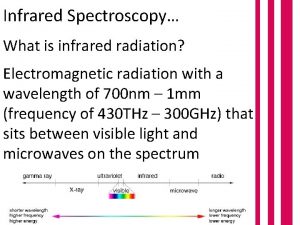
Infrared Spectroscopy 13 20 Introduction to Infrared Spectroscopy




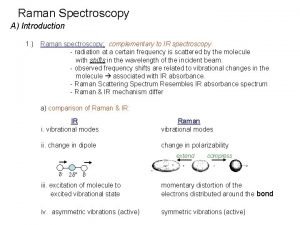
![Types of vibrations [5, 11] Stretching vibrations Asymmetric (nu) 2925 720 cm-1 For stretching Types of vibrations [5, 11] Stretching vibrations Asymmetric (nu) 2925 720 cm-1 For stretching](https://slidetodoc.com/presentation_image_h2/acf0b494b8f3680dd2efb4b939ef78f0/image-24.jpg)

- Slides: 39

Infrared Spectroscopy



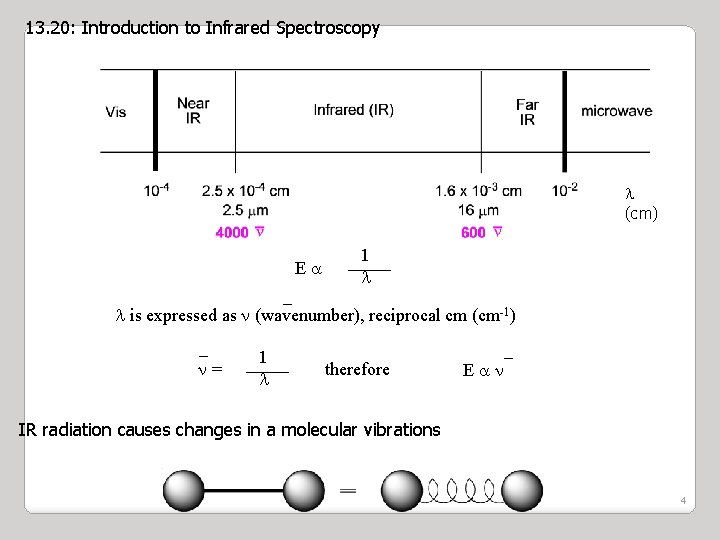
13. 20: Introduction to Infrared Spectroscopy (cm) Ea 1 _ is expressed as (wavenumber), reciprocal cm (cm-1) _ = 1 therefore _ Ea IR radiation causes changes in a molecular vibrations 4

Wavenumbers are expressed in units of reciprocal centimeters (cm-1) i. e. the reciprocal of the wavelength ( ) expressed in centimeters. (cm-1) = 1 / (cm) Wave Numbers can be converted to a frequency ( ) by multiplying them by the speed of light (c) in cm/sec (Hz) = c = c / (cm /sec /cm = 1/sec) Recall: E = h c / Thus, wavenumbers are directly proportional to energy 2/12/2022 5

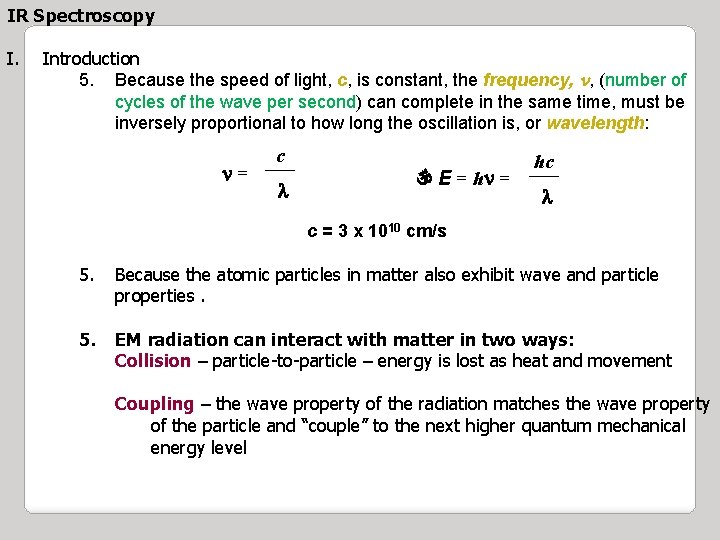
IR Spectroscopy I. Introduction 5. Because the speed of light, c, is constant, the frequency, n, (number of cycles of the wave per second) can complete in the same time, must be inversely proportional to how long the oscillation is, or wavelength: n= c ___ l E = hn = hc ___ l c = 3 x 1010 cm/s 5. Because the atomic particles in matter also exhibit wave and particle properties. 5. EM radiation can interact with matter in two ways: Collision – particle-to-particle – energy is lost as heat and movement Coupling – the wave property of the radiation matches the wave property of the particle and “couple” to the next higher quantum mechanical energy level

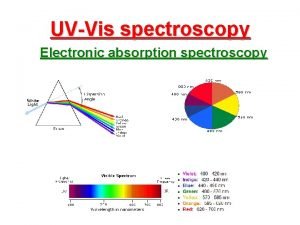
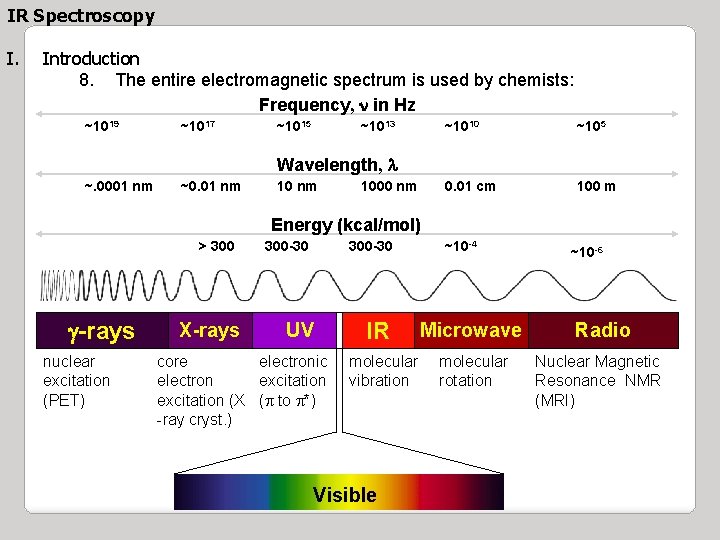
IR Spectroscopy I. Introduction 8. The entire electromagnetic spectrum is used by chemists: Frequency, n in Hz ~1019 ~1017 ~1015 ~1013 ~1010 ~105 0. 01 cm 100 m Wavelength, l ~. 0001 nm ~0. 01 nm 1000 nm Energy (kcal/mol) > 300 g-rays nuclear excitation (PET) X-rays 300 -30 UV core electronic electron excitation (X (p to p*) -ray cryst. ) IR molecular vibration Visible ~10 -4 ~10 -6 Microwave Radio molecular rotation Nuclear Magnetic Resonance NMR (MRI)

