IR Infrared Spectroscopy What is IR Spectroscopy Infrared






- Slides: 15

IR – Infrared Spectroscopy

What is IR Spectroscopy? • Infrared spectroscopy is the analysis of infrared light interacting with a molecule. • IR spectroscopy measures the vibrations of the atoms caused by the infrared light and from these measurements it is possible to identify the functional groups of the compound, i. e. identify the compound. • Every functional group absorbs at different and specific frequencies of IR radiation, therefore each specific frequency corresponds to a functional group. So when and if a frequency appears on the IR spectra, we can therefore identify the functional group. • IR region is between 4000 – 400 cm-1 on the electromagnetic spectra.

• It is the high speed quantitative analysis of a sample, without the destruction or consumption of the sample. • IR spectrums are obtained by detecting changes in absorption intensity of the radiation by the sample. There are changes in absorption intensity as the functional groups within the sample absorb the radiation at different intensities. • The frequency at which a bond vibrates depends on a number of factors, including the mass of the atom and the strength of the bond. The bonds can vibrate with different amounts of energy at the dependable frequency. Mass of atom • Lighter atoms vibrate at higher frequencies. • Heavier atoms vibrate at lower frequencies. (Heavier atoms move more slowly due to their weight i. e. molecular weight) v. Greater the mass, lower the frequency.

Strength of the bond • Stronger bonds vibrate at higher frequencies • Weaker bonds vibrate at lower frequencies • At room temperature bonds vibrate with the lowest amount of energy, but if radiation (source of energy) of the right frequency is supplied to the bond, the bond can absorb the energy and vibrate with greater amplitude. (greater vibrations i. e. larger and faster vibrations). • The greater the energy supplied to the bond, the greater the vibrations. Radiation Bond Vibration

Why carry out IR analyse on a sample? • For compound identification • For the identification of functional groups within unknown samples, with the aim of identifying the sample. • IR analysis can be carried out on solid, liquid and gas samples.

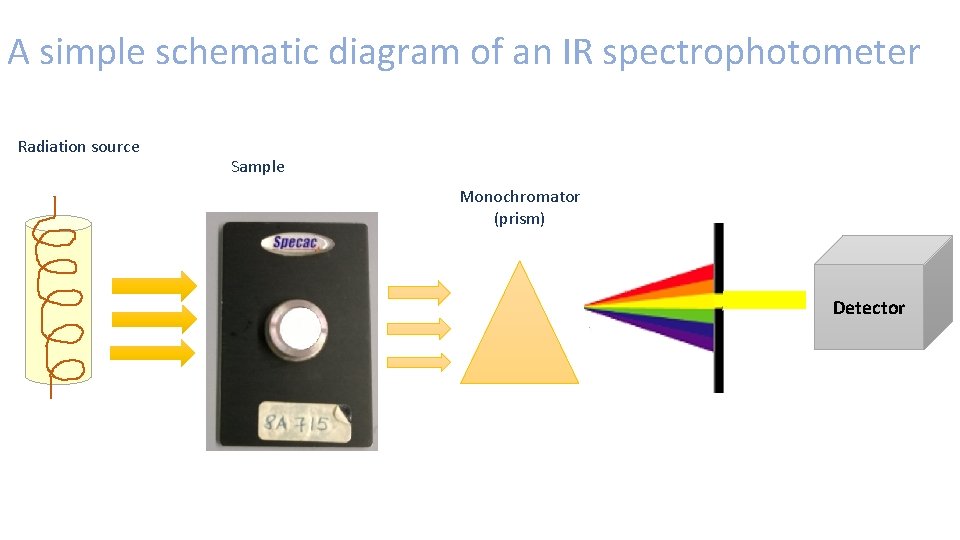
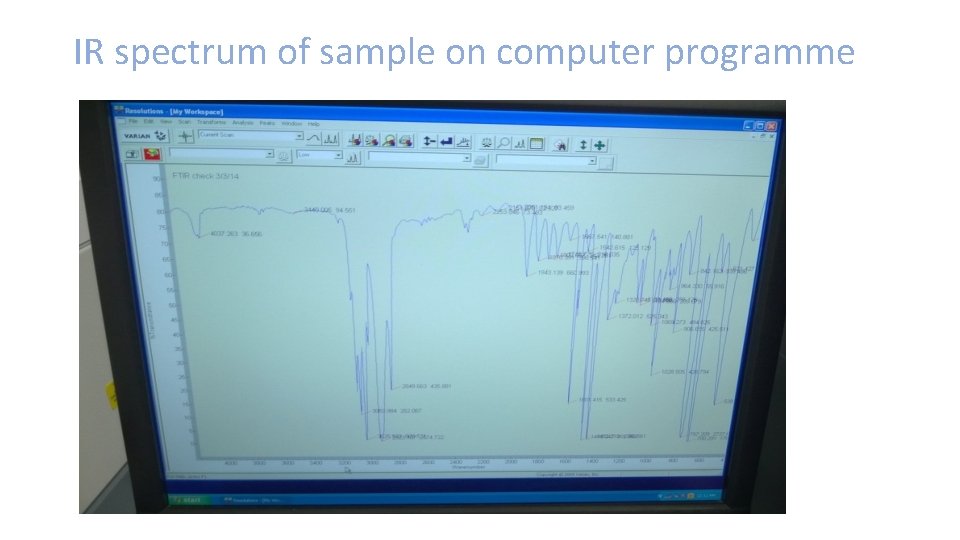
IR spectrophotometer • Consists of 3 basic components; radiation source, monochromator and detector. • The IR radiation source consists of a coil wire surrounded by a ceramic capsule which is electrically heated to give off IR radiation over a range of frequencies. • The monochromator is a device used to disperse a broad spectrum of radiation. • The radiation passes through the sample and is then dispersed by a monochromator into its’ different frequencies. The radiation not absorbed by the sample arrives at the detector, which generates an electrical signal and results in the form of a spectra which appears on the computer programme. • The spectra shows frequency peaks, representing the wavelengths the different functional groups within the sample were absorbed by the light. Which gives the ability to identify the sample being analysed. (As frequencies correspond to a specific functional groups).

