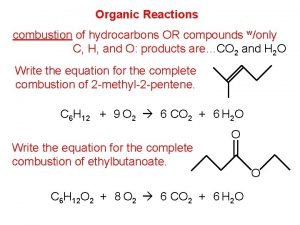
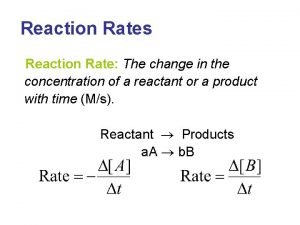
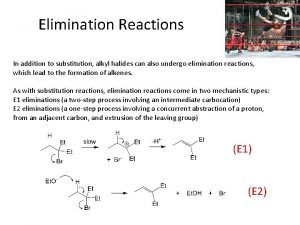
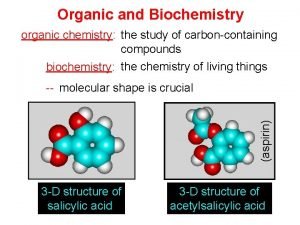
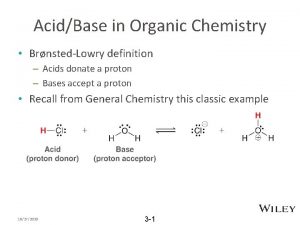
Organic Chemistry Reaction Exercises Organic chemistry nowadays almost







- Slides: 64

Organic Chemistry Reaction Exercises ”Organic chemistry nowadays almost drives me mad. It gives me the impression of a primeval tropical forest, full of the most remarkable things, a monstrous and boundless thicket, a dreadful, endless jungle with no way of escape, into which one may well dread to enter for there seems no way out. ” Friedrich Wöhler (1800 – 1882) founded organic chemistry in 1828. An organic chemistry reaction exercise based on the web site http: //murov. info/rxnmaponline. pdf (for more info, see the last 5 slides). Several exercises are suggested for students to improve competency in organic reactions at that site. The first one is: For each numbered reaction, classify the reaction by mechanism (e. g. , substitution, nucleophilic) and list the reagents, conditions, regioselectivity, stereoselectivity and restrictions associated with the reaction. This exercise is intended to facilitate the suggestion that students list the reagents needed to enable the reactions. The reactions appear in an order determined by a random number generator (1 - 107). For suggestions and comments, please e-mail murovs@yosemite. edu.

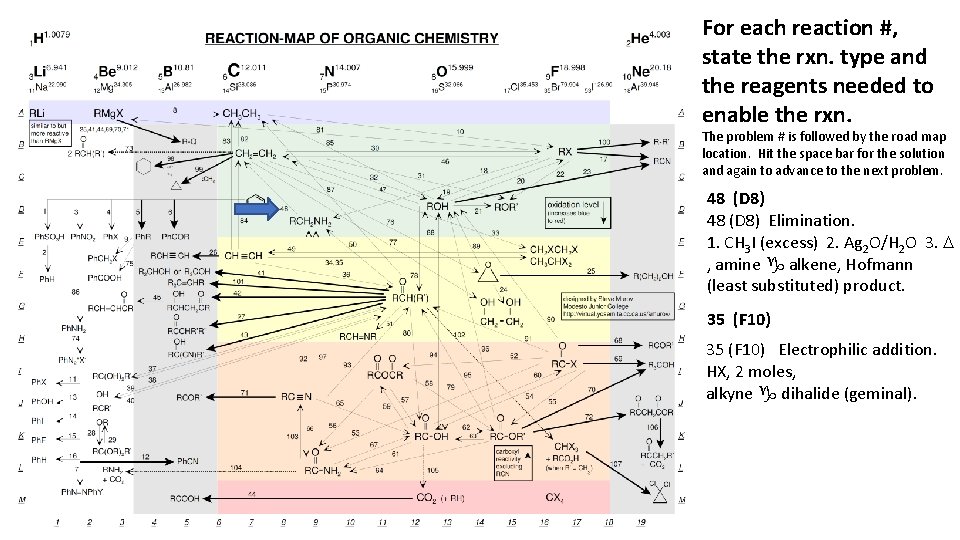
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 48 (D 8) Elimination. 1. CH 3 I (excess) 2. Ag 2 O/H 2 O 3. D , amine g alkene, Hofmann (least substituted) product. 35 (F 10) Electrophilic addition. HX, 2 moles, alkyne g dihalide (geminal).

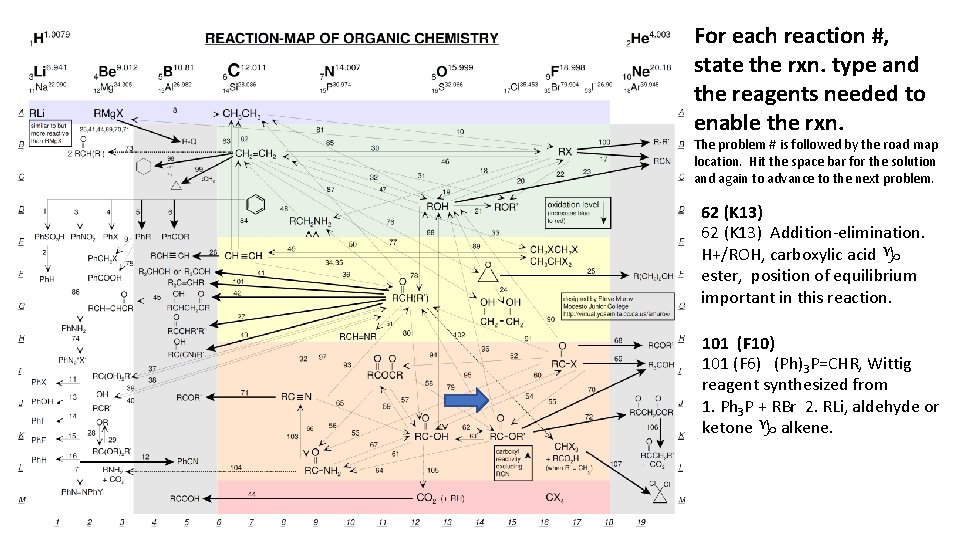
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 62 (K 13) Addition-elimination. H+/ROH, carboxylic acid g ester, position of equilibrium important in this reaction. 101 (F 10) 101 (F 6) (Ph)3 P=CHR, Wittig reagent synthesized from 1. Ph 3 P + RBr 2. RLi, aldehyde or ketone g alkene.

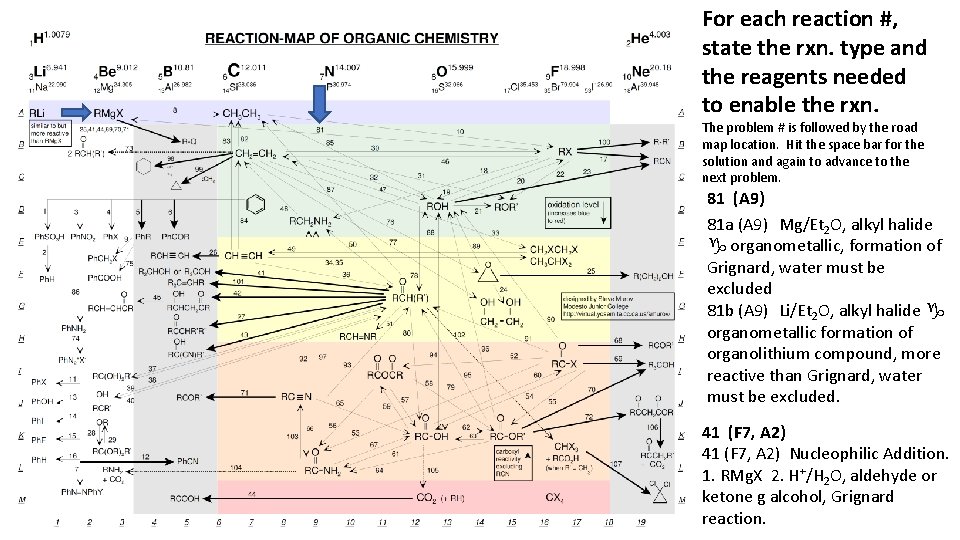
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 81 (A 9) 81 a (A 9) Mg/Et 2 O, alkyl halide g organometallic, formation of Grignard, water must be excluded 81 b (A 9) Li/Et 2 O, alkyl halide g organometallic formation of organolithium compound, more reactive than Grignard, water must be excluded. 41 (F 7, A 2) Nucleophilic Addition. 1. RMg. X 2. H+/H 2 O, aldehyde or ketone g alcohol, Grignard reaction.

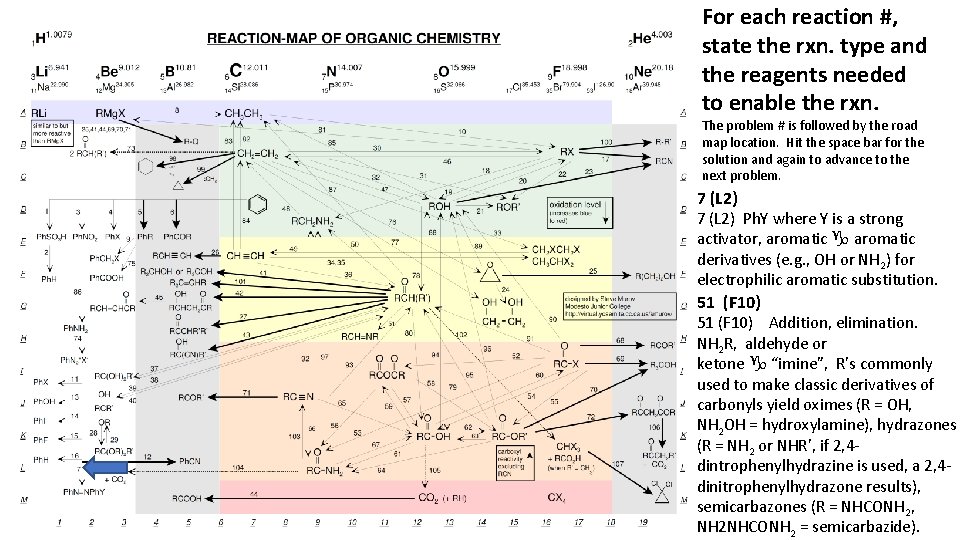
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 7 (L 2) Ph. Y where Y is a strong activator, aromatic g aromatic derivatives (e. g. , OH or NH 2) for electrophilic aromatic substitution. 51 (F 10) Addition, elimination. NH 2 R, aldehyde or ketone g “imine”, R’s commonly used to make classic derivatives of carbonyls yield oximes (R = OH, NH 2 OH = hydroxylamine), hydrazones (R = NH 2 or NHR’, if 2, 4 dintrophenylhydrazine is used, a 2, 4 dinitrophenylhydrazone results), semicarbazones (R = NHCONH 2, NH 2 NHCONH 2 = semicarbazide).

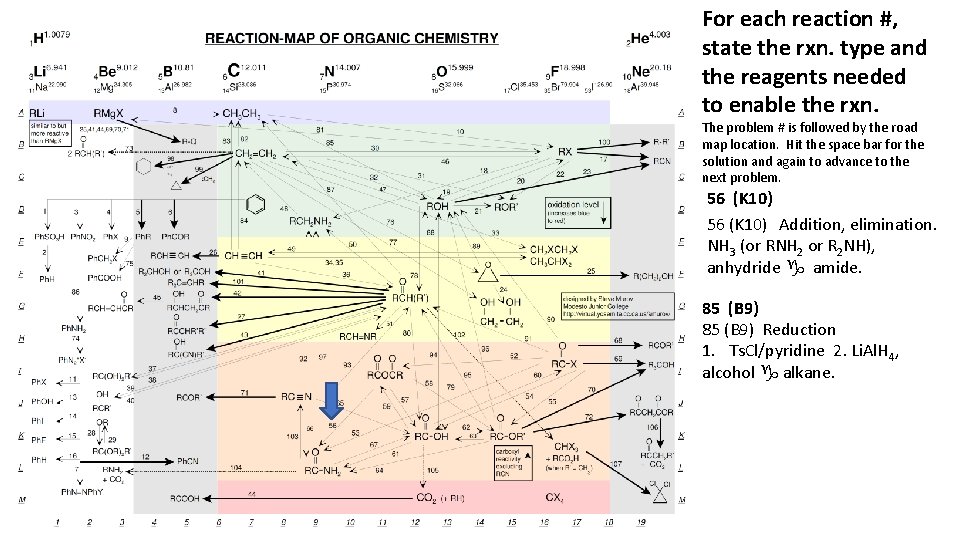
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 56 (K 10) Addition, elimination. NH 3 (or RNH 2 or R 2 NH), anhydride g amide. 85 (B 9) Reduction 1. Ts. Cl/pyridine 2. Li. Al. H 4, alcohol g alkane.

