Infrared Spectroscopy What is infrared radiation Electromagnetic radiation






- Slides: 11

Infrared Spectroscopy… What is infrared radiation? Electromagnetic radiation with a wavelength of 700 nm – 1 mm (frequency of 430 THz – 300 GHz) that sits between visible light and microwaves on the spectrum

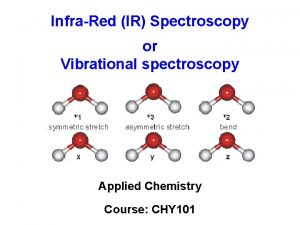
Infrared Spectroscopy… Infrared Radiation (heat) makes everything hotter because it increases the kinetic energy of molecules. This motion is mainly within the bonds of the molecule. There are 2 main movement types: • Compression/Rarefaction • Bending

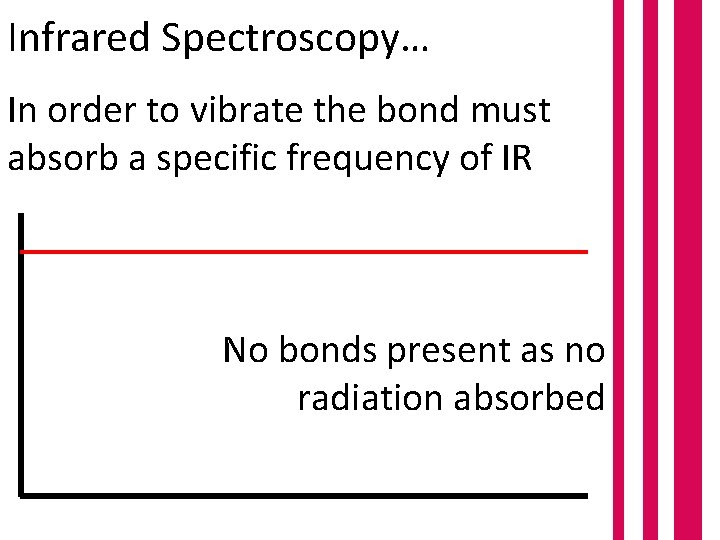
Infrared Spectroscopy… In order to vibrate the bond must absorb a specific frequency of IR No bonds present as no radiation absorbed

Infrared Spectroscopy… In order to vibrate the bond must absorb a specific frequency of IR What is the absorption of this carbonyl bond? 1700 cm-1 3500 3000 2500 2000 1500 1000 500

Infrared Spectroscopy… Each functional group has a different absorption which allows us to identify whether or not it is present: An OH bond has an absorption range of 2500 -3600 cm-1 Which functional groups contain an OH bond?

Infrared Spectroscopy… Alcohols cause an OH peak between 3300 cm-1 and 3600 cm-1

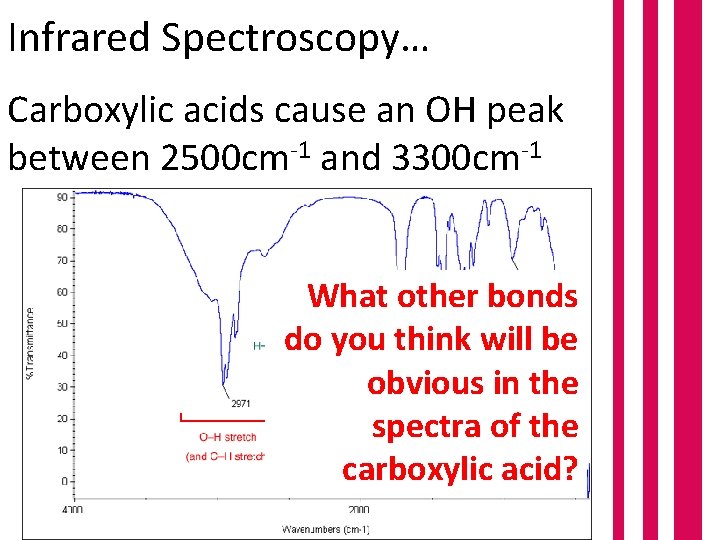
Infrared Spectroscopy… Carboxylic acids cause an OH peak between 2500 cm-1 and 3300 cm-1 The peak is described a broad because it is wide at the top and pointy at the bottom

Infrared Spectroscopy… Carboxylic acids cause an OH peak between 2500 cm-1 and 3300 cm-1 What other bonds do you think will be obvious in the spectra of the carboxylic acid?

Infrared Spectroscopy… Carboxy groups (C=O) cause a peak between 1630 cm-1 and 1820 cm-1

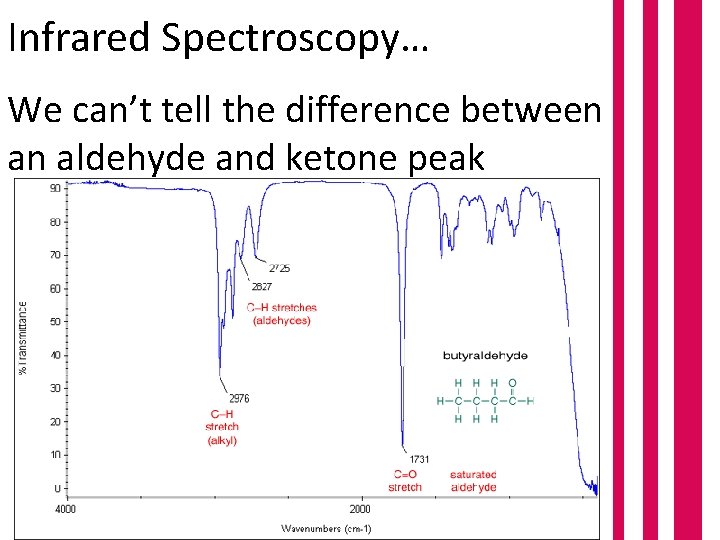
Infrared Spectroscopy… We can’t tell the difference between an aldehyde and ketone peak

Infrared Spectroscopy… Isomers are often distinguished using IR spectra as their different functional groups give rise to unique absorption peaks. Let's have a look…
 Near infrared spectroscopy instrumentation
Near infrared spectroscopy instrumentation Ir spectroscopy instrumentation
Ir spectroscopy instrumentation Site:slidetodoc.com
Site:slidetodoc.com Infrared spectroscopy theory
Infrared spectroscopy theory Infrared spectroscopy ppt
Infrared spectroscopy ppt Herschel infrared discovery
Herschel infrared discovery Infrared radiation hazards
Infrared radiation hazards Electromagentic spectrum
Electromagentic spectrum Intensity of em waves
Intensity of em waves When electromagnetic radiation of wavelength 300
When electromagnetic radiation of wavelength 300 Vertical physics
Vertical physics Facts about electromagnetic radiation
Facts about electromagnetic radiation