A simple schematic diagram of an IR spectrophotometer Radiation source Sample Monochromator (prism) Detector

IR spectrum of sample on computer programme

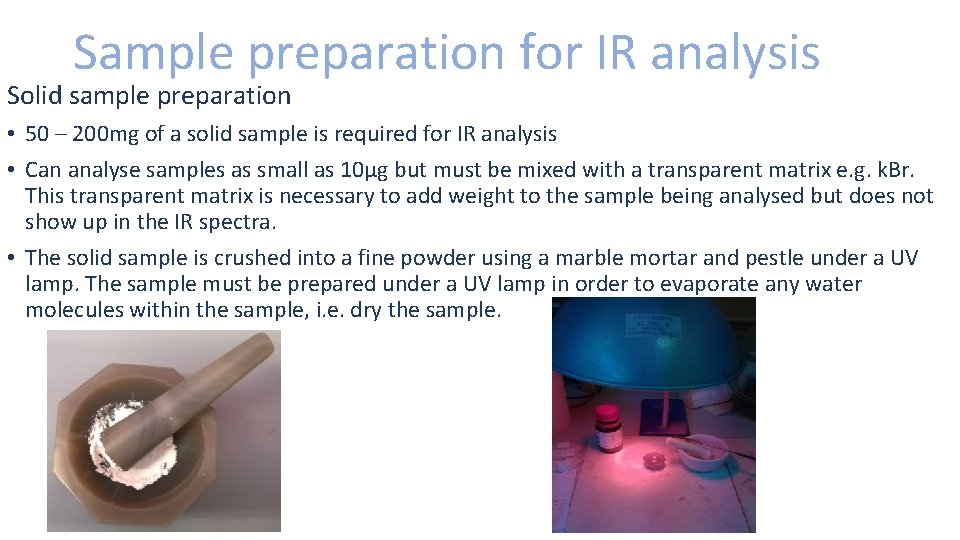
Sample preparation for IR analysis Solid sample preparation • 50 – 200 mg of a solid sample is required for IR analysis • Can analyse samples as small as 10µg but must be mixed with a transparent matrix e. g. k. Br. This transparent matrix is necessary to add weight to the sample being analysed but does not show up in the IR spectra. • The solid sample is crushed into a fine powder using a marble mortar and pestle under a UV lamp. The sample must be prepared under a UV lamp in order to evaporate any water molecules within the sample, i. e. dry the sample.