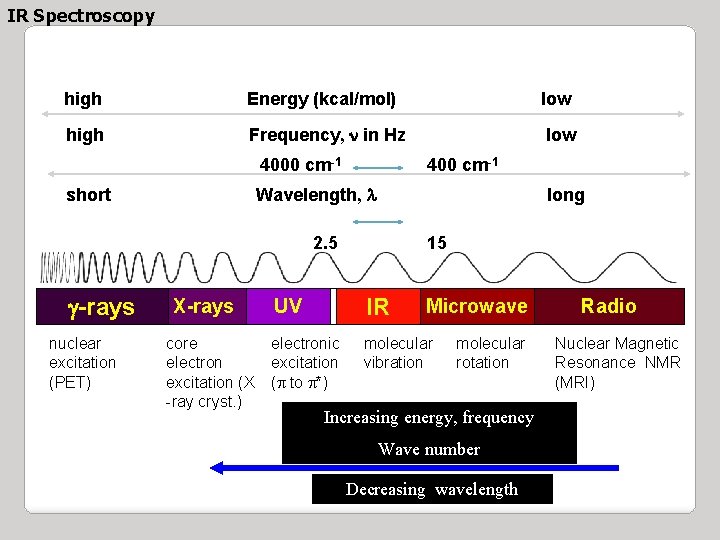
IR Spectroscopy high Energy (kcal/mol) low high Frequency, n in Hz low 4000 cm-1 400 cm-1 Wavelength, l short long 2. 5 g-rays nuclear excitation (PET) X-rays core electron excitation (X -ray cryst. ) 15 UV IR electronic excitation (p to p*) molecular vibration Microwave molecular rotation Increasing energy, frequency Wave number Decreasing wavelength Radio Nuclear Magnetic Resonance NMR (MRI)

How do we know: how atoms are connected together? Which bonds are single, double, or triple? What functional groups exist in the molecule? If we have a specific stereoisomer? The field of organic structure determination attempts to answer these questions.






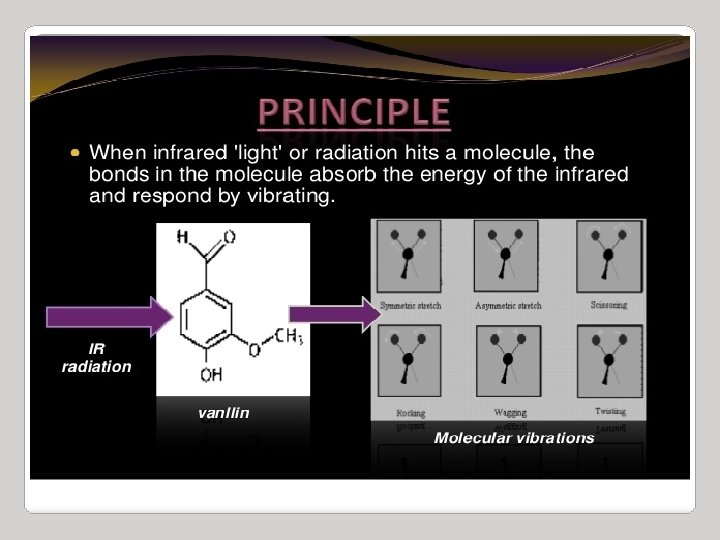
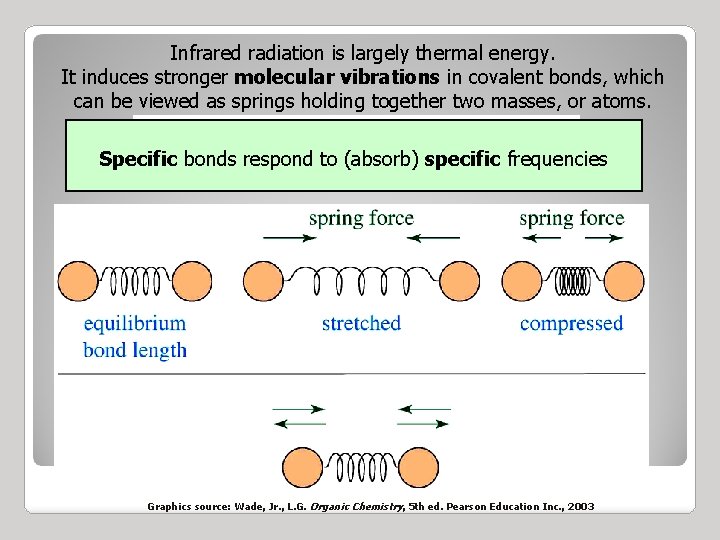
Infrared radiation is largely thermal energy. It induces stronger molecular vibrations in covalent bonds, which can be viewed as springs holding together two masses, or atoms. Specific bonds respond to (absorb) specific frequencies Graphics source: Wade, Jr. , L. G. Organic Chemistry, 5 th ed. Pearson Education Inc. , 2003

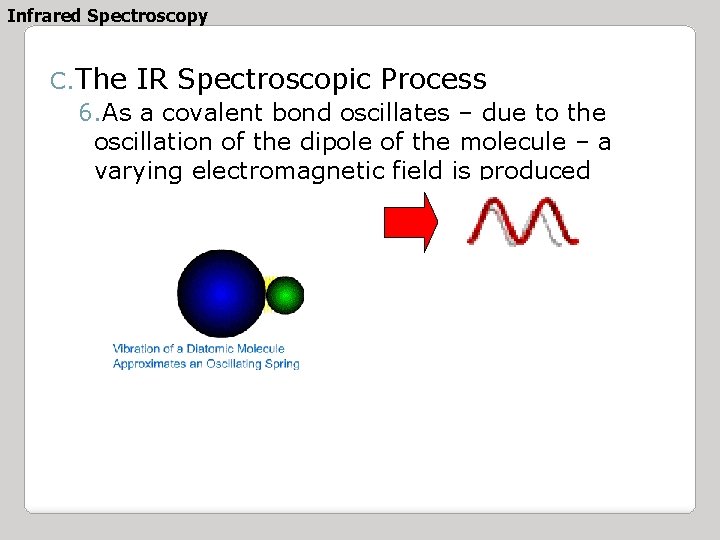
Infrared Spectroscopy C. The IR Spectroscopic Process 6. As a covalent bond oscillates – due to the oscillation of the dipole of the molecule – a varying electromagnetic field is produced