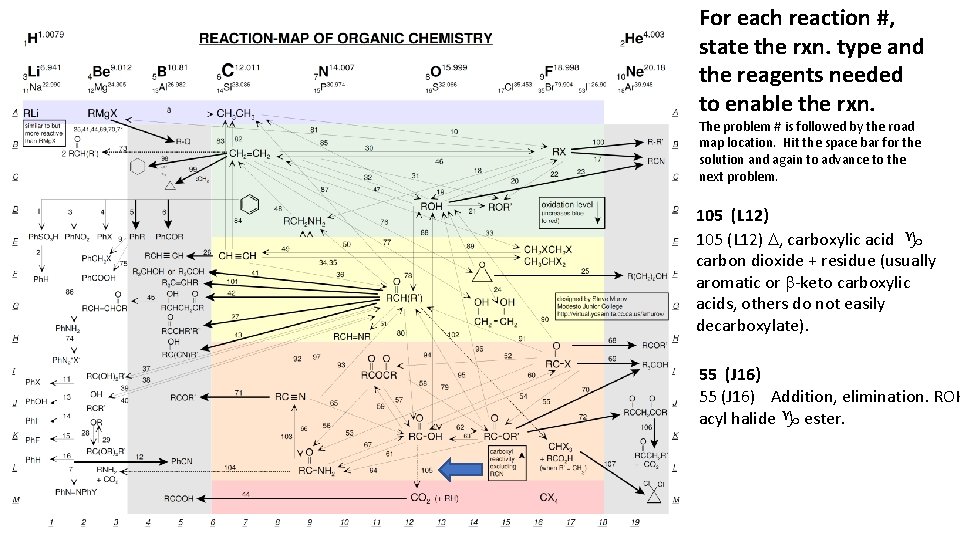
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 105 (L 12) D, carboxylic acid g carbon dioxide + residue (usually aromatic or b-keto carboxylic acids, others do not easily decarboxylate). 55 (J 16) Addition, elimination. ROH acyl halide g ester.

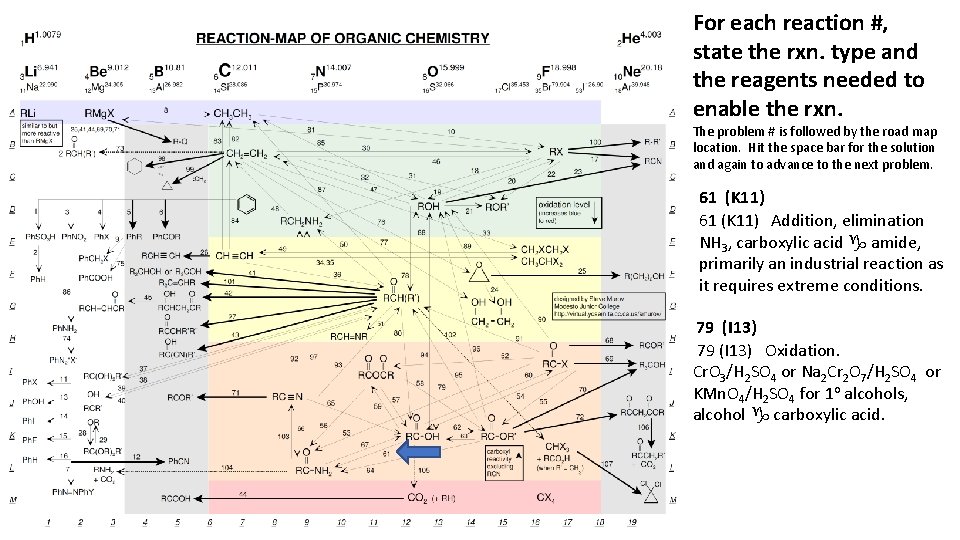
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 61 (K 11) Addition, elimination NH 3, carboxylic acid g amide, primarily an industrial reaction as it requires extreme conditions. 79 (I 13) Oxidation. Cr. O 3/H 2 SO 4 or Na 2 Cr 2 O 7/H 2 SO 4 or KMn. O 4/H 2 SO 4 for 1 o alcohols, alcohol g carboxylic acid.

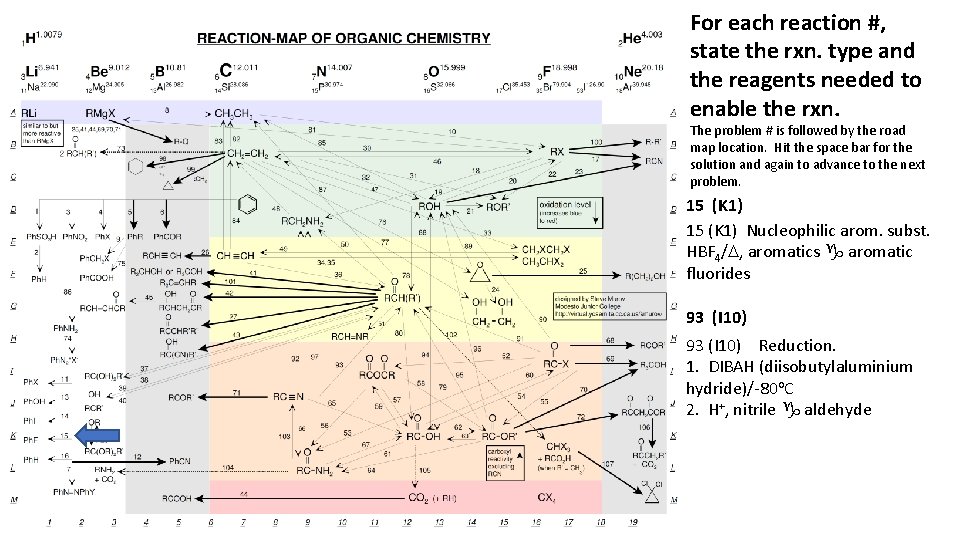
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 15 (K 1) Nucleophilic arom. subst. HBF 4/D, aromatics g aromatic fluorides 93 (I 10) Reduction. 1. DIBAH (diisobutylaluminium hydride)/-80 o. C 2. H+, nitrile g aldehyde

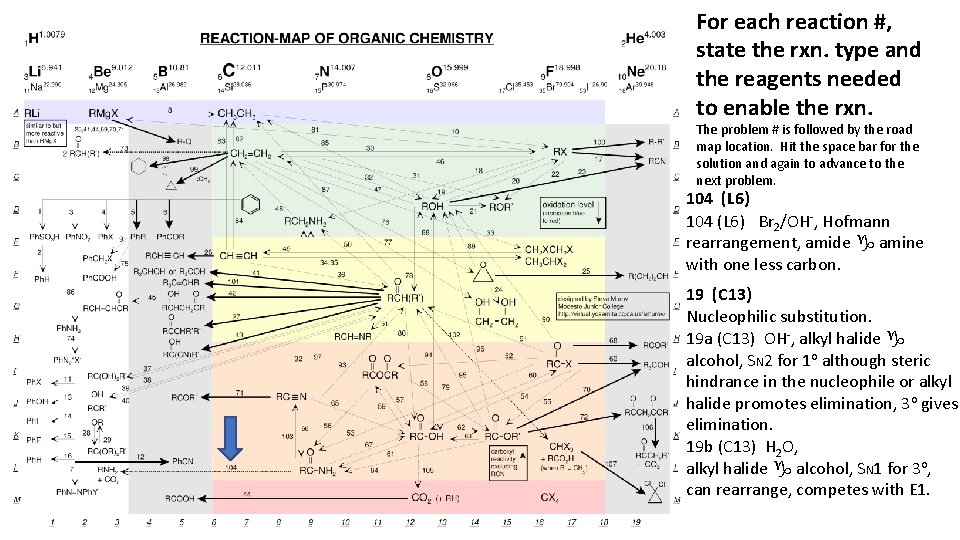
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 104 (L 6) Br 2/OH-, Hofmann rearrangement, amide g amine with one less carbon. 19 (C 13) Nucleophilic substitution. 19 a (C 13) OH-, alkyl halide g alcohol, SN 2 for 1 o although steric hindrance in the nucleophile or alkyl halide promotes elimination, 3 o gives elimination. 19 b (C 13) H 2 O, alkyl halide g alcohol, SN 1 for 3 o, can rearrange, competes with E 1.

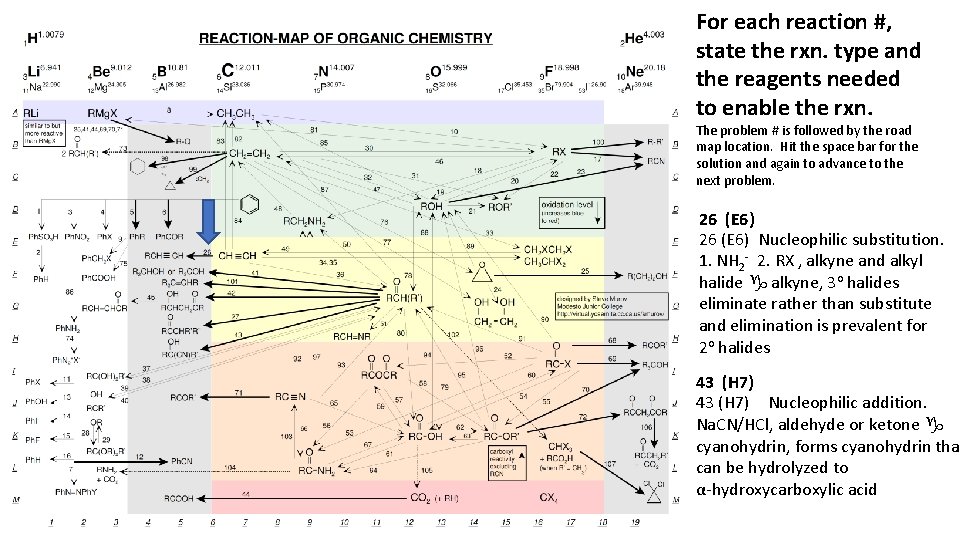
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 26 (E 6) Nucleophilic substitution. 1. NH 2 - 2. RX , alkyne and alkyl halide g alkyne, 3 o halides eliminate rather than substitute and elimination is prevalent for 2 o halides 43 (H 7) Nucleophilic addition. Na. CN/HCl, aldehyde or ketone g cyanohydrin, forms cyanohydrin that can be hydrolyzed to α-hydroxycarboxylic acid

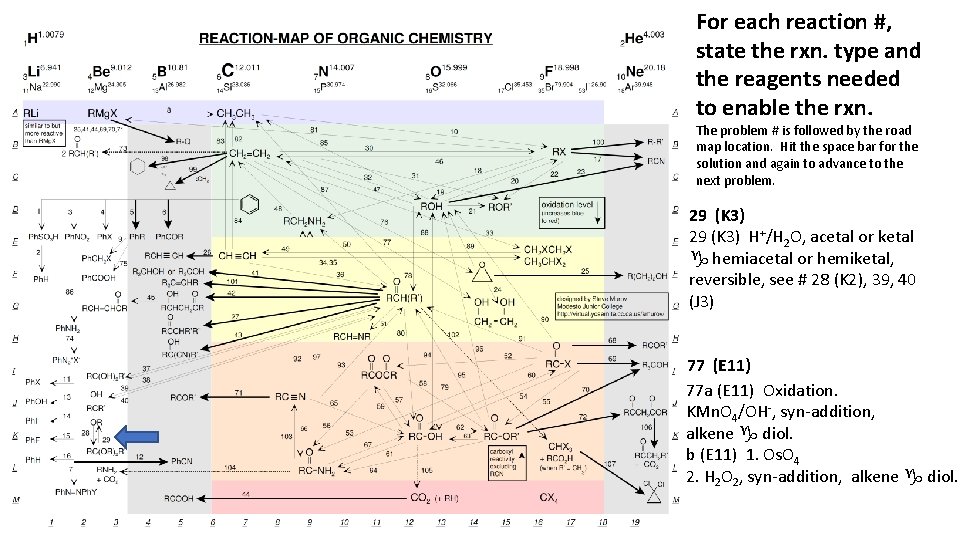
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 29 (K 3) H+/H 2 O, acetal or ketal g hemiacetal or hemiketal, reversible, see # 28 (K 2), 39, 40 (J 3) 77 (E 11) 77 a (E 11) Oxidation. KMn. O 4/OH-, syn-addition, alkene g diol. b (E 11) 1. Os. O 4 2. H 2 O 2, syn-addition, alkene g diol.

