Today 1 Infrared Spectroscopy IR 2 Introduction to




- Slides: 9

Today: 1) Infrared Spectroscopy (IR) 2) Introduction to Exp. 10: Caffeine from Tea Leaves 1

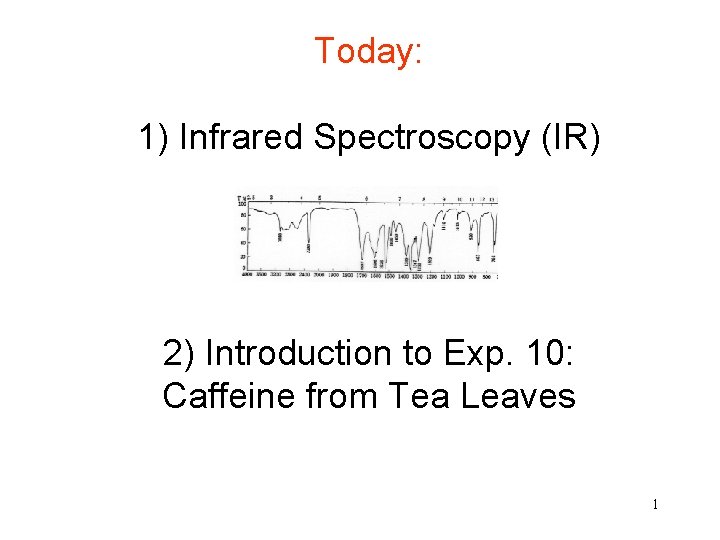
Infrared Spectroscopy (IR) 1) What is the approximate range of wavelengths visible to humans? And where would you find the range of IR light? How about its energy compared to visible light? 2) What are the "wavenumbers", commonly used in IR to define absorptions? 3) What type of information do we obtain from an IR spectrum? 4) How can we identify a compound from its IR, e. g. how can we know that we obtained eugenol? 5) How will your IR tell you if your eugenol sample contains impurities? 2

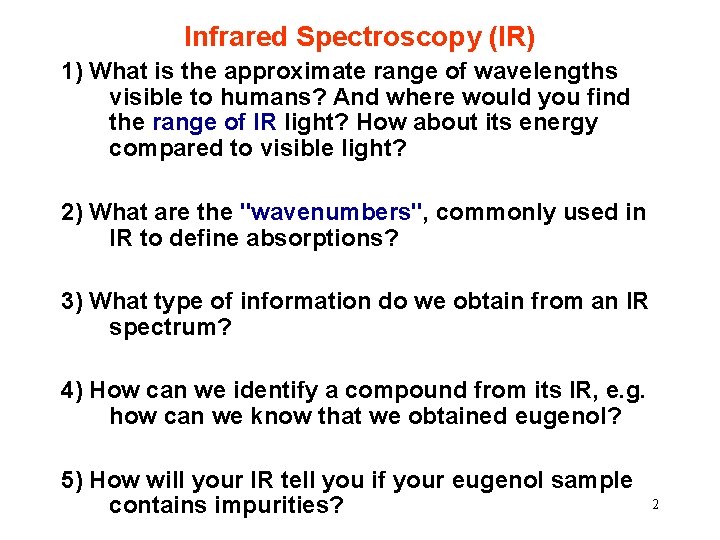
IR: Study Ch. 9, esp. from p. 536 Excellent website: http: //www. chem. ucla. edu/~webspectra/index. html 3

Exp. 10: Isolation of Caffeine from Tea Leaves 1. Caffeine is an alkaloid. What are “alkaloids”? Examples of other alkaloids? 2. Do you recognize any functional groups in caffeine? 3. Caffeine is slightly basic. Which group is responsible for its basic character? 4. Caffeine is usually found as a salt (citrate, malate, other) in plants. What could be the advantage of this for the plant? 4

1. Caffeine has an LD 50 (orally, in mice) of 127 mg/kg. What is the LD 50? 2. What is the solubility of caffeine a) in cold water? b) in hot water? c) In room temp. CH 2 Cl 2? 3. What is the purpose of adding Na 2 CO 3 to the tea solution at the beginning of the experiment? (Hint: see 4. ) 4. What else is in tea leaves besides caffeine…? 5

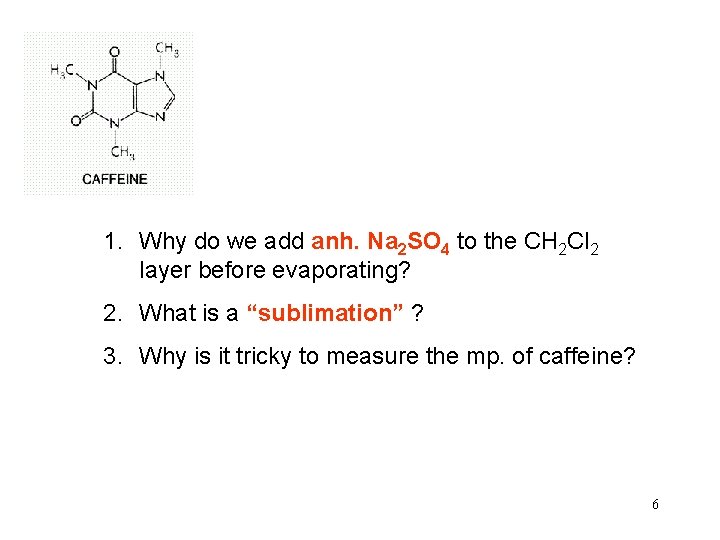
1. Why do we add anh. Na 2 SO 4 to the CH 2 Cl 2 layer before evaporating? 2. What is a “sublimation” ? 3. Why is it tricky to measure the mp. of caffeine? 6

Experimental Hints • Weigh the teabags without the tag and the string. But do NOT open the tea bags. • During heating take care not to tear the tea bags. • During the extraction (in which layer do you expect caffeine? ) emulsions can form: • Remedies: – wait a few more minutes for separation of layers to occur and separate as well as possible – Add more CH 2 Cl 2 – Fill into centrifuge tubes (two, balance!) and centrifugate 7

Caffeine Report Form Weight of tea bags Weight of crude caffeine % of crude caffeine in your tea sample Weight of sublimed caffeine What does caffeine look like? For your report find the approximate caffeine content of your “favorite” beverages: coffee, tea, coca cola. . etc. 8

Next Experiment: Exp. 6: Column Chromatography of Ferrocene Midterm October 27! will include Exp. 6 material Start reviewing lab lectures, practice questions, experiments. Review calculations, graphs. 9