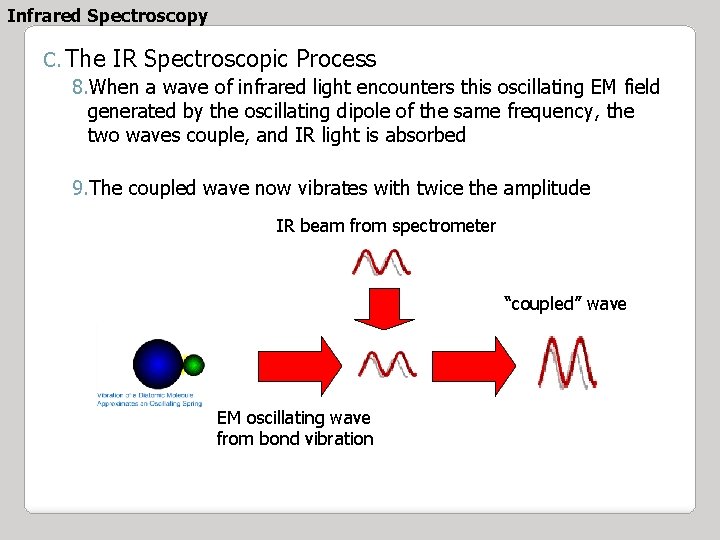
Infrared Spectroscopy C. The IR Spectroscopic Process 8. When a wave of infrared light encounters this oscillating EM field generated by the oscillating dipole of the same frequency, the two waves couple, and IR light is absorbed 9. The coupled wave now vibrates with twice the amplitude IR beam from spectrometer “coupled” wave EM oscillating wave from bond vibration

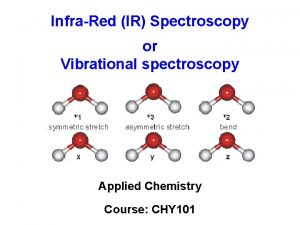
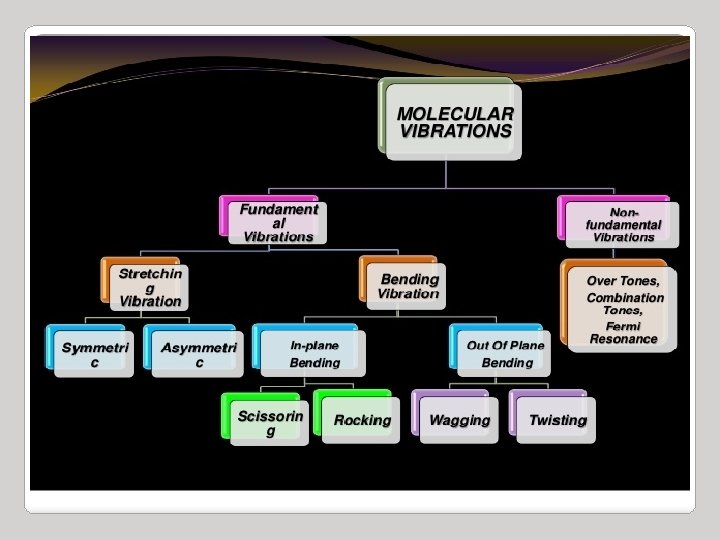
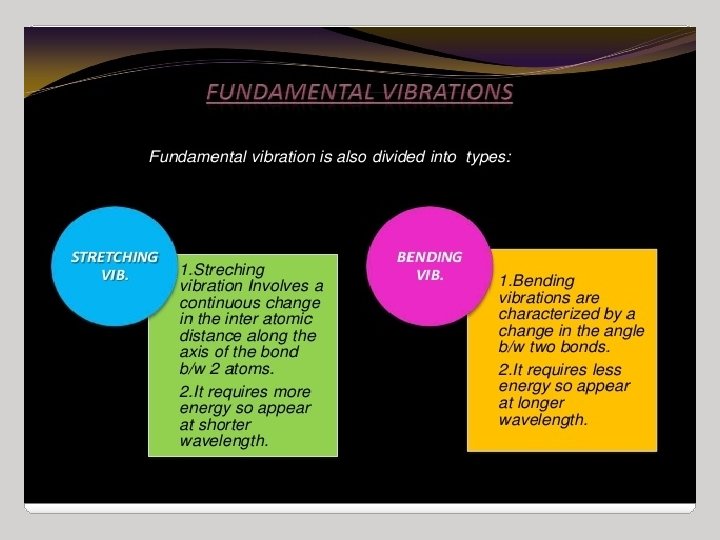
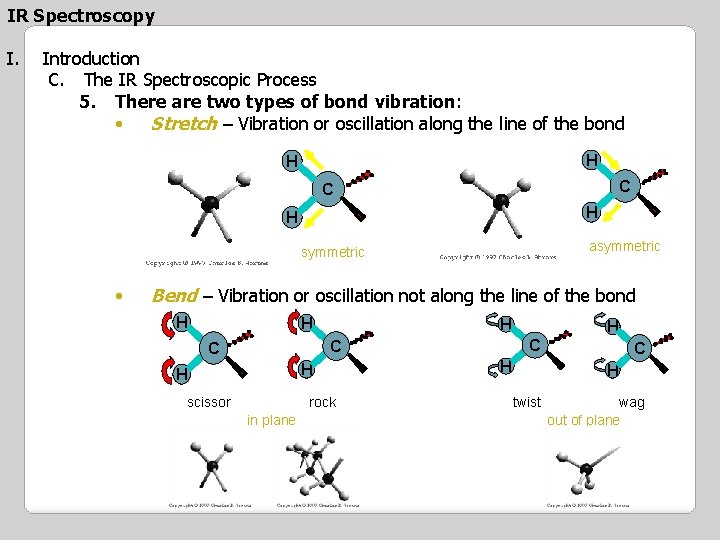
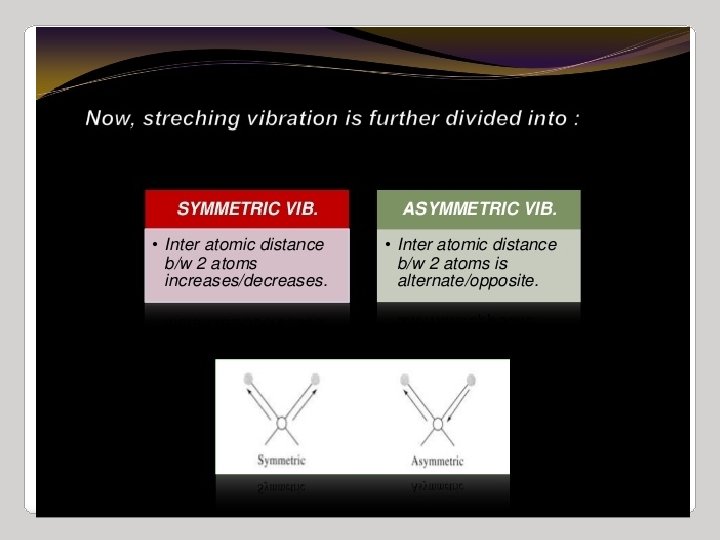
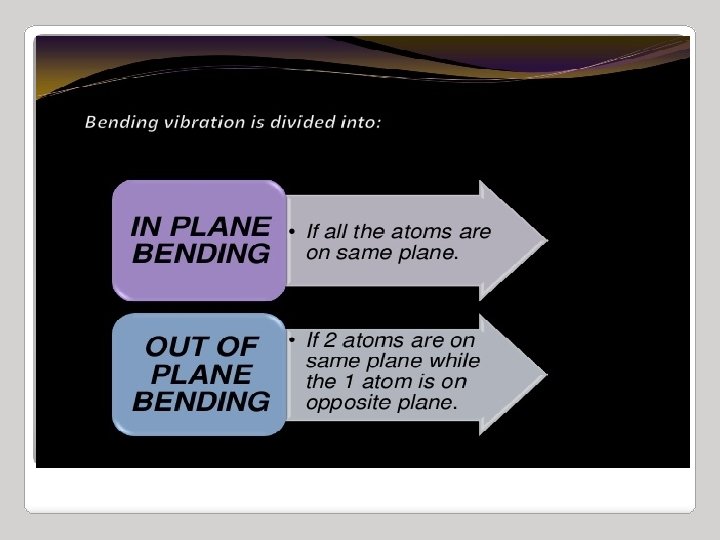
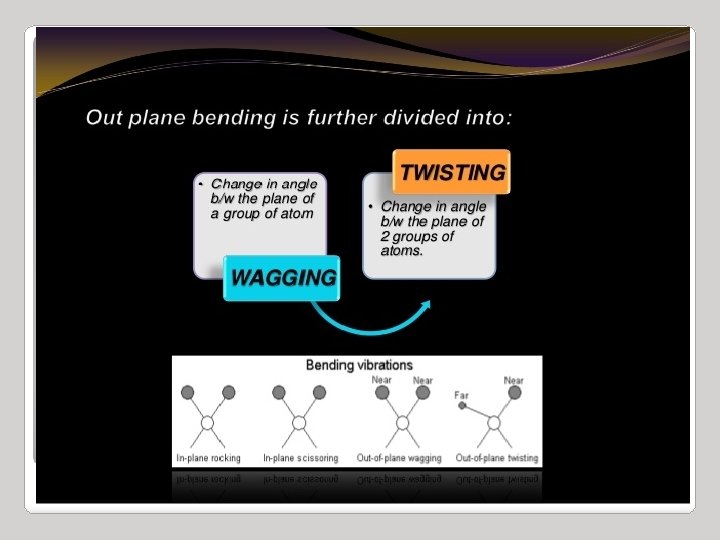
IR Spectroscopy I. Introduction C. The IR Spectroscopic Process 5. There are two types of bond vibration: • Stretch – Vibration or oscillation along the line of the bond H H C C H H asymmetric • Bend – Vibration or oscillation not along the line of the bond H H H C C C H H scissor rock in plane H H C H twist wag out of plane