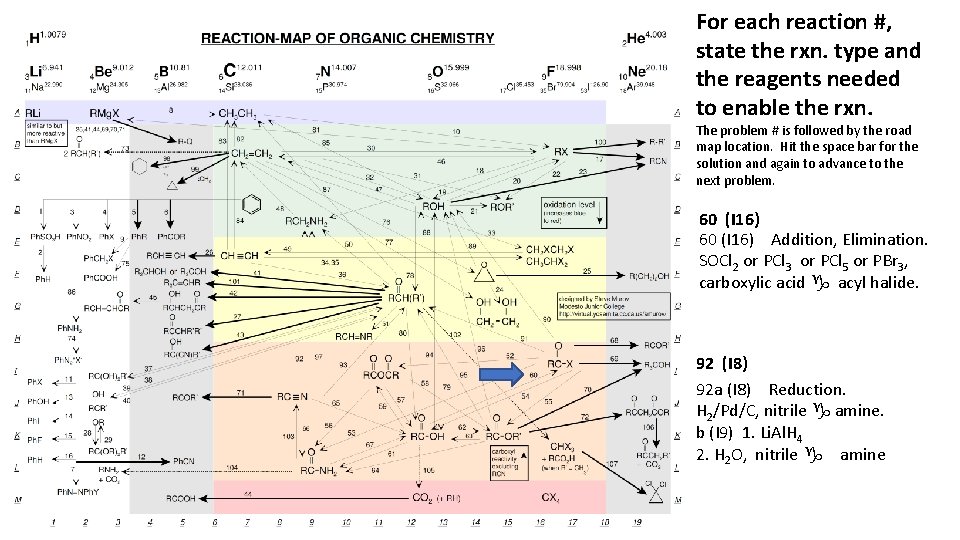
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 60 (I 16) Addition, Elimination. SOCl 2 or PCl 3 or PCl 5 or PBr 3, carboxylic acid g acyl halide. 92 (I 8) 92 a (I 8) Reduction. H 2/Pd/C, nitrile g amine. b (I 9) 1. Li. Al. H 4 2. H 2 O, nitrile g amine

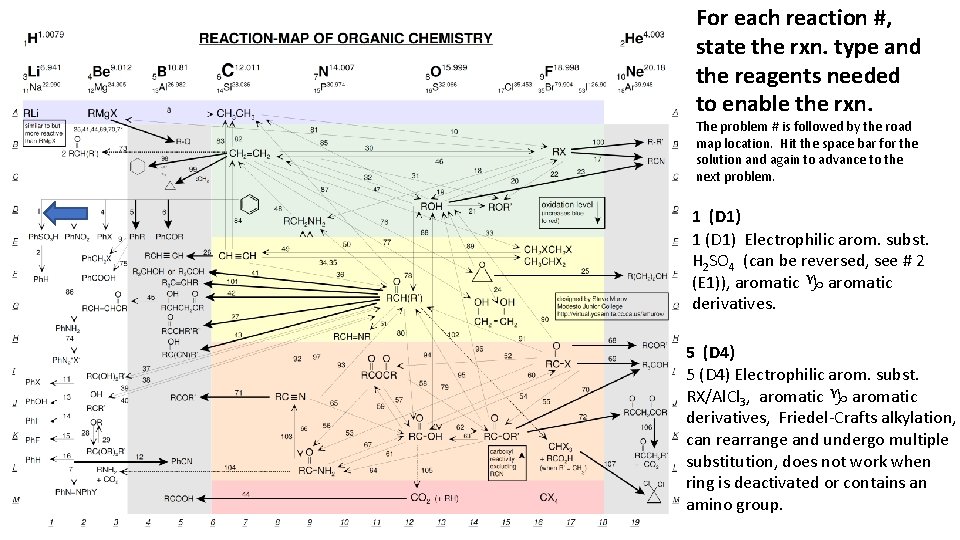
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 1 (D 1) Electrophilic arom. subst. H 2 SO 4 (can be reversed, see # 2 (E 1)), aromatic g aromatic derivatives. 5 (D 4) Electrophilic arom. subst. RX/Al. Cl 3, aromatic g aromatic derivatives, Friedel-Crafts alkylation, can rearrange and undergo multiple substitution, does not work when ring is deactivated or contains an amino group.

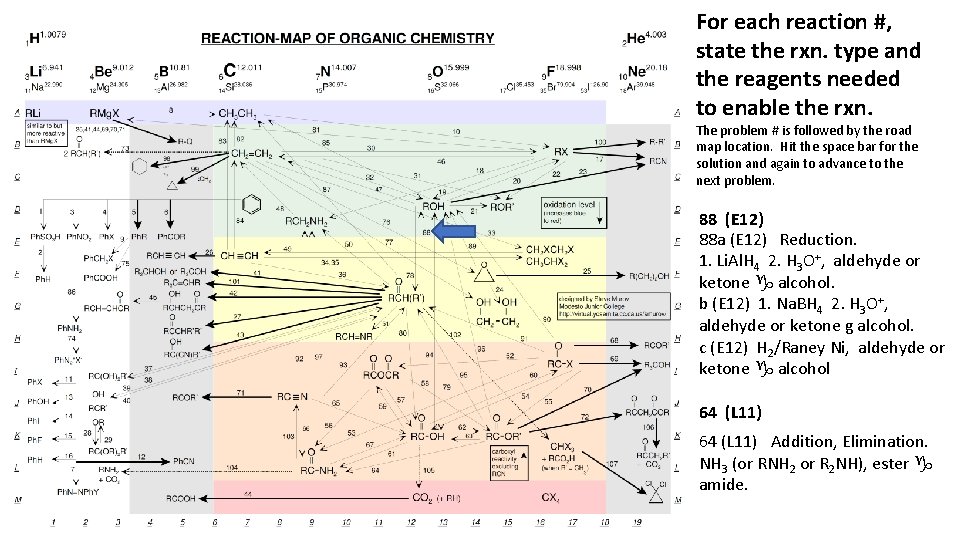
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 88 (E 12) 88 a (E 12) Reduction. 1. Li. Al. H 4 2. H 3 O+, aldehyde or ketone g alcohol. b (E 12) 1. Na. BH 4 2. H 3 O+, aldehyde or ketone g alcohol. c (E 12) H 2/Raney Ni, aldehyde or ketone g alcohol 64 (L 11) Addition, Elimination. NH 3 (or RNH 2 or R 2 NH), ester g amide.

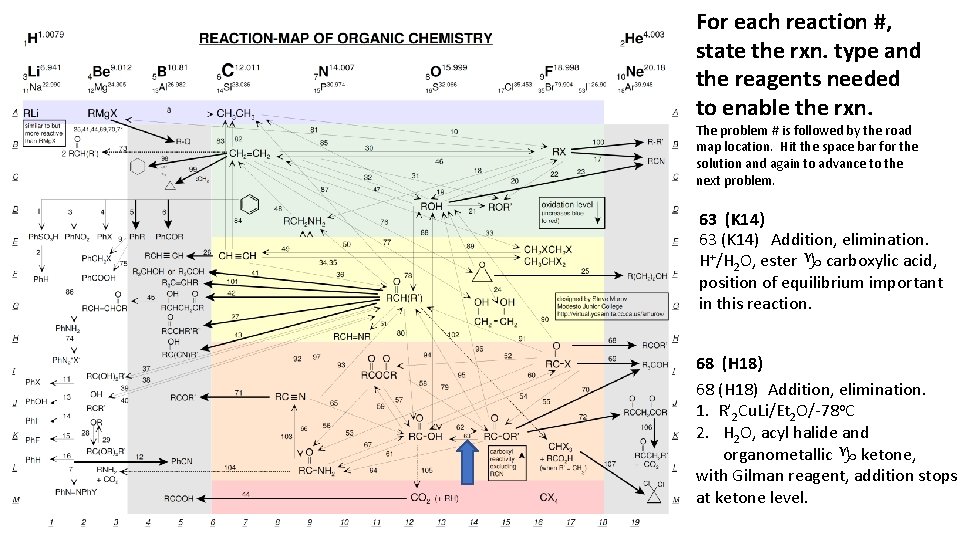
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 63 (K 14) Addition, elimination. H+/H 2 O, ester g carboxylic acid, position of equilibrium important in this reaction. 68 (H 18) Addition, elimination. 1. R’ 2 Cu. Li/Et 2 O/-78 o. C 2. H 2 O, acyl halide and organometallic g ketone, with Gilman reagent, addition stops at ketone level.

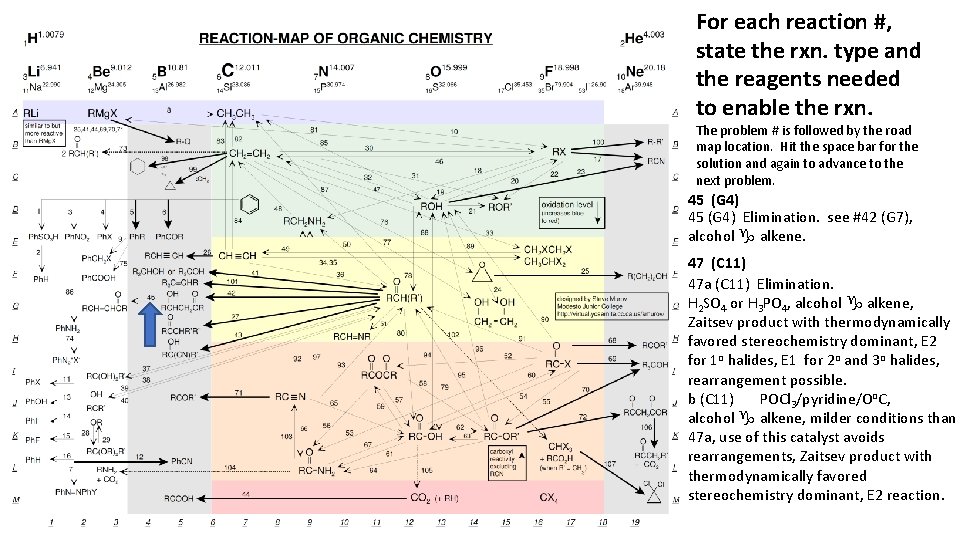
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 45 (G 4) Elimination. see #42 (G 7), alcohol g alkene. 47 (C 11) 47 a (C 11) Elimination. H 2 SO 4 or H 3 PO 4, alcohol g alkene, Zaitsev product with thermodynamically favored stereochemistry dominant, E 2 for 1 o halides, E 1 for 2 o and 3 o halides, rearrangement possible. b (C 11) POCl 3/pyridine/Oo. C, alcohol g alkene, milder conditions than 47 a, use of this catalyst avoids rearrangements, Zaitsev product with thermodynamically favored stereochemistry dominant, E 2 reaction.

