Infrared Spectroscopy Theory of Infrared Absorption Spectroscopy IR
Infrared Spectroscopy
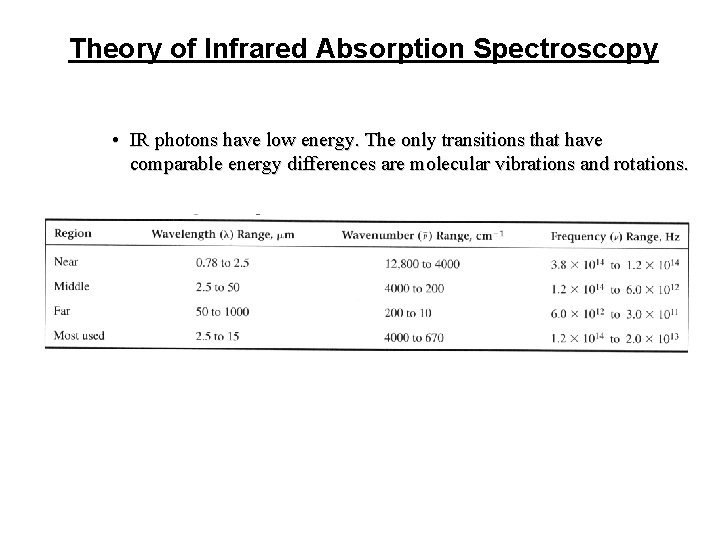
Theory of Infrared Absorption Spectroscopy • IR photons have low energy. The only transitions that have comparable energy differences are molecular vibrations and rotations.

Theory of Infrared Absorption Spectroscopy • In order for IR absorbance to occur two conditions must be met: 1. There must be a change in the dipole moment of the molecule as a result of a molecular vibration (or rotation). The change (or oscillation) in the dipole moment allows interaction with the alternating electrical component of the IR radiation wave. Symmetric molecules (or bonds) do not absorb IR radiation since there is no dipole moment. 2. If the frequency of the radiation matches the natural frequency of the vibration (or rotation), the IR photon is absorbed and the amplitude of the vibration increases.

Theory of Infrared Absorption Spectroscopy • In order for IR absorbance to occur two conditions must be met: 1. There must be a change in the dipole moment of the molecule as a result of a molecular vibration (or rotation). The change (or oscillation) in the dipole moment allows interaction with the alternating electrical component of the IR radiation wave. Symmetric molecules (or bonds) do not absorb IR radiation since there is no dipole moment. 2. If the frequency of the radiation matches the natural frequency of the vibration (or rotation), the IR photon is absorbed and the amplitude of the vibration increases.
