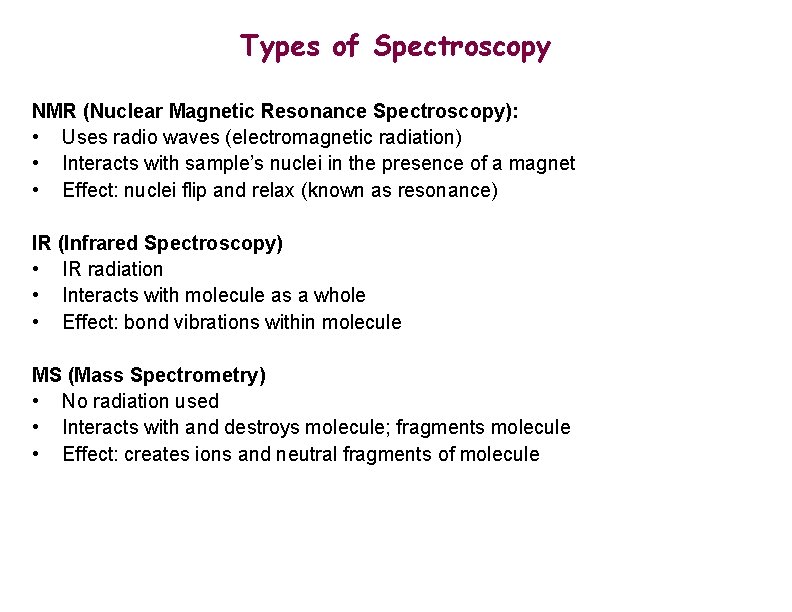
Types of Spectroscopy NMR (Nuclear Magnetic Resonance Spectroscopy): • Uses radio waves (electromagnetic radiation) • Interacts with sample’s nuclei in the presence of a magnet • Effect: nuclei flip and relax (known as resonance) IR (Infrared Spectroscopy) • IR radiation • Interacts with molecule as a whole • Effect: bond vibrations within molecule MS (Mass Spectrometry) • No radiation used • Interacts with and destroys molecule; fragments molecule • Effect: creates ions and neutral fragments of molecule

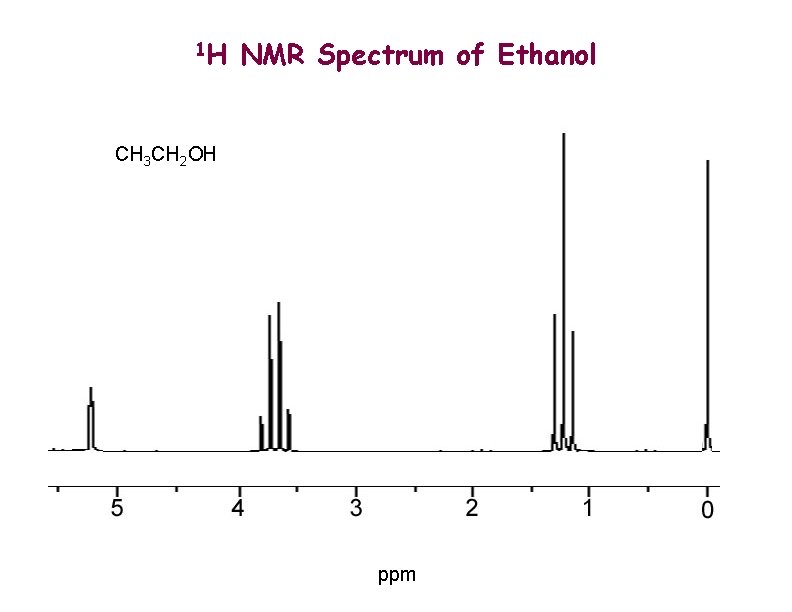
1 H NMR Spectrum of Ethanol CH 3 CH 2 OH ppm