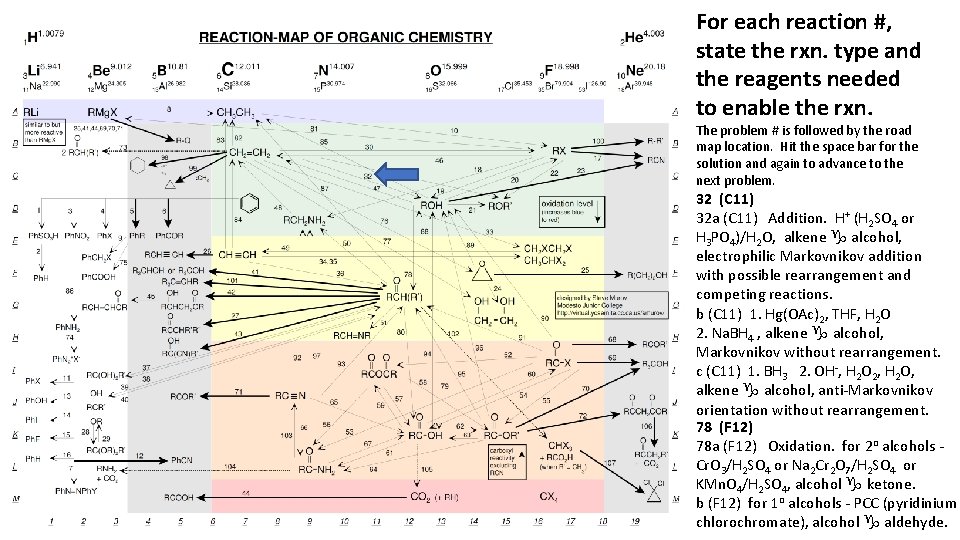
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 32 (C 11) 32 a (C 11) Addition. H+ (H 2 SO 4 or H 3 PO 4)/H 2 O, alkene g alcohol, electrophilic Markovnikov addition with possible rearrangement and competing reactions. b (C 11) 1. Hg(OAc)2, THF, H 2 O 2. Na. BH 4 , alkene g alcohol, Markovnikov without rearrangement. c (C 11) 1. BH 3 2. OH-, H 2 O 2, H 2 O, alkene g alcohol, anti-Markovnikov orientation without rearrangement. 78 (F 12) 78 a (F 12) Oxidation. for 2 o alcohols Cr. O 3/H 2 SO 4 or Na 2 Cr 2 O 7/H 2 SO 4 or KMn. O 4/H 2 SO 4, alcohol g ketone. b (F 12) for 1 o alcohols - PCC (pyridinium chlorochromate), alcohol g aldehyde.

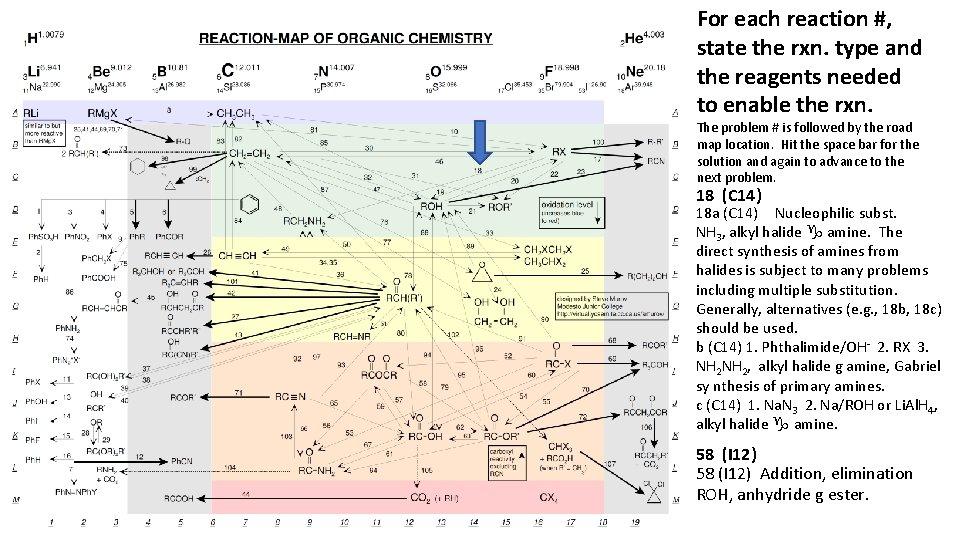
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 18 (C 14) 18 a (C 14) Nucleophilic subst. NH 3, alkyl halide g amine. The direct synthesis of amines from halides is subject to many problems including multiple substitution. Generally, alternatives (e. g. , 18 b, 18 c) should be used. b (C 14) 1. Phthalimide/OH- 2. RX 3. NH 2, alkyl halide g amine, Gabriel sy nthesis of primary amines. c (C 14) 1. Na. N 3 2. Na/ROH or Li. Al. H 4, alkyl halide g amine. 58 (I 12) Addition, elimination ROH, anhydride g ester.

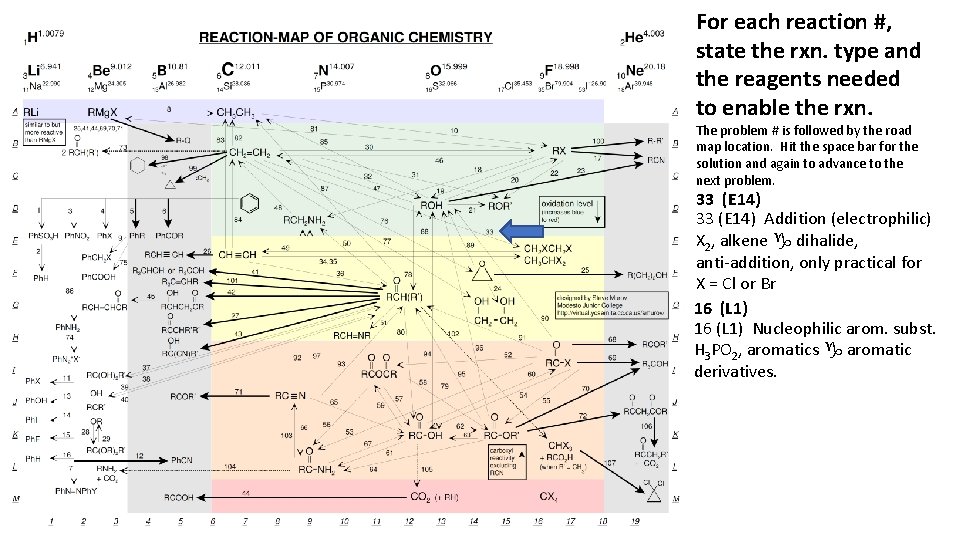
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 33 (E 14) Addition (electrophilic) X 2, alkene g dihalide, anti-addition, only practical for X = Cl or Br 16 (L 1) Nucleophilic arom. subst. H 3 PO 2, aromatics g aromatic derivatives.

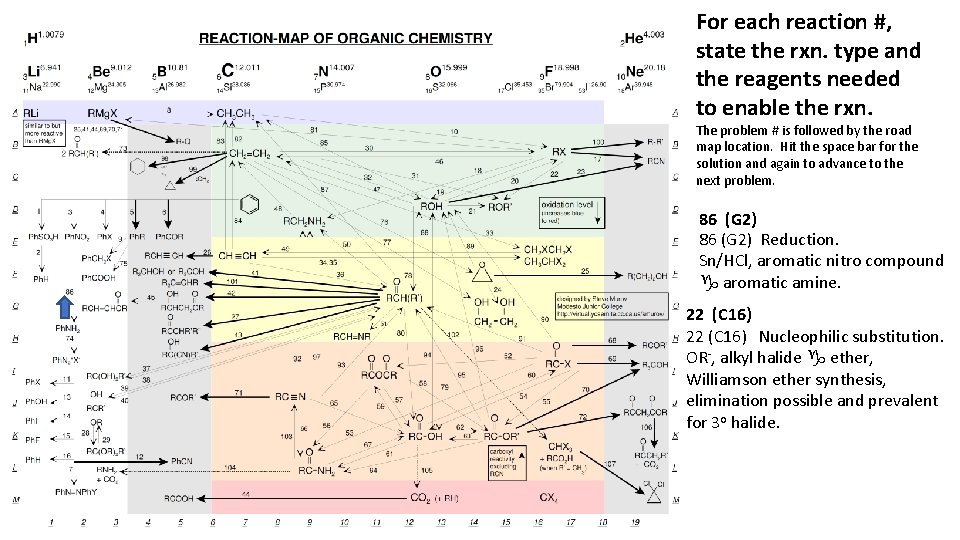
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 86 (G 2) Reduction. Sn/HCl, aromatic nitro compound g aromatic amine. 22 (C 16) Nucleophilic substitution. OR-, alkyl halide g ether, Williamson ether synthesis, elimination possible and prevalent for 3 o halide.

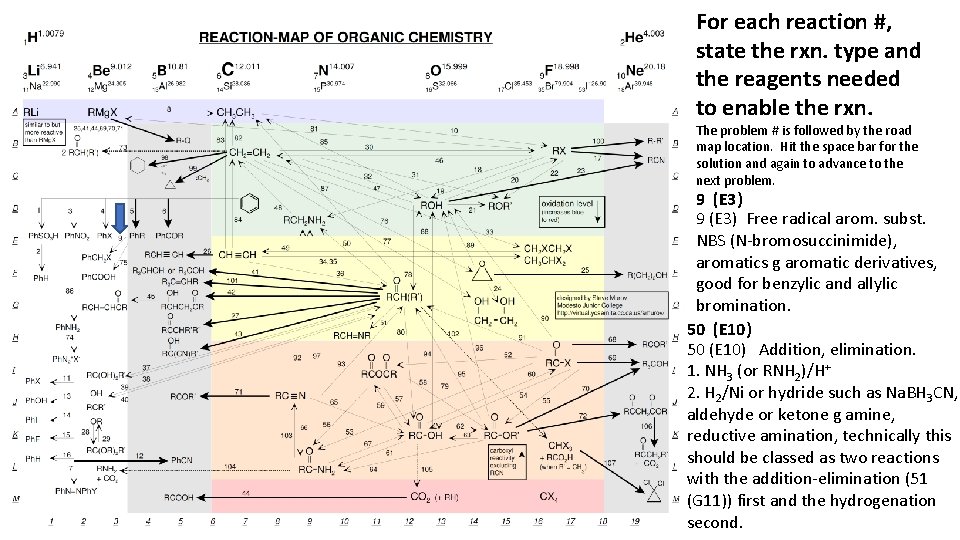
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 9 (E 3) Free radical arom. subst. NBS (N-bromosuccinimide), aromatics g aromatic derivatives, good for benzylic and allylic bromination. 50 (E 10) Addition, elimination. 1. NH 3 (or RNH 2)/H+ 2. H 2/Ni or hydride such as Na. BH 3 CN, aldehyde or ketone g amine, reductive amination, technically this should be classed as two reactions with the addition-elimination (51 (G 11)) first and the hydrogenation second.