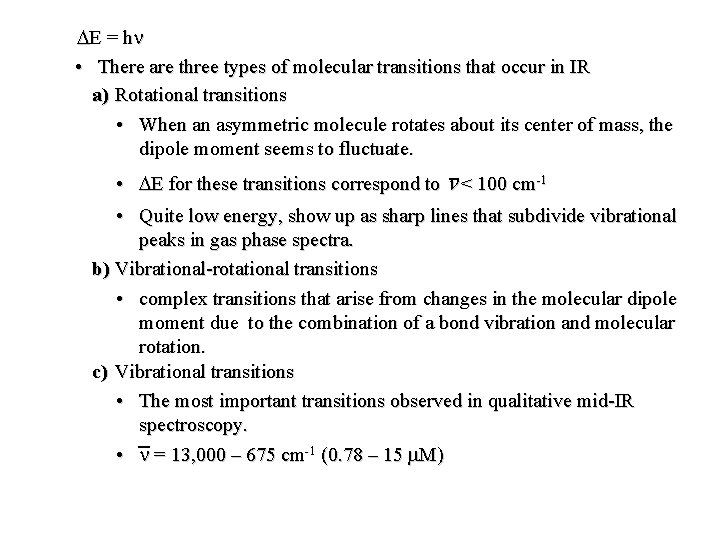
DE = h n • There are three types of molecular transitions that occur in IR a) Rotational transitions • When an asymmetric molecule rotates about its center of mass, the dipole moment seems to fluctuate. • DE for these transitions correspond to n < 100 cm-1 • Quite low energy, show up as sharp lines that subdivide vibrational peaks in gas phase spectra. b) Vibrational-rotational transitions • complex transitions that arise from changes in the molecular dipole moment due to the combination of a bond vibration and molecular rotation. c) Vibrational transitions • The most important transitions observed in qualitative mid-IR spectroscopy. • n = 13, 000 – 675 cm-1 (0. 78 – 15 m. M)
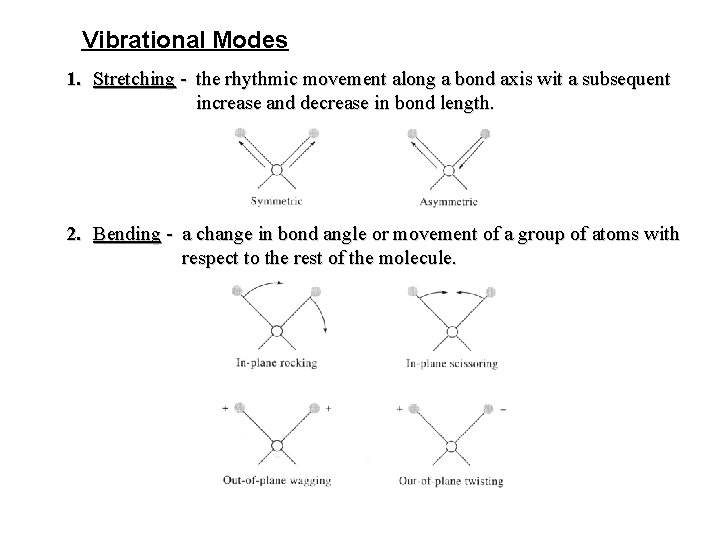
Vibrational Modes 1. Stretching - the rhythmic movement along a bond axis wit a subsequent increase and decrease in bond length. 2. Bending - a change in bond angle or movement of a group of atoms with respect to the rest of the molecule.
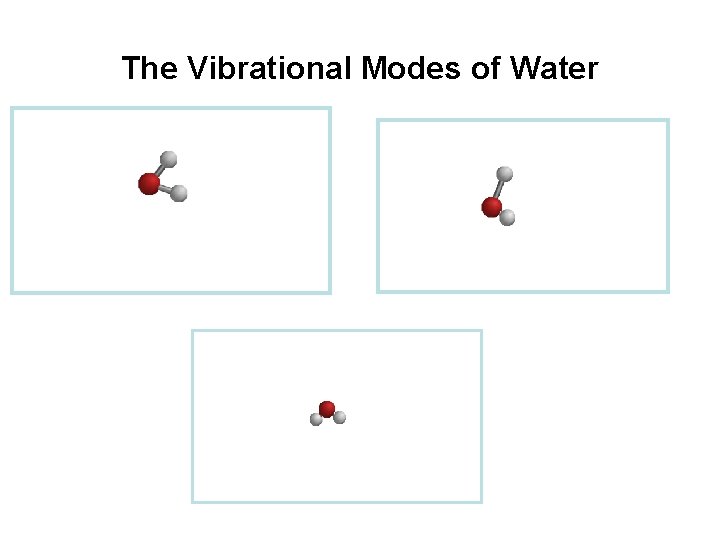
The Vibrational Modes of Water
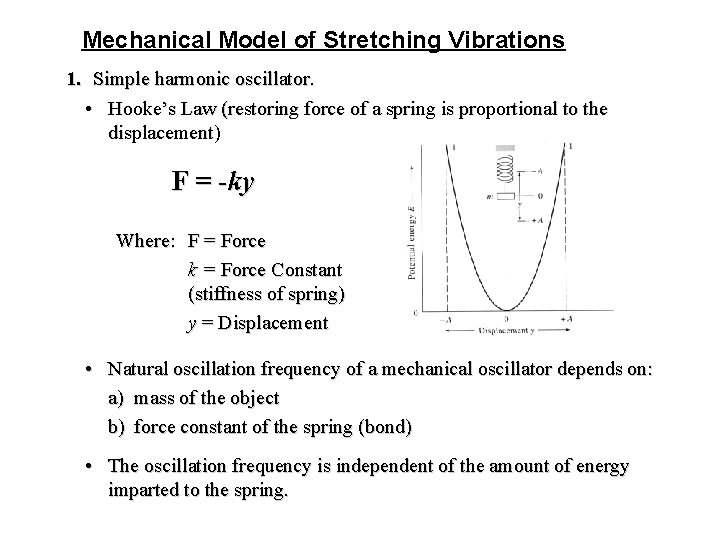
Mechanical Model of Stretching Vibrations 1. Simple harmonic oscillator. • Hooke’s Law (restoring force of a spring is proportional to the displacement) F = -ky Where: F = Force k = Force Constant (stiffness of spring) y = Displacement • Natural oscillation frequency of a mechanical oscillator depends on: a) mass of the object b) force constant of the spring (bond) • The oscillation frequency is independent of the amount of energy imparted to the spring.