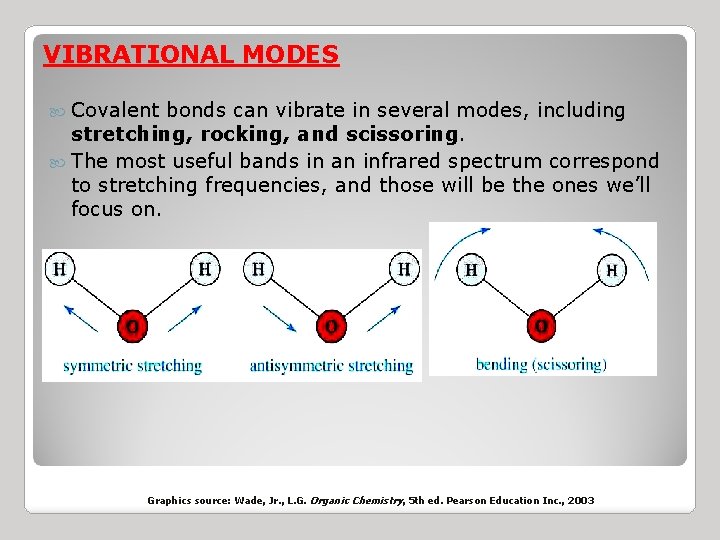
VIBRATIONAL MODES Covalent bonds can vibrate in several modes, including stretching, rocking, and scissoring. The most useful bands in an infrared spectrum correspond to stretching frequencies, and those will be the ones we’ll focus on. Graphics source: Wade, Jr. , L. G. Organic Chemistry, 5 th ed. Pearson Education Inc. , 2003



![Types of vibrations 5 11 Stretching vibrations Asymmetric nu 2925 720 cm1 For stretching Types of vibrations [5, 11] Stretching vibrations Asymmetric (nu) 2925 720 cm-1 For stretching](https://slidetodoc.com/presentation_image_h2/acf0b494b8f3680dd2efb4b939ef78f0/image-24.jpg)
Types of vibrations [5, 11] Stretching vibrations Asymmetric (nu) 2925 720 cm-1 For stretching vibration = N -1 For bending vibration [(3 N - 6)-(N -1)]=2 N -5 for non-linear [(3 N - 5)-(N -1)] =2 N – 4 for linear ‘N’ is the number of atoms in the bond. Bending vibrations In-plane Symmetric (nu) 2850 Scissoring (s) 1465 Out plane Twisting (tau) In-plane Wagging (ω) Rocking (ρ ) 1350 Vibrational energy depends on : 1. masses of the atoms 2. strength of bonds 3. arrangement of atoms within the molecule 1150 24