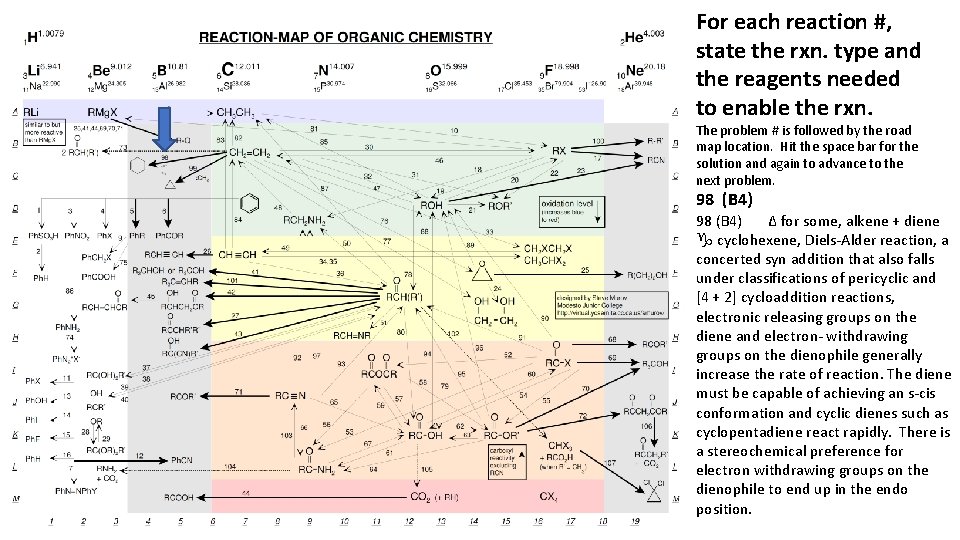
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 98 (B 4) Δ for some, alkene + diene g cyclohexene, Diels-Alder reaction, a concerted syn addition that also falls under classifications of pericyclic and [4 + 2] cycloaddition reactions, electronic releasing groups on the diene and electron- withdrawing groups on the dienophile generally increase the rate of reaction. The diene must be capable of achieving an s-cis conformation and cyclic dienes such as cyclopentadiene react rapidly. There is a stereochemical preference for electron withdrawing groups on the dienophile to end up in the endo position.

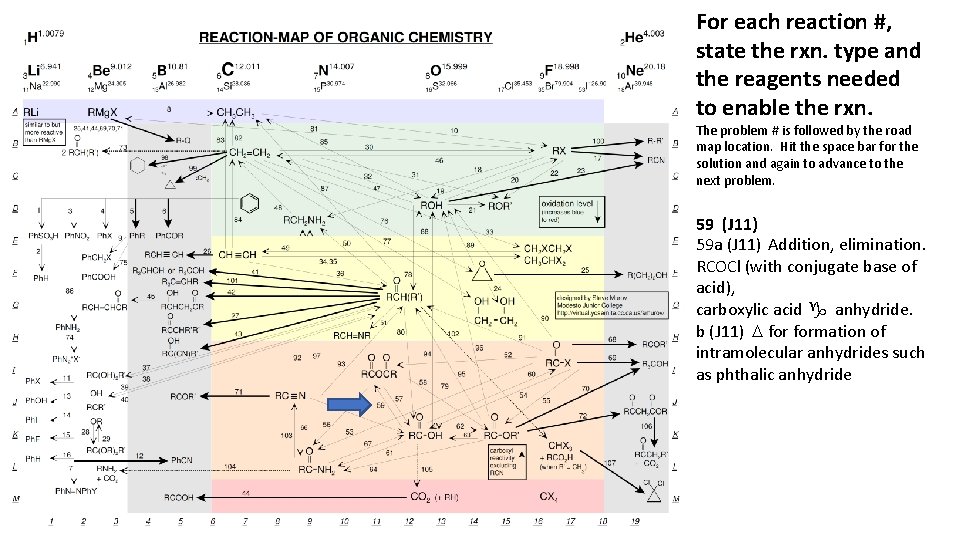
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 59 (J 11) 59 a (J 11) Addition, elimination. RCOCl (with conjugate base of acid), carboxylic acid g anhydride. b (J 11) D formation of intramolecular anhydrides such as phthalic anhydride

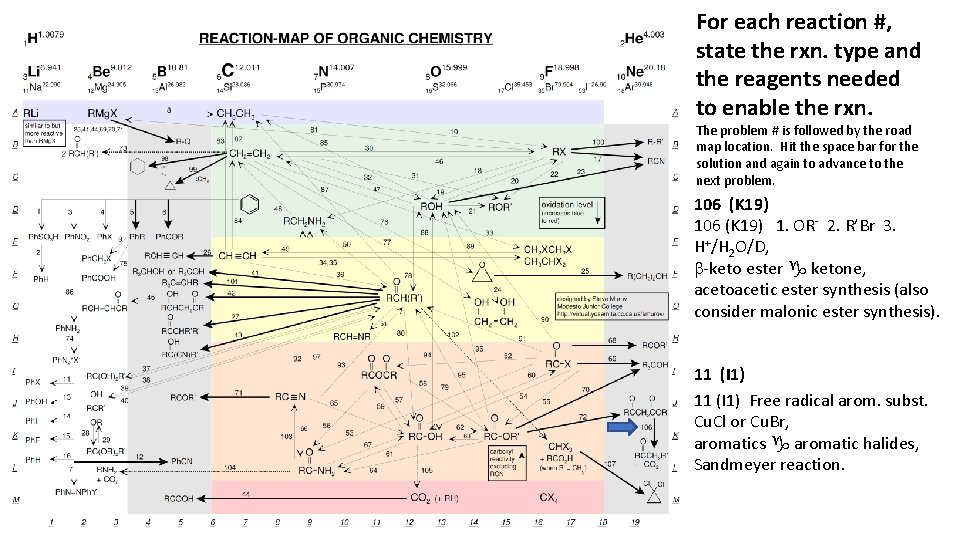
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 106 (K 19) 1. OR- 2. R’Br 3. H+/H 2 O/D, b-keto ester g ketone, acetoacetic ester synthesis (also consider malonic ester synthesis). 11 (I 1) Free radical arom. subst. Cu. Cl or Cu. Br, aromatics g aromatic halides, Sandmeyer reaction.

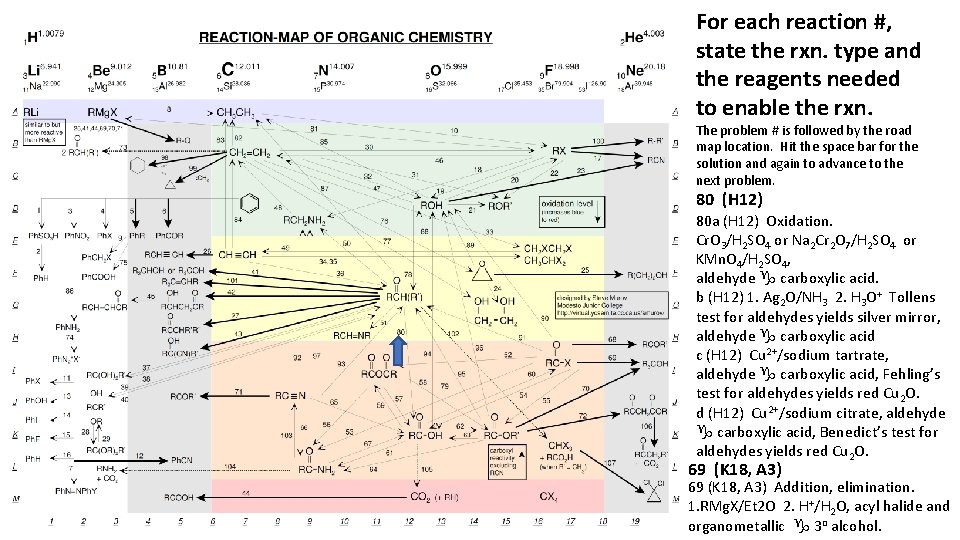
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 80 (H 12) 80 a (H 12) Oxidation. Cr. O 3/H 2 SO 4 or Na 2 Cr 2 O 7/H 2 SO 4 or KMn. O 4/H 2 SO 4, aldehyde g carboxylic acid. b (H 12) 1. Ag 2 O/NH 3 2. H 3 O+ Tollens test for aldehydes yields silver mirror, aldehyde g carboxylic acid c (H 12) Cu 2+/sodium tartrate, aldehyde g carboxylic acid, Fehling’s test for aldehydes yields red Cu 2 O. d (H 12) Cu 2+/sodium citrate, aldehyde g carboxylic acid, Benedict’s test for aldehydes yields red Cu 2 O. 69 (K 18, A 3) Addition, elimination. 1. RMg. X/Et 2 O 2. H+/H 2 O, acyl halide and organometallic g 3 o alcohol.

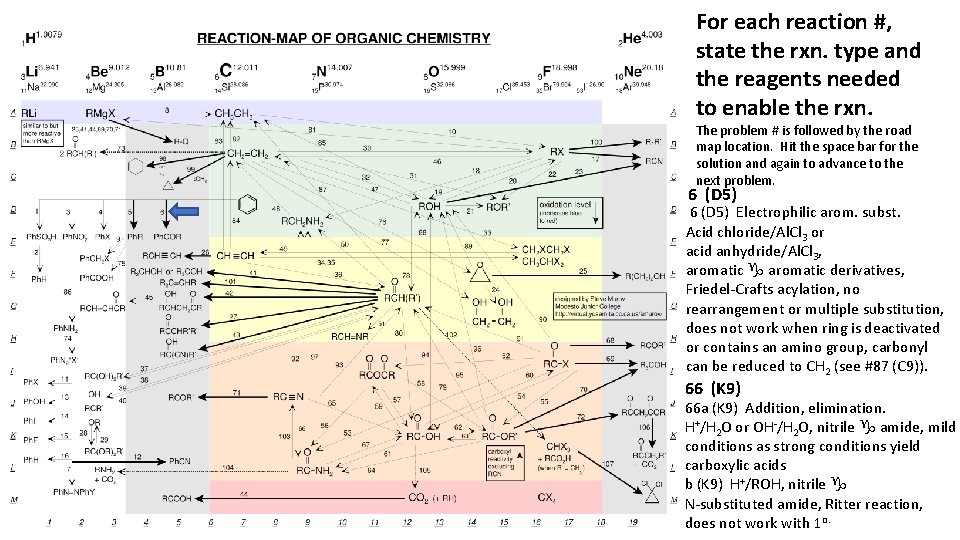
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 6 (D 5) Electrophilic arom. subst. Acid chloride/Al. Cl 3 or acid anhydride/Al. Cl 3, aromatic g aromatic derivatives, Friedel-Crafts acylation, no rearrangement or multiple substitution, does not work when ring is deactivated or contains an amino group, carbonyl can be reduced to CH 2 (see #87 (C 9)). 66 (K 9) 66 a (K 9) Addition, elimination. H+/H 2 O or OH-/H 2 O, nitrile g amide, mild conditions as strong conditions yield carboxylic acids b (K 9) H+/ROH, nitrile g N-substituted amide, Ritter reaction, does not work with 1 o.

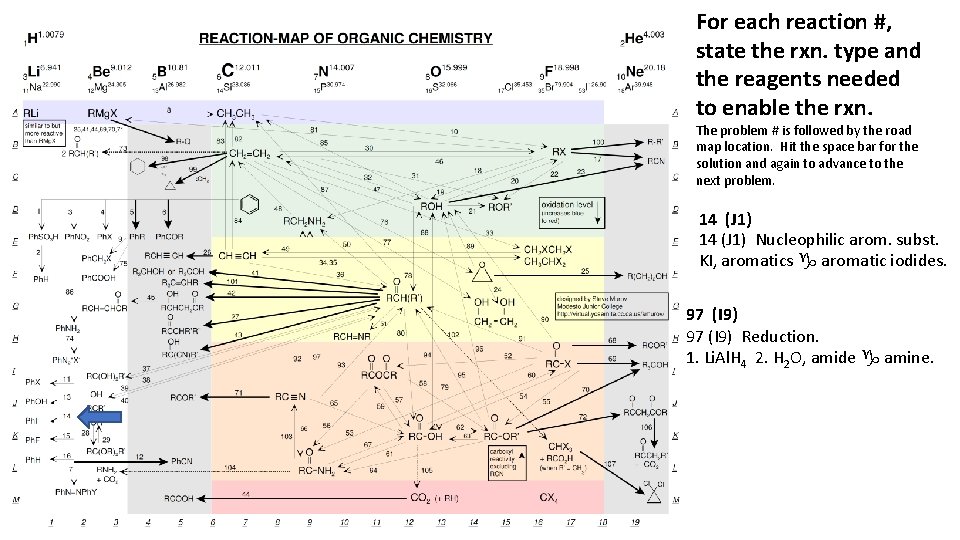
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 14 (J 1) Nucleophilic arom. subst. KI, aromatics g aromatic iodides. 97 (I 9) Reduction. 1. Li. Al. H 4 2. H 2 O, amide g amine.