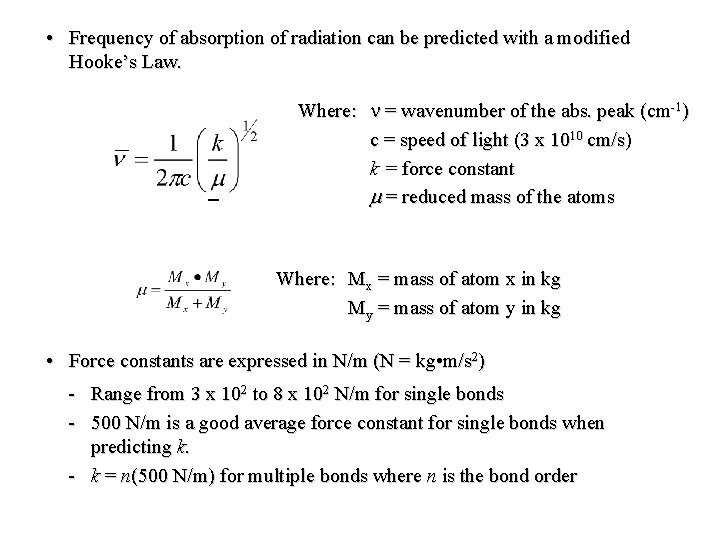
• Frequency of absorption of radiation can be predicted with a modified Hooke’s Law. Where: n = wavenumber of the abs. peak (cm-1) c = speed of light (3 x 1010 cm/s) k = force constant m = reduced mass of the atoms Where: Mx = mass of atom x in kg My = mass of atom y in kg • Force constants are expressed in N/m (N = kg • m/s 2) - Range from 3 x 102 to 8 x 102 N/m for single bonds - 500 N/m is a good average force constant for single bonds when predicting k. - k = n(500 N/m) for multiple bonds where n is the bond order
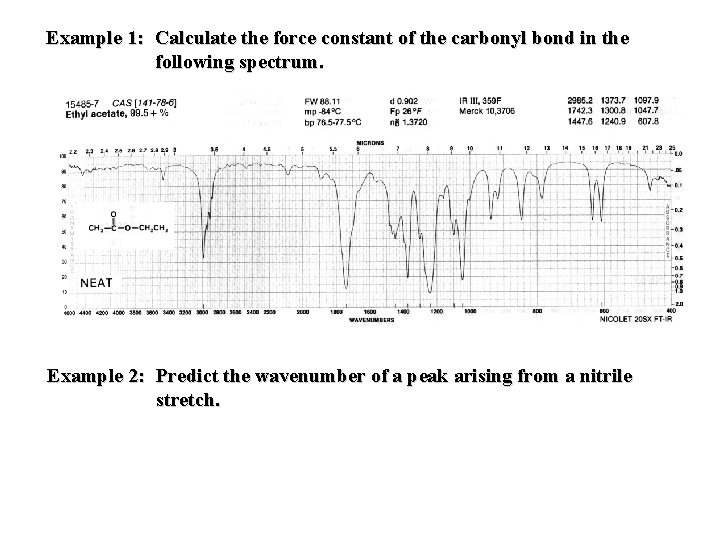
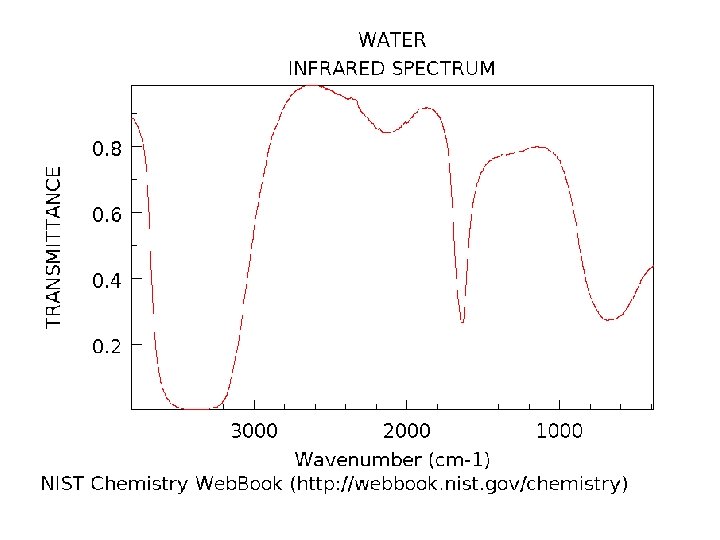
Example 1: Calculate the force constant of the carbonyl bond in the following spectrum. Example 2: Predict the wavenumber of a peak arising from a nitrile stretch.