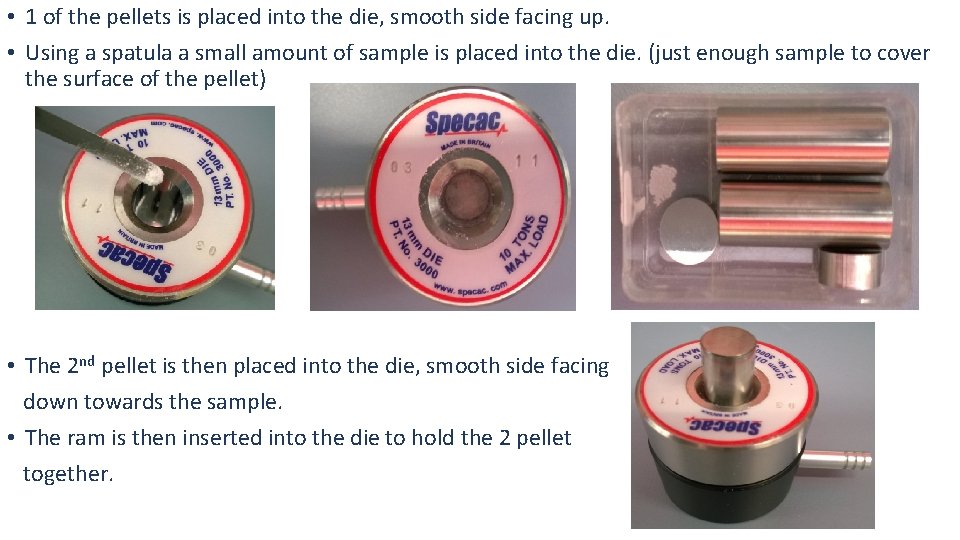
• 1 of the pellets is placed into the die, smooth side facing up. • Using a spatula a small amount of sample is placed into the die. (just enough sample to cover the surface of the pellet) • The 2 nd pellet is then placed into the die, smooth side facing down towards the sample. • The ram is then inserted into the die to hold the 2 pellet together.

• The die is then placed into the hydraulic press and held in position by tightening the bleed screw. • In order to bind the sample and make an IR disc, pressure is required. Using the handle the pressure is pumped to approx. 10 tons (10 kg x 1000) and is left for 1 minute. • After 1 minute the pressure is released using the hand screw and the die is removed from the hydraulic press. • The die is then carefully disassemble.

• Between the 2 pellets a thin transparent IR disc should have formed. • The disc is carefully removed from the pellet, to avoid breakage and placed in the appropriate cell/disc holder. • The sample is now ready to be placed into the IR spectrophotometer for IR analysis.

Liquid sample preparation • 1 -2 drops of neat sample is placed between 2 salt plates. • Salt plates (Na. Cl) are the most popular choice for non-aqueous liquids because they are transparent to IR radiation. This means the plates will not absorb any of the radiation and will not appear in the resulting IR spectra, therefore does not affect results. • The salt discs are squeezed together to form a thin film. The discs are held together by capillary attraction from the sample.

• The plates are then placed into a screw-tight holder, which can then be inserted into the IR spectrophotometer for IR analysis. Sample holder inserted here • Once analysis is complete the salt plates are cleaned with acetone, water is not used as can dissolve the salt plates.

IR spectroscopy is the analysis of infrared light interacting with a molecule. Compound identification – as specific frequencies correspond to different functional groups. IR spectroscopy measures the vibrations of the atoms caused by the infrared light and from these measurements it is possible to identify the functional groups of the compound. What have I learned? IR spectrophotometer consists of a radiation source, monochromator and a detector. Analysis can be carried out on solids, liquids and gases. IR region is between 4000 – 400 cm-1