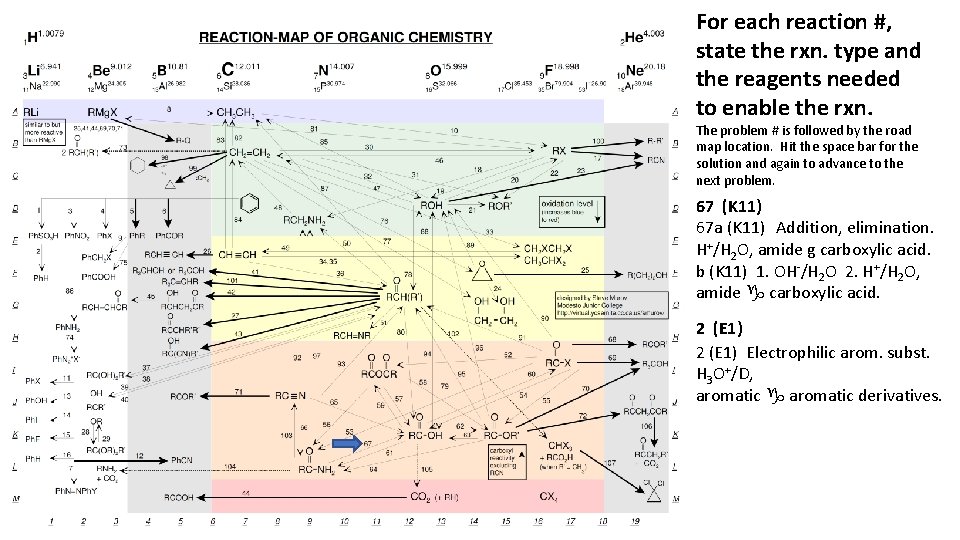
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 67 (K 11) 67 a (K 11) Addition, elimination. H+/H 2 O, amide g carboxylic acid. b (K 11) 1. OH-/H 2 O 2. H+/H 2 O, amide g carboxylic acid. 2 (E 1) Electrophilic arom. subst. H 3 O+/D, aromatic g aromatic derivatives.

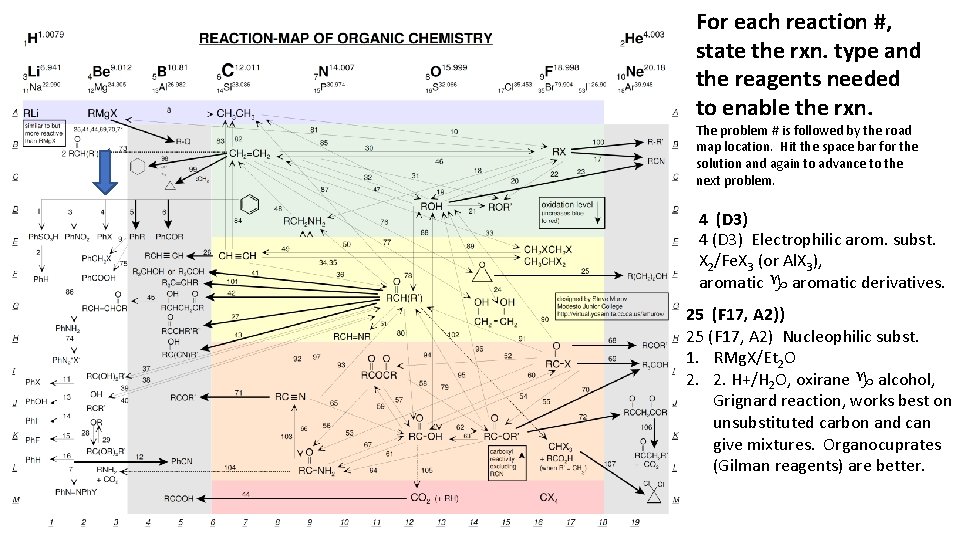
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 4 (D 3) Electrophilic arom. subst. X 2/Fe. X 3 (or Al. X 3), aromatic g aromatic derivatives. 25 (F 17, A 2)) 25 (F 17, A 2) Nucleophilic subst. 1. RMg. X/Et 2 O 2. 2. H+/H 2 O, oxirane g alcohol, Grignard reaction, works best on unsubstituted carbon and can give mixtures. Organocuprates (Gilman reagents) are better.

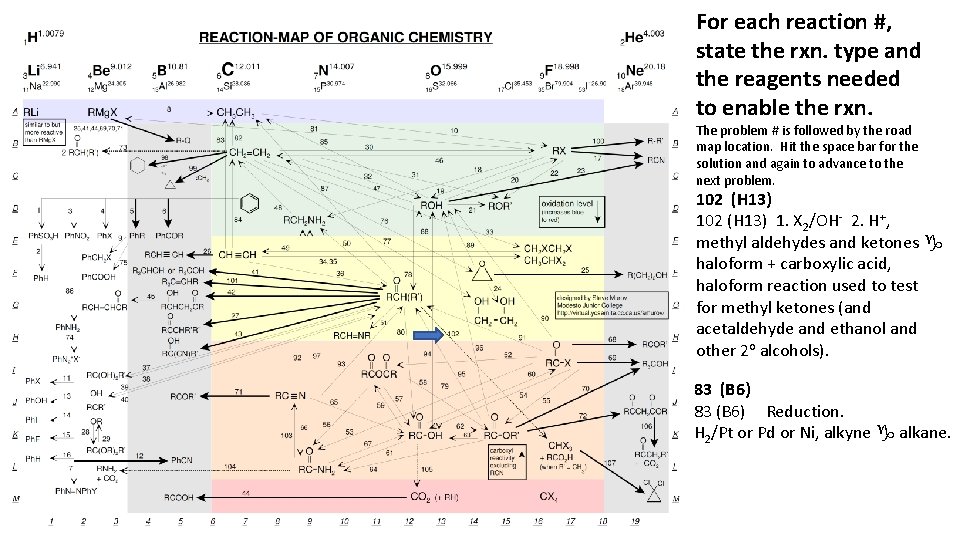
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 102 (H 13) 1. X 2/OH- 2. H+, methyl aldehydes and ketones g haloform + carboxylic acid, haloform reaction used to test for methyl ketones (and acetaldehyde and ethanol and other 2 o alcohols). 83 (B 6) Reduction. H 2/Pt or Pd or Ni, alkyne g alkane.

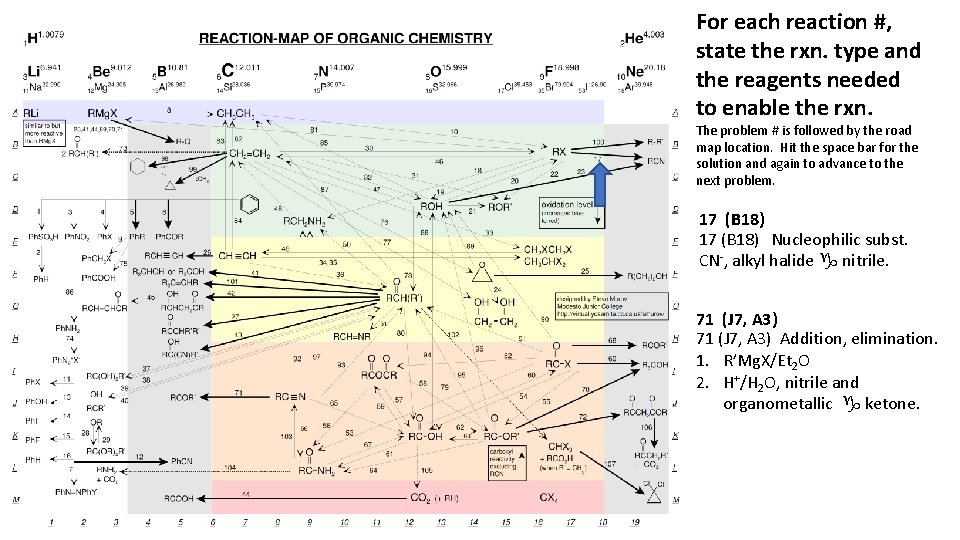
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 17 (B 18) Nucleophilic subst. CN-, alkyl halide g nitrile. 71 (J 7, A 3) Addition, elimination. 1. R’Mg. X/Et 2 O 2. H+/H 2 O, nitrile and organometallic g ketone.

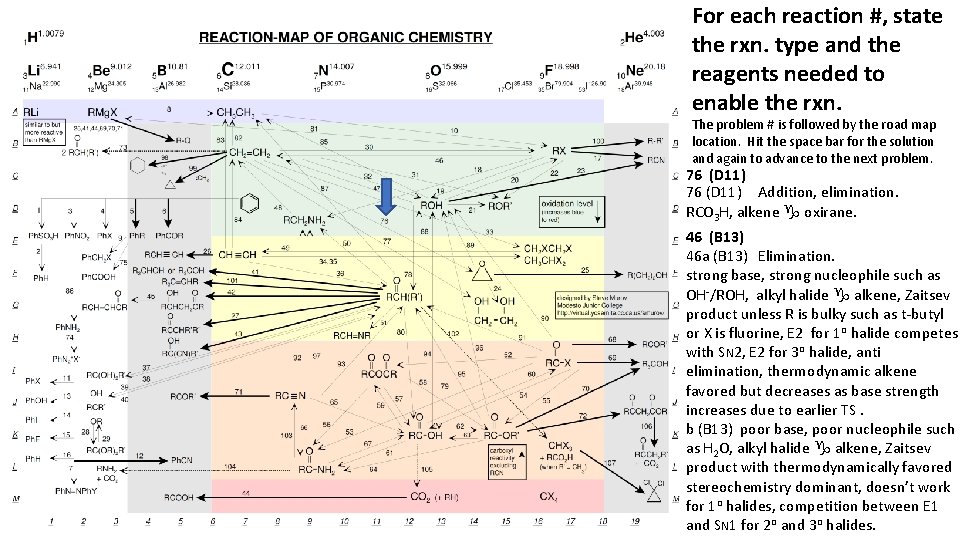
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 76 (D 11) Addition, elimination. RCO 3 H, alkene g oxirane. 46 (B 13) 46 a (B 13) Elimination. strong base, strong nucleophile such as OH-/ROH, alkyl halide g alkene, Zaitsev product unless R is bulky such as t-butyl or X is fluorine, E 2 for 1 o halide competes with SN 2, E 2 for 3 o halide, anti elimination, thermodynamic alkene favored but decreases as base strength increases due to earlier TS. b (B 13) poor base, poor nucleophile such as H 2 O, alkyl halide g alkene, Zaitsev product with thermodynamically favored stereochemistry dominant, doesn’t work for 1 o halides, competition between E 1 and SN 1 for 2 o and 3 o halides.