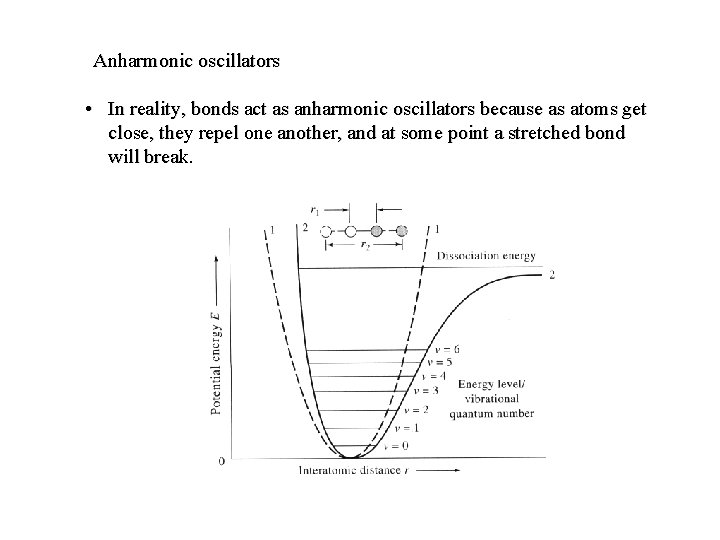
Anharmonic oscillators • In reality, bonds act as anharmonic oscillators because as atoms get close, they repel one another, and at some point a stretched bond will break.

IR Sources and Detectors Sources - inert solids that heat electrically to 1500 – 2200 K. • Emit blackbody radiation produced by atomic and molecular oscillations excited in the solid by thermal energy. • The inert solid “glows” when heated. • Common sources: 1. Nernst glower - constructed of a rod of a rare earth oxide (lanthanide) with platinum leads. 2. Globar - Silicon carbide rod with water cooled contacts to prevent arcing. 3. Incandescent wire - tightly wound wire heated electrically. Longer life but lower intensity.
Detectors – measure minute changes in temperature. 1. Thermal transducer • Constructed of a bimetal junction, which has a temperature dependant potential (V). (similar to a thermocouple) • Have a slow response time, so they are not well suited to FT-IR. 2. Pyroelectric transducer • Constructed of crystalline wafers of triglycine sulfate (TGS) that have a strong temperature dependent polarization. • Have a fast response time and are well suited for FT-IR. 3. Photoconducting transducer • Constructed of a semiconducting material (lead sulfide, mercury/cadmium telluride, or indium antimonide) deposited on a glass surface and sealed in an evacuated envelope to protect the semiconducting material from the environment. • Absorption of radiation promotes nonconducting valence electrons to a conducting state, thus decreasing the resistance (W) of the semiconductor. • Fast response time, but require cooling by liquid N 2.

Multiplexing (FT) Spectrometers • Collect data in the time domain and convert to the frequency domain by Fourier Transform. • Detectors are not fast enough to respond to power variations at high frequency (1012 to 1015 Hz) so the signal is modulated by a Michelson interferometer to a lower frequency that is directly proportional to the high frequency.
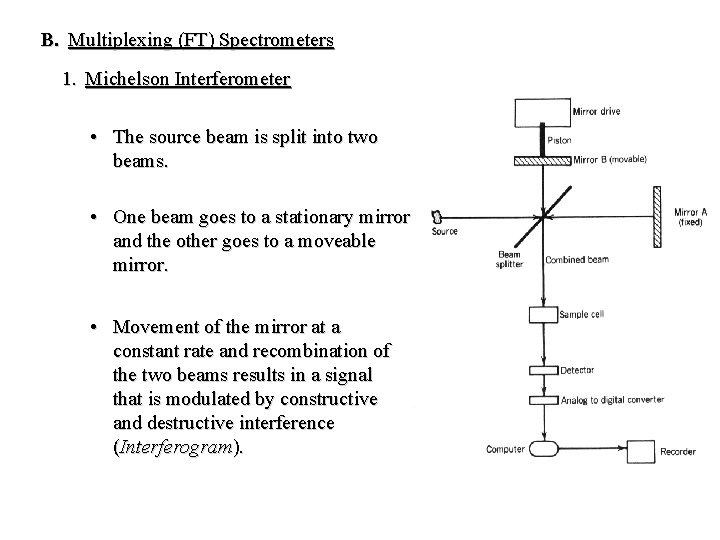
B. Multiplexing (FT) Spectrometers 1. Michelson Interferometer • The source beam is split into two beams. • One beam goes to a stationary mirror and the other goes to a moveable mirror. • Movement of the mirror at a constant rate and recombination of the two beams results in a signal that is modulated by constructive and destructive interference (Interferogram).