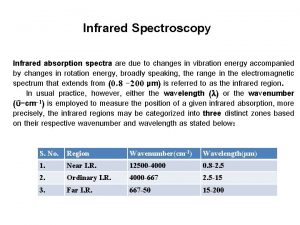
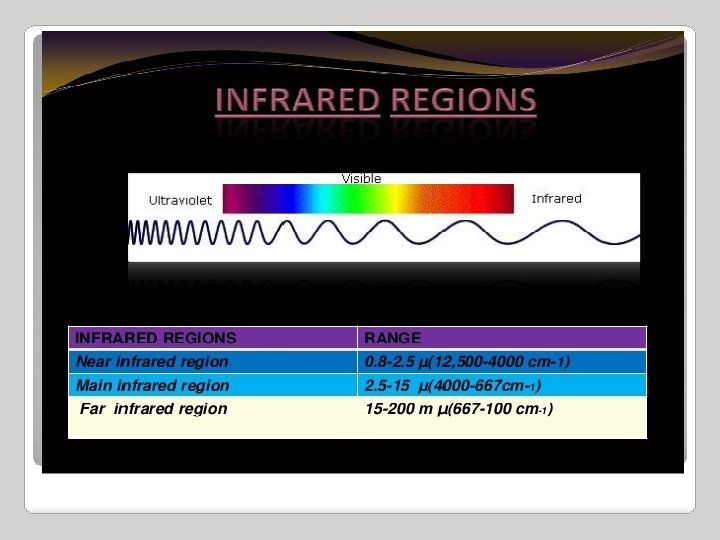
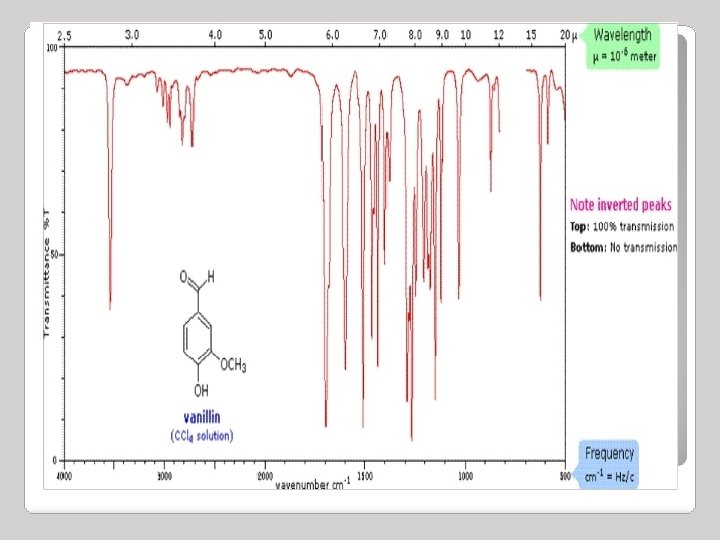
Infrared Spectroscopy Region of infrared that is most useful lies between 2. 5 -16 mm (4000 -400 cm-1) depends on transitions between vibrational energy states Stretching: higher energy / higher wave number (cm-1) Bending: lower energy / lower wave number (cm-1)

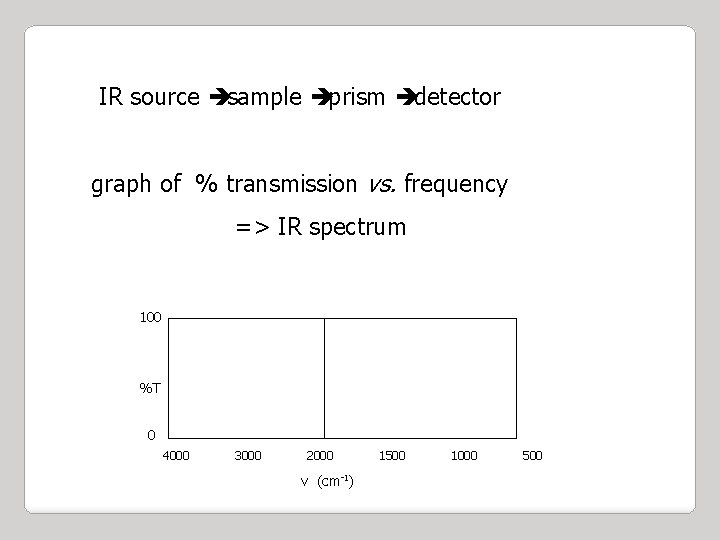
IR source èsample èprism èdetector graph of % transmission vs. frequency => IR spectrum 100 %T 0 4000 3000 2000 v (cm-1) 1500 1000 500

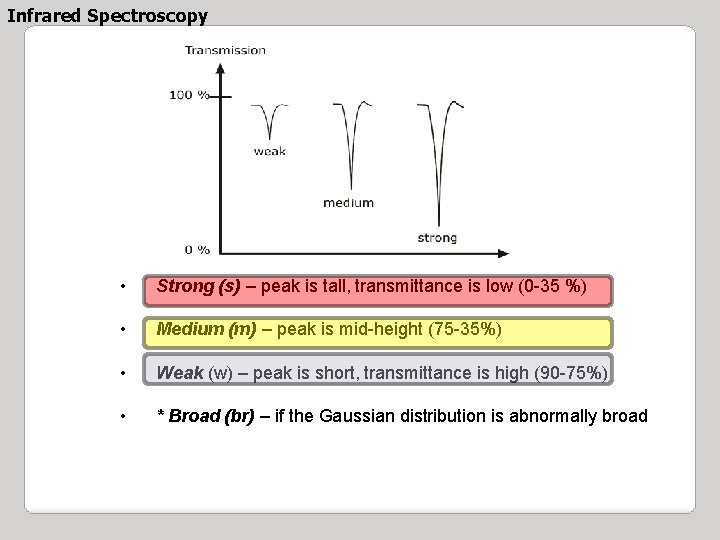
Infrared Spectroscopy • Strong (s) – peak is tall, transmittance is low (0 -35 %) • Medium (m) – peak is mid-height (75 -35%) • Weak (w) – peak is short, transmittance is high (90 -75%) • * Broad (br) – if the Gaussian distribution is abnormally broad