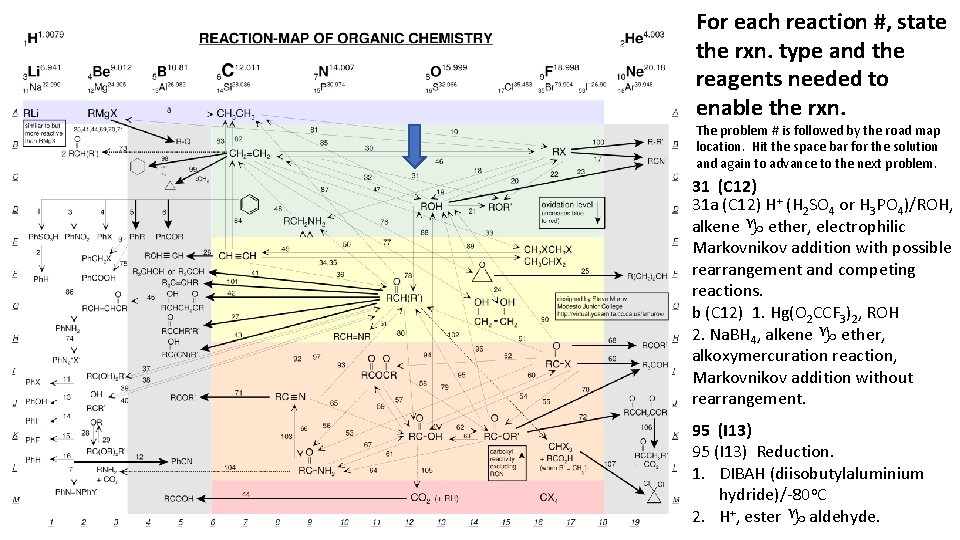
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 31 (C 12) 31 a (C 12) H+ (H 2 SO 4 or H 3 PO 4)/ROH, alkene g ether, electrophilic Markovnikov addition with possible rearrangement and competing reactions. b (C 12) 1. Hg(O 2 CCF 3)2, ROH 2. Na. BH 4, alkene g ether, alkoxymercuration reaction, Markovnikov addition without rearrangement. 95 (I 13) Reduction. 1. DIBAH (diisobutylaluminium hydride)/-80 o. C 2. H+, ester g aldehyde.

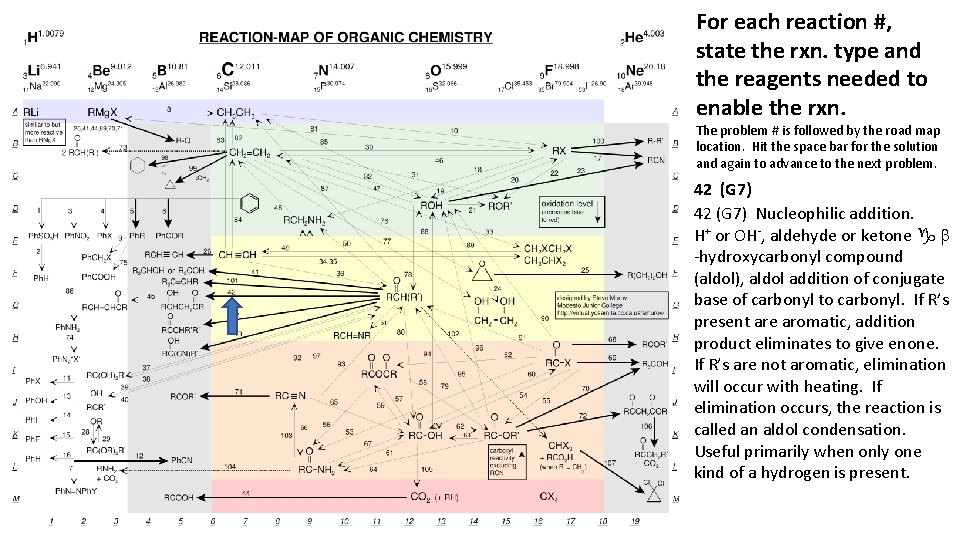
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 42 (G 7) Nucleophilic addition. H+ or OH-, aldehyde or ketone g b -hydroxycarbonyl compound (aldol), aldol addition of conjugate base of carbonyl to carbonyl. If R’s present are aromatic, addition product eliminates to give enone. If R’s are not aromatic, elimination will occur with heating. If elimination occurs, the reaction is called an aldol condensation. Useful primarily when only one kind of a hydrogen is present.

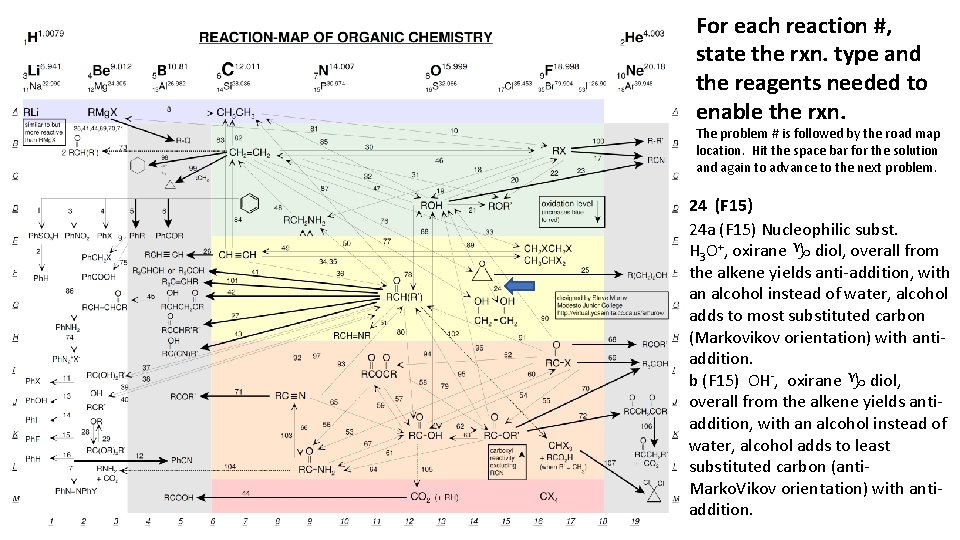
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 24 (F 15) 24 a (F 15) Nucleophilic subst. H 3 O+, oxirane g diol, overall from the alkene yields anti-addition, with an alcohol instead of water, alcohol adds to most substituted carbon (Markovikov orientation) with antiaddition. b (F 15) OH-, oxirane g diol, overall from the alkene yields antiaddition, with an alcohol instead of water, alcohol adds to least substituted carbon (anti. Marko. Vikov orientation) with antiaddition.

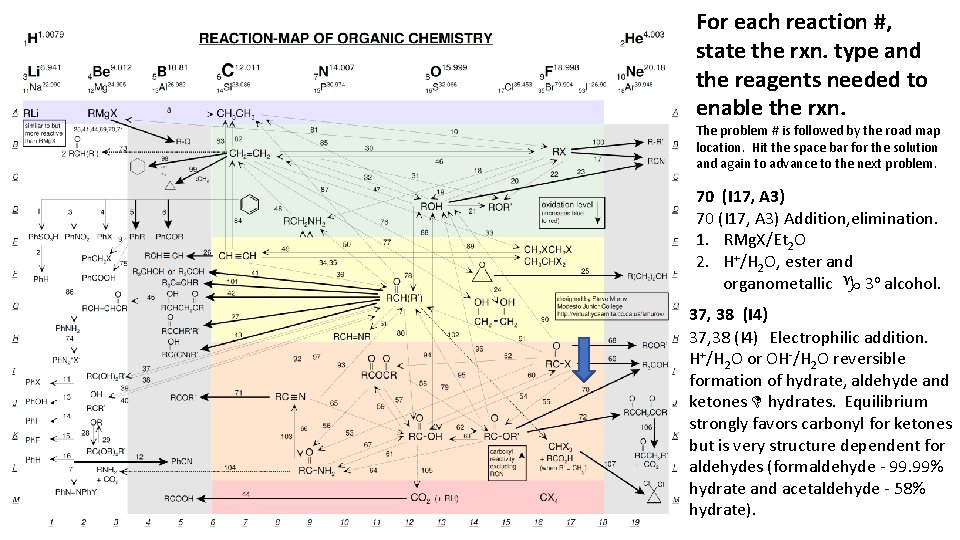
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 70 (I 17, A 3) Addition, elimination. 1. RMg. X/Et 2 O 2. H+/H 2 O, ester and organometallic g 3 o alcohol. 37, 38 (I 4) Electrophilic addition. H+/H 2 O or OH-/H 2 O reversible formation of hydrate, aldehyde and ketones D hydrates. Equilibrium strongly favors carbonyl for ketones but is very structure dependent for aldehydes (formaldehyde - 99. 99% hydrate and acetaldehyde - 58% hydrate).

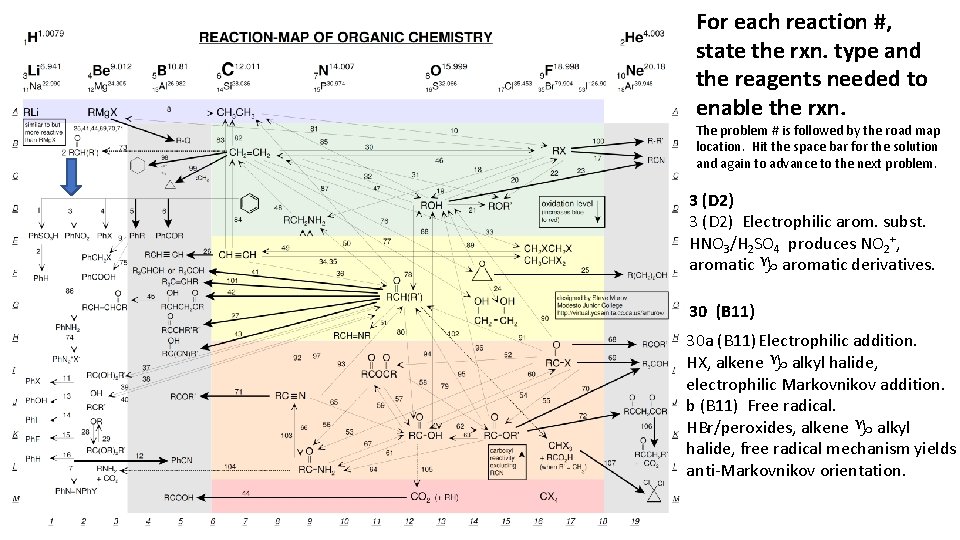
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 3 (D 2) Electrophilic arom. subst. HNO 3/H 2 SO 4 produces NO 2+, aromatic g aromatic derivatives. 30 (B 11) 30 a (B 11) Electrophilic addition. HX, alkene g alkyl halide, electrophilic Markovnikov addition. b (B 11) Free radical. HBr/peroxides, alkene g alkyl halide, free radical mechanism yields anti-Markovnikov orientation.