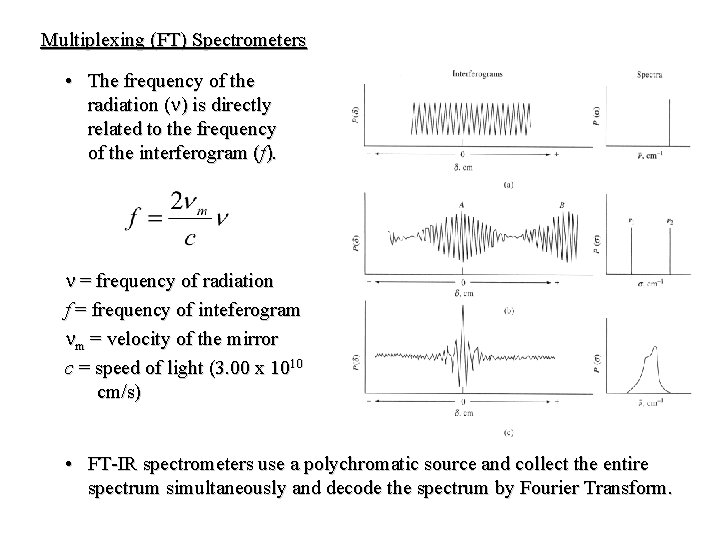
Multiplexing (FT) Spectrometers • The frequency of the radiation (n) is directly related to the frequency of the interferogram (f). n = frequency of radiation f = frequency of inteferogram nm = velocity of the mirror c = speed of light (3. 00 x 1010 cm/s) • FT-IR spectrometers use a polychromatic source and collect the entire spectrum simultaneously and decode the spectrum by Fourier Transform.

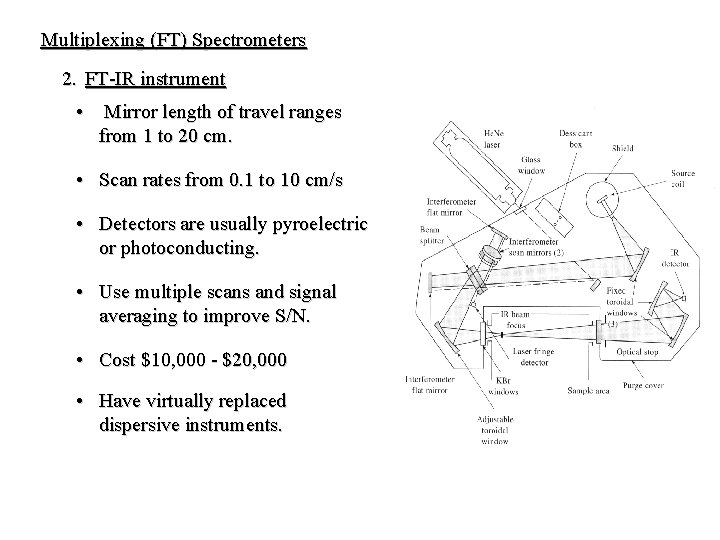
Multiplexing (FT) Spectrometers 2. FT-IR instrument • Mirror length of travel ranges from 1 to 20 cm. • Scan rates from 0. 1 to 10 cm/s • Detectors are usually pyroelectric or photoconducting. • Use multiple scans and signal averaging to improve S/N. • Cost $10, 000 - $20, 000 • Have virtually replaced dispersive instruments.

Performance Characteristics • Range: 7800 to 350 cm-1 (less expensive) 25, 000 to 10 cm-1 (Near to far IR, expensive) • Resolution: 8 cm-1 to 0. 01 cm-1 • Qualitative: Very good, functional groups are identifiable • Quantitative: Dispersive – poor FTIR - fair
- Slides: 21