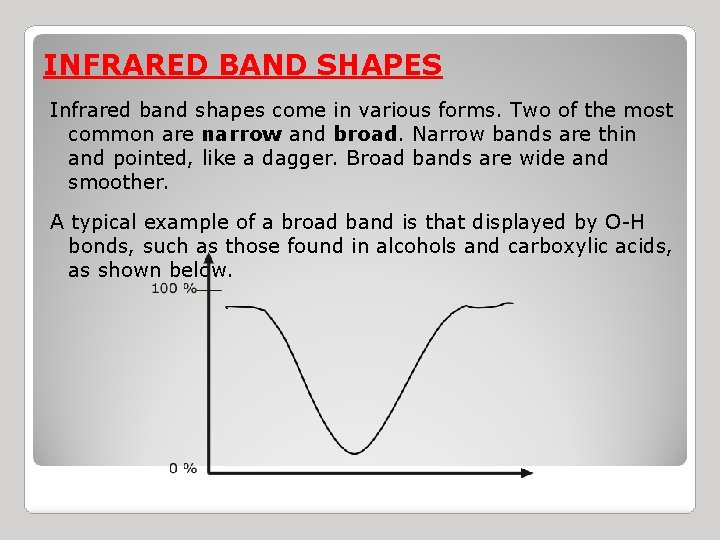
INFRARED BAND SHAPES Infrared band shapes come in various forms. Two of the most common are narrow and broad. Narrow bands are thin and pointed, like a dagger. Broad bands are wide and smoother. A typical example of a broad band is that displayed by O-H bonds, such as those found in alcohols and carboxylic acids, as shown below.

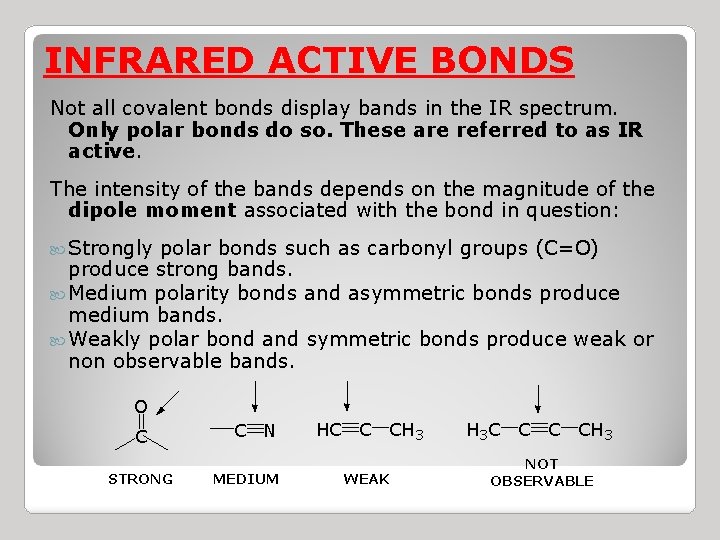
INFRARED ACTIVE BONDS Not all covalent bonds display bands in the IR spectrum. Only polar bonds do so. These are referred to as IR active. The intensity of the bands depends on the magnitude of the dipole moment associated with the bond in question: Strongly polar bonds such as carbonyl groups (C=O) produce strong bands. Medium polarity bonds and asymmetric bonds produce medium bands. Weakly polar bond and symmetric bonds produce weak or non observable bands.

Infrared spectroscopy can be used to find out about covalent bonds in molecules. IR is used to tell: 1. what type of bonds are present 2. some structural information

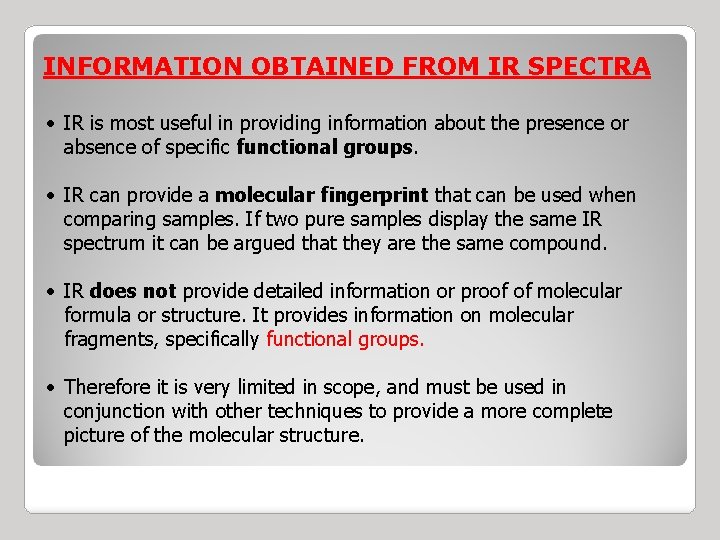
INFORMATION OBTAINED FROM IR SPECTRA • IR is most useful in providing information about the presence or absence of specific functional groups. • IR can provide a molecular fingerprint that can be used when comparing samples. If two pure samples display the same IR spectrum it can be argued that they are the same compound. • IR does not provide detailed information or proof of molecular formula or structure. It provides information on molecular fragments, specifically functional groups. • Therefore it is very limited in scope, and must be used in conjunction with other techniques to provide a more complete picture of the molecular structure.

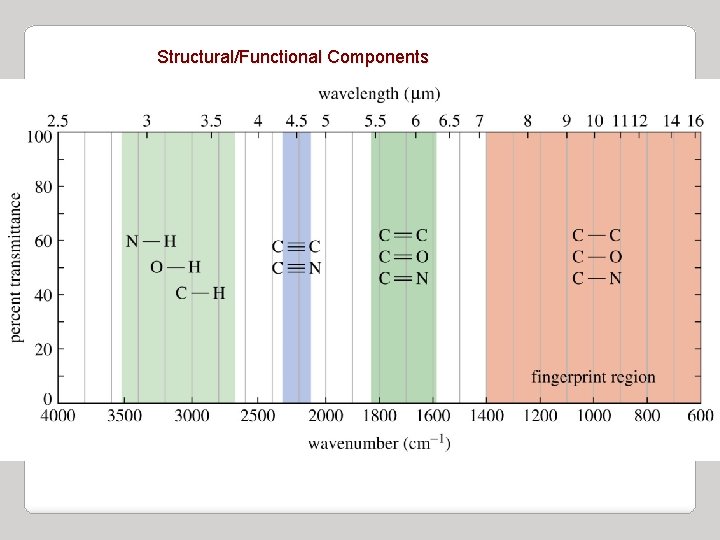
Structural/Functional Components

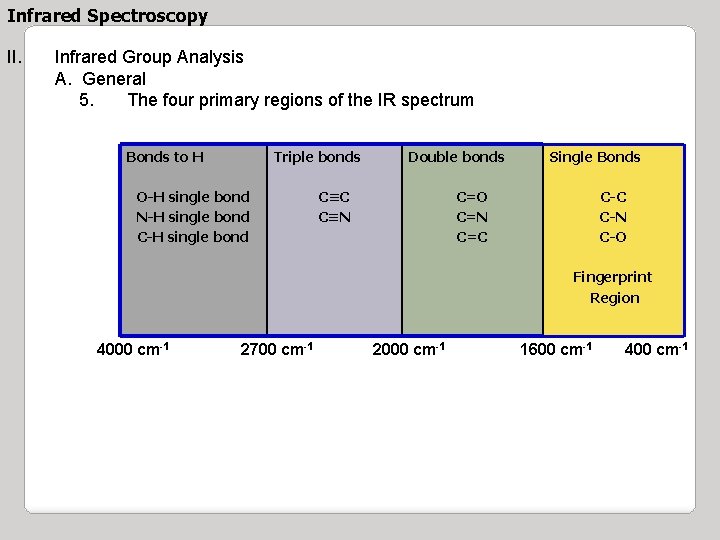
Infrared Spectroscopy II. Infrared Group Analysis A. General 5. The four primary regions of the IR spectrum Bonds to H Triple bonds O-H single bond N-H single bond C-H single bond Double bonds C≡C C≡N Single Bonds C=O C=N C=C C-N C-O Fingerprint Region 4000 cm-1 2700 cm-1 2000 cm-1 1600 cm-1 400 cm-1

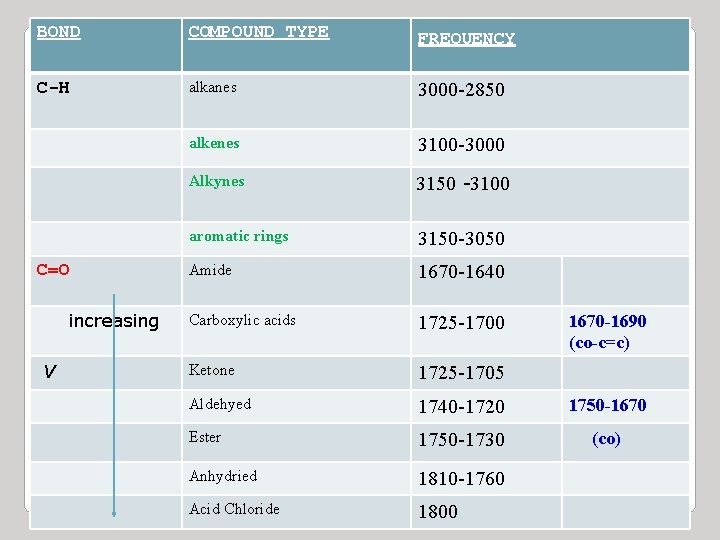
BOND COMPOUND TYPE FREQUENCY C-H alkanes 3000 -2850 alkenes 3100 -3000 Alkynes 3150 -3100 aromatic rings 3150 -3050 Amide 1670 -1640 Carboxylic acids 1725 -1700 Ketone 1725 -1705 Aldehyed 1740 -1720 1750 -1670 Ester 1750 -1730 (co) Anhydried 1810 -1760 Acid Chloride 1800 C=O increasing V 1670 -1690 (co-c=c)

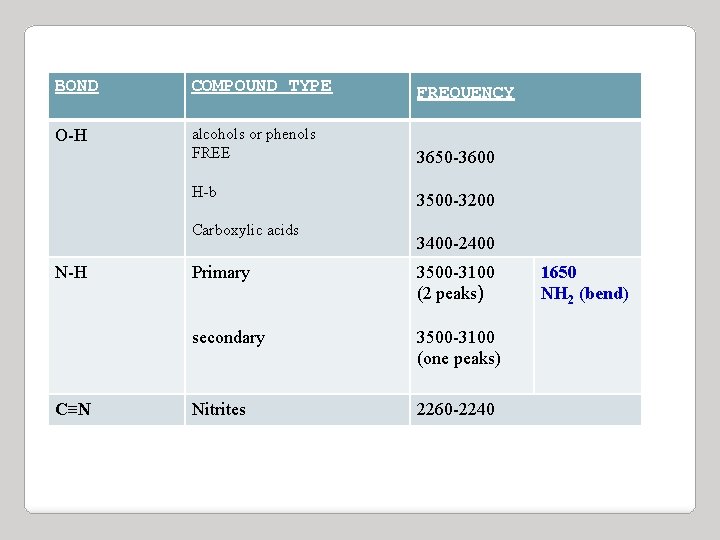
BOND COMPOUND TYPE FREQUENCY O-H alcohols or phenols FREE 3650 -3600 H-b Carboxylic acids N-H C≡N 3500 -3200 3400 -2400 Primary 3500 -3100 (2 peaks) secondary 3500 -3100 (one peaks) Nitrites 2260 -2240 1650 NH 2 (bend)

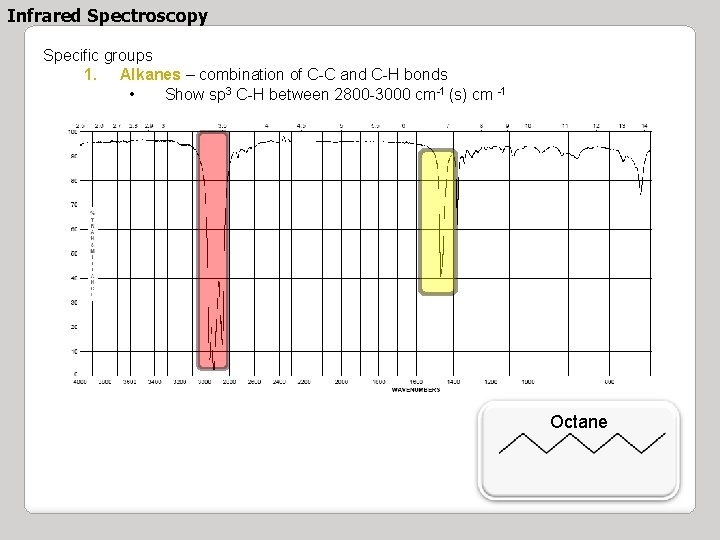
Infrared Spectroscopy Specific groups 1. Alkanes – combination of C-C and C-H bonds • Show sp 3 C-H between 2800 -3000 cm-1 (s) cm -1 Octane

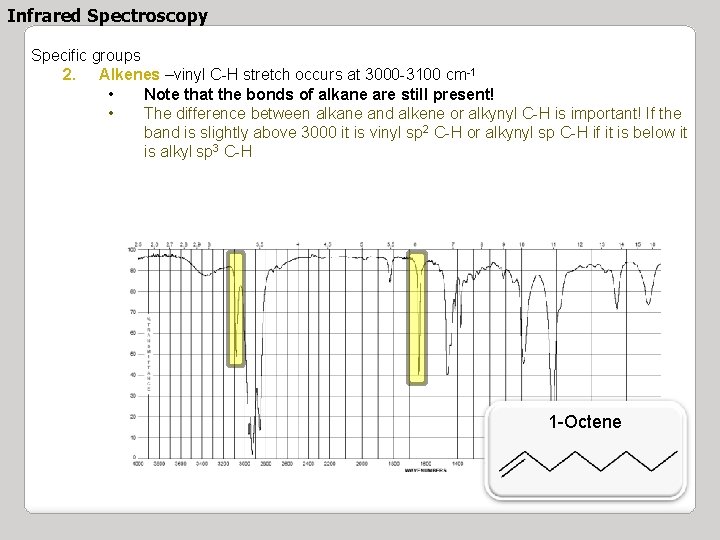
Infrared Spectroscopy Specific groups 2. Alkenes –vinyl C-H stretch occurs at 3000 -3100 cm-1 • Note that the bonds of alkane are still present! • The difference between alkane and alkene or alkynyl C-H is important! If the band is slightly above 3000 it is vinyl sp 2 C-H or alkynyl sp C-H if it is below it is alkyl sp 3 C-H 1 -Octene

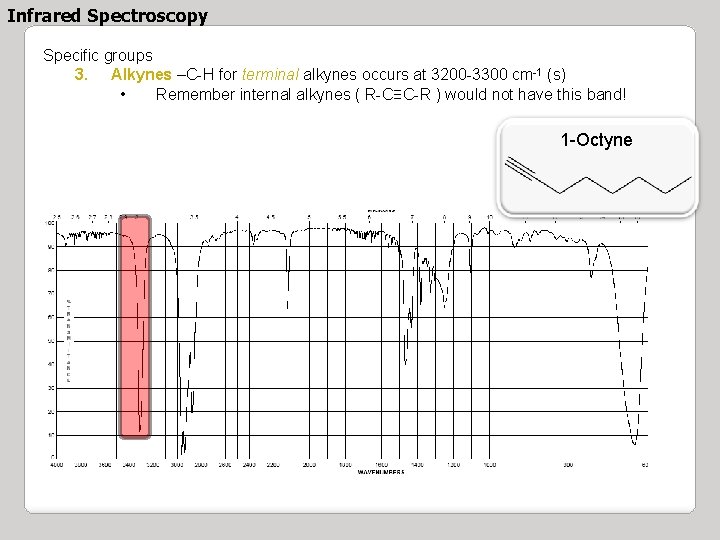
Infrared Spectroscopy Specific groups 3. Alkynes –C-H for terminal alkynes occurs at 3200 -3300 cm-1 (s) • Remember internal alkynes ( R-C≡C-R ) would not have this band! 1 -Octyne

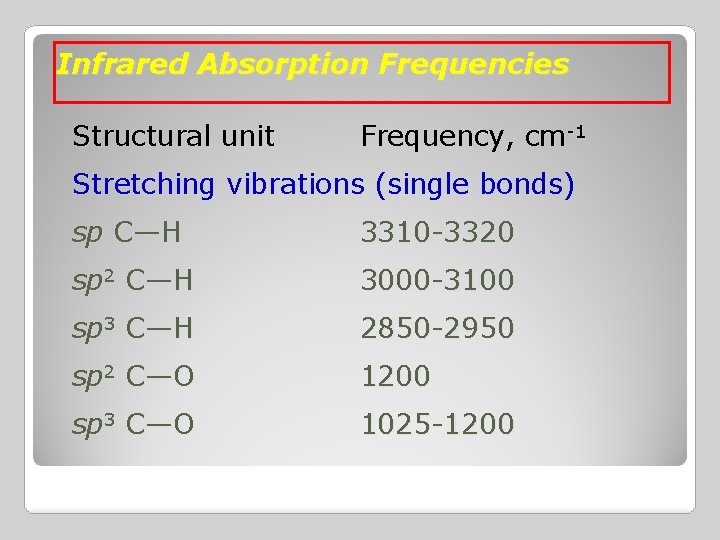
Infrared Absorption Frequencies Structural unit Frequency, cm-1 Stretching vibrations (single bonds) sp C—H 3310 -3320 sp 2 C—H 3000 -3100 sp 3 C—H 2850 -2950 sp 2 C—O 1200 sp 3 C—O 1025 -1200
 Near infrared spectroscopy instrumentation
Near infrared spectroscopy instrumentation Ethyl benzoate ir
Ethyl benzoate ir Infrared spectroscopy theory
Infrared spectroscopy theory Infrared spectroscopy ppt
Infrared spectroscopy ppt Infrared spectroscopy
Infrared spectroscopy Applications of uv and visible spectroscopy
Applications of uv and visible spectroscopy Charge d'un électron
Charge d'un électron Infrared vs bluetooth
Infrared vs bluetooth Infrared vs bluetooth
Infrared vs bluetooth Pengertian sensor infrared
Pengertian sensor infrared Bill nye reflection and refraction
Bill nye reflection and refraction Ir waves
Ir waves Infrared spektroskopisi
Infrared spektroskopisi Signage posted at a handwashing station must include
Signage posted at a handwashing station must include Shortwave infrared camera
Shortwave infrared camera Bluetooth vs infrared
Bluetooth vs infrared Hybridboard
Hybridboard Infrared radiation discovery
Infrared radiation discovery Thermal infrared
Thermal infrared Drainage network
Drainage network Touch screen advantages and disadvantages
Touch screen advantages and disadvantages Nirops
Nirops Wide field infrared survey explorer
Wide field infrared survey explorer Light spectrum frequencies
Light spectrum frequencies Infrared radiation hazards
Infrared radiation hazards Infrared sensor principle
Infrared sensor principle Infrared building envelope
Infrared building envelope Made up school
Made up school Scott can industries
Scott can industries What is spectroscopy
What is spectroscopy Auxochrome
Auxochrome Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Spectroscopy definition
Spectroscopy definition Beer lambert law in uv spectroscopy
Beer lambert law in uv spectroscopy Sample holder in ir spectroscopy
Sample holder in ir spectroscopy Atomic absorption spectroscopy adalah
Atomic absorption spectroscopy adalah Difference between ir and raman spectroscopy
Difference between ir and raman spectroscopy Difference between ir and raman spectroscopy
Difference between ir and raman spectroscopy Process nmr associates
Process nmr associates Objectives of spectroscopy
Objectives of spectroscopy