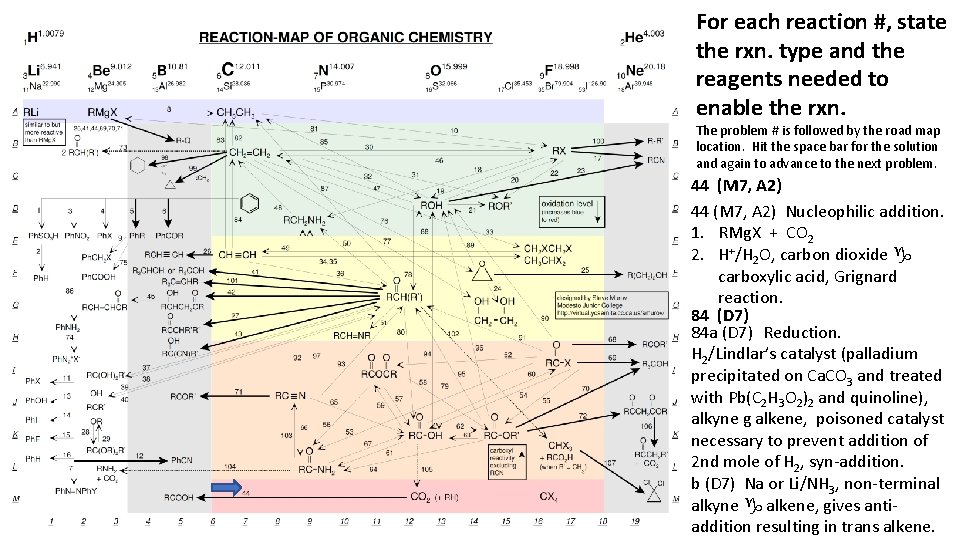
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 44 (M 7, A 2) Nucleophilic addition. 1. RMg. X + CO 2 2. H+/H 2 O, carbon dioxide g carboxylic acid, Grignard reaction. 84 (D 7) 84 a (D 7) Reduction. H 2/Lindlar’s catalyst (palladium precipitated on Ca. CO 3 and treated with Pb(C 2 H 3 O 2)2 and quinoline), alkyne g alkene, poisoned catalyst necessary to prevent addition of 2 nd mole of H 2, syn-addition. b (D 7) Na or Li/NH 3, non-terminal alkyne g alkene, gives antiaddition resulting in trans alkene.

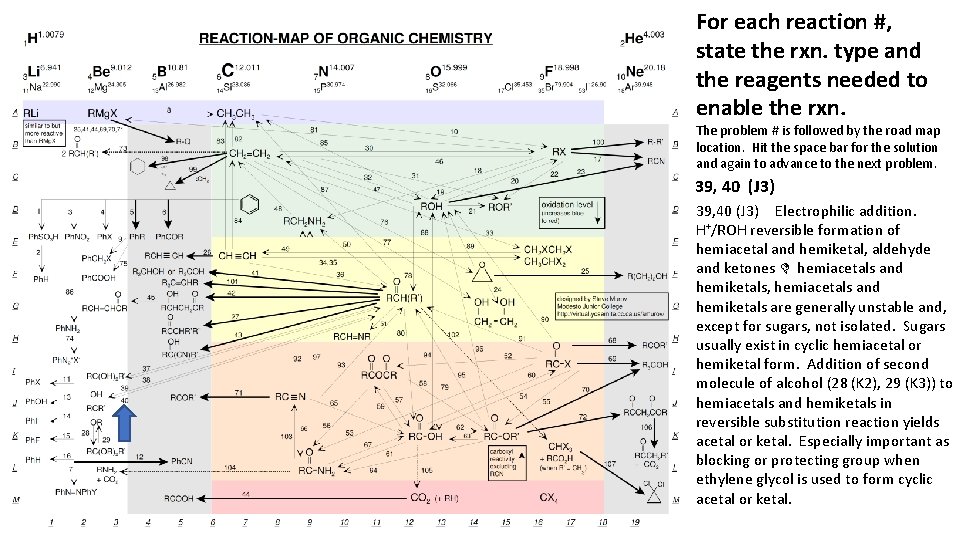
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 39, 40 (J 3) Electrophilic addition. H+/ROH reversible formation of hemiacetal and hemiketal, aldehyde and ketones D hemiacetals and hemiketals, hemiacetals and hemiketals are generally unstable and, except for sugars, not isolated. Sugars usually exist in cyclic hemiacetal or hemiketal form. Addition of second molecule of alcohol (28 (K 2), 29 (K 3)) to hemiacetals and hemiketals in reversible substitution reaction yields acetal or ketal. Especially important as blocking or protecting group when ethylene glycol is used to form cyclic acetal or ketal.

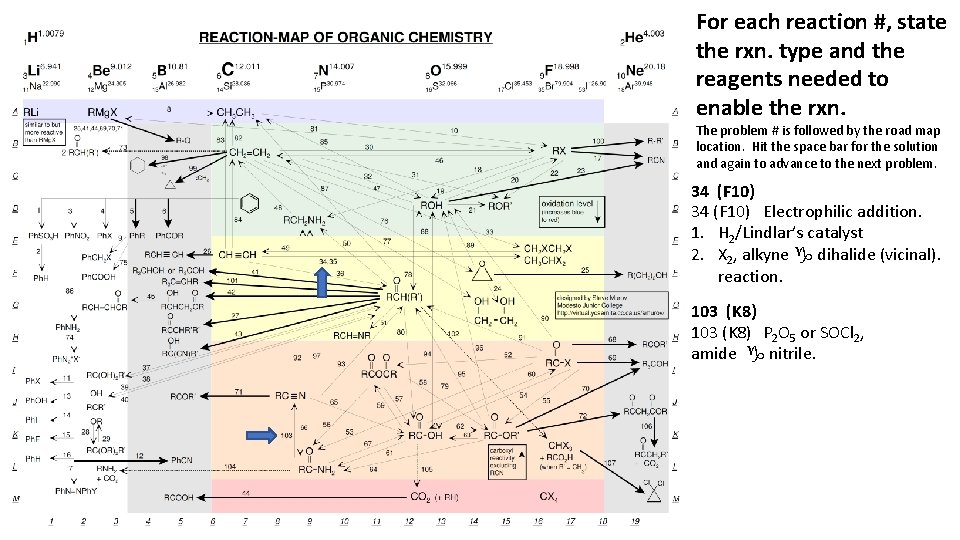
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 34 (F 10) Electrophilic addition. 1. H 2/Lindlar’s catalyst 2. X 2, alkyne g dihalide (vicinal). reaction. 103 (K 8) P 2 O 5 or SOCl 2, amide g nitrile.

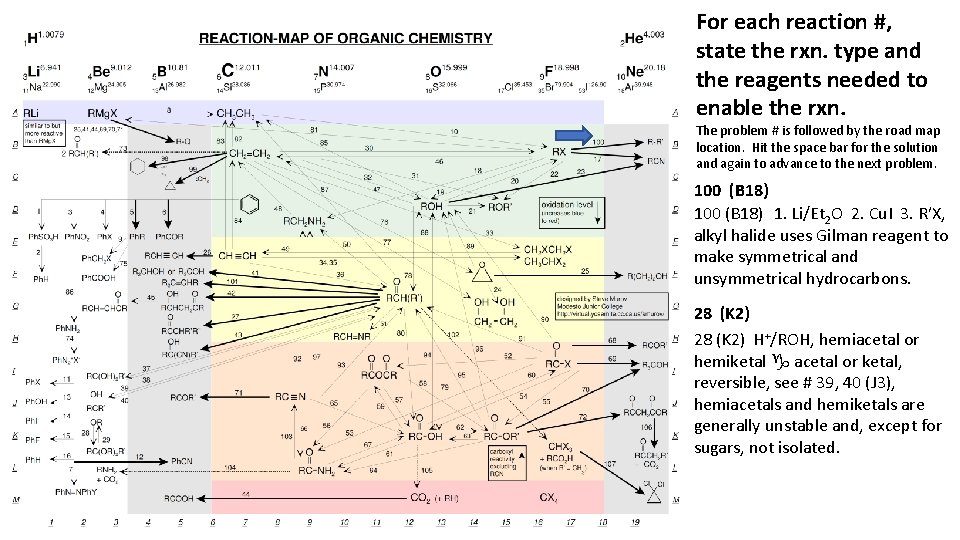
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 100 (B 18) 1. Li/Et 2 O 2. Cu. I 3. RʹX, alkyl halide uses Gilman reagent to make symmetrical and unsymmetrical hydrocarbons. 28 (K 2) H+/ROH, hemiacetal or hemiketal g acetal or ketal, reversible, see # 39, 40 (J 3), hemiacetals and hemiketals are generally unstable and, except for sugars, not isolated.

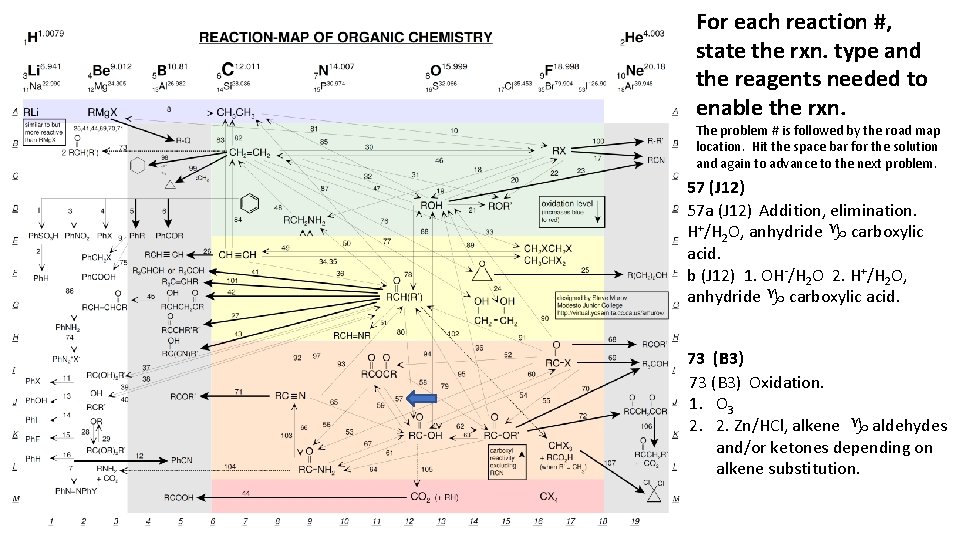
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 57 (J 12) 57 a (J 12) Addition, elimination. H+/H 2 O, anhydride g carboxylic acid. b (J 12) 1. OH-/H 2 O 2. H+/H 2 O, anhydride g carboxylic acid. 73 (B 3) Oxidation. 1. O 3 2. 2. Zn/HCl, alkene g aldehydes and/or ketones depending on alkene substitution.

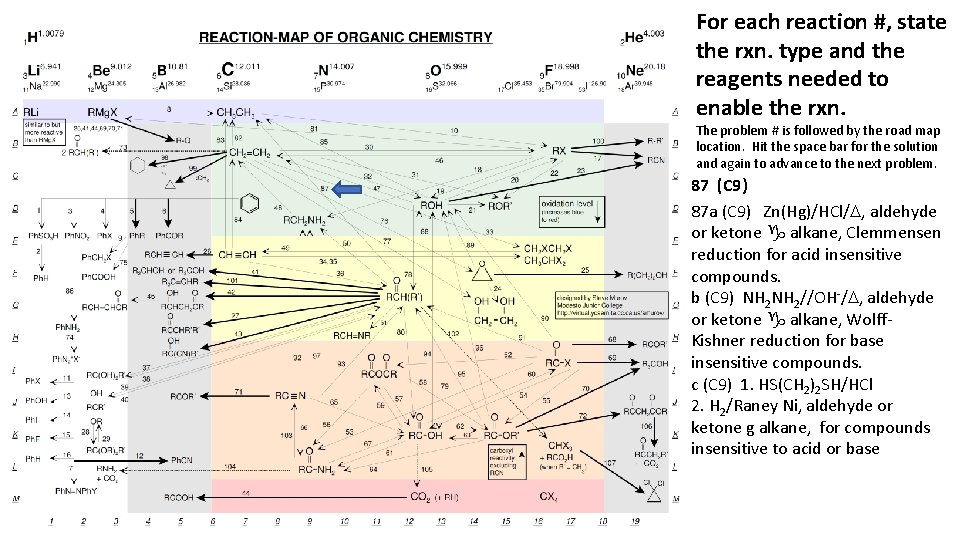
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 87 (C 9) 87 a (C 9) Zn(Hg)/HCl/D, aldehyde or ketone g alkane, Clemmensen reduction for acid insensitive compounds. b (C 9) NH 2//OH-/D, aldehyde or ketone g alkane, Wolff. Kishner reduction for base insensitive compounds. c (C 9) 1. HS(CH 2)2 SH/HCl 2. H 2/Raney Ni, aldehyde or ketone g alkane, for compounds insensitive to acid or base