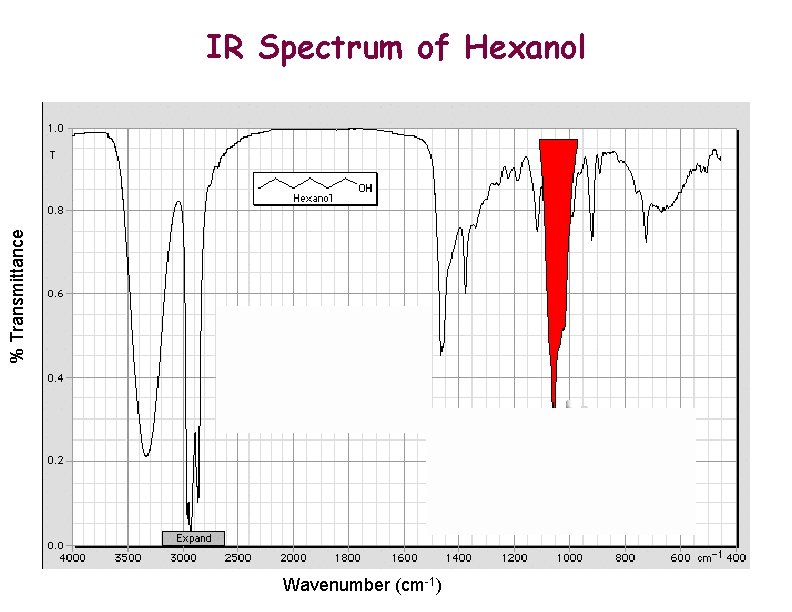
% Transmittance IR Spectrum of Hexanol Wavenumber (cm-1)

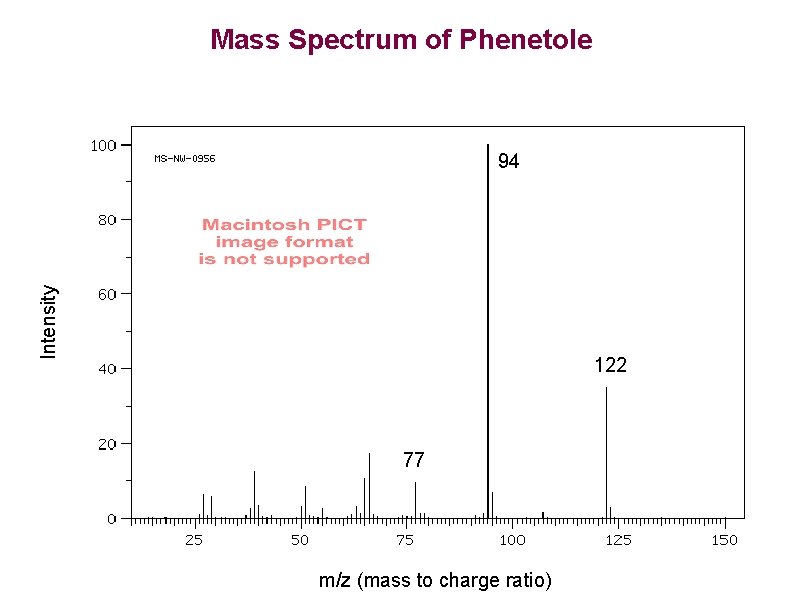
Mass Spectrum of Phenetole Intensity 94 122 77 m/z (mass to charge ratio)

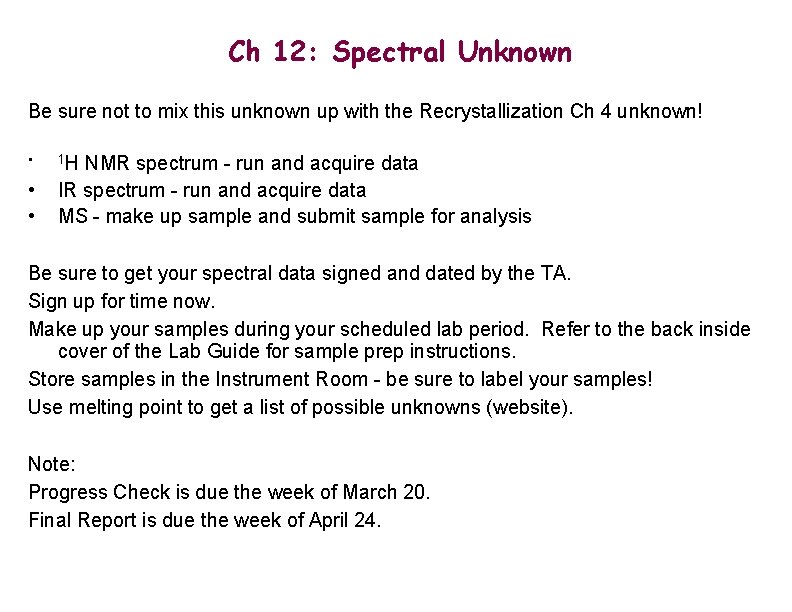
Ch 12: Spectral Unknown Be sure not to mix this unknown up with the Recrystallization Ch 4 unknown! • • • 1 H NMR spectrum - run and acquire data IR spectrum - run and acquire data MS - make up sample and submit sample for analysis Be sure to get your spectral data signed and dated by the TA. Sign up for time now. Make up your samples during your scheduled lab period. Refer to the back inside cover of the Lab Guide for sample prep instructions. Store samples in the Instrument Room - be sure to label your samples! Use melting point to get a list of possible unknowns (website). Note: Progress Check is due the week of March 20. Final Report is due the week of April 24.

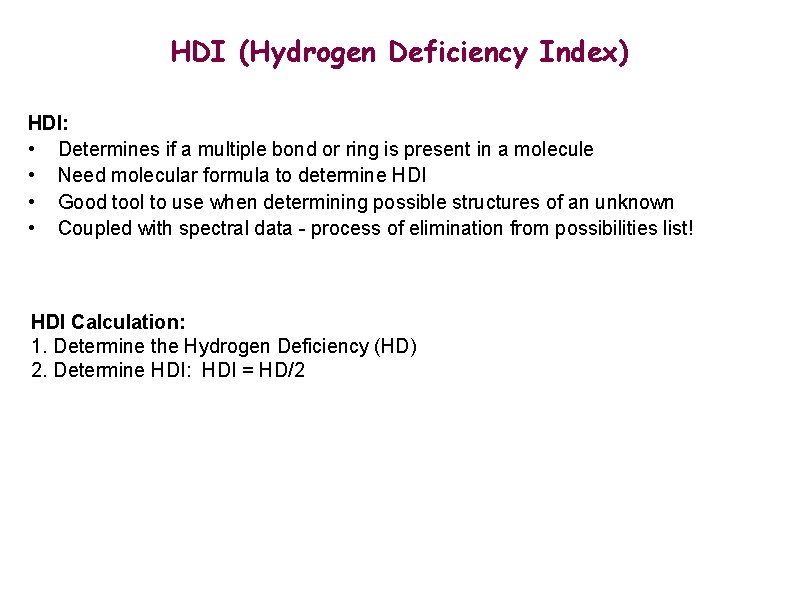
HDI (Hydrogen Deficiency Index) HDI: • Determines if a multiple bond or ring is present in a molecule • Need molecular formula to determine HDI • Good tool to use when determining possible structures of an unknown • Coupled with spectral data - process of elimination from possibilities list! HDI Calculation: 1. Determine the Hydrogen Deficiency (HD) 2. Determine HDI: HDI = HD/2

Hydrogen Deficiency Hydrocarbons (C & H): Example: Determine the HDI of a compound whose MF is C 5 H 10. What are possible structures for this compound? Calculation (in class):

HDI Hydrocarbons (C & H): Example: Determine the HDI of a compound whose MF is C 5 H 10. What are possible structures for this compound? The possible structures of the compound are:

Nitrogen and HDI Nitrogen Organic Compounds (C, H & N) Example: Given a MF of C 4 H 7 N, what is the HDI of this compound? Rule: Count N as NH and rewrite formula: C 4 H 6 NH Calculation (in class): Possible Compounds:

Oxygen and HDI Oxygen Organic Compounds (C, H & O) Example: Given a MF of C 6 H 12 O, what is the HDI of this compound? Rule: MF stays the same (unlike nitrogen); Oxygen doesn’t affect the HDI calculation Calculation (in class): Possible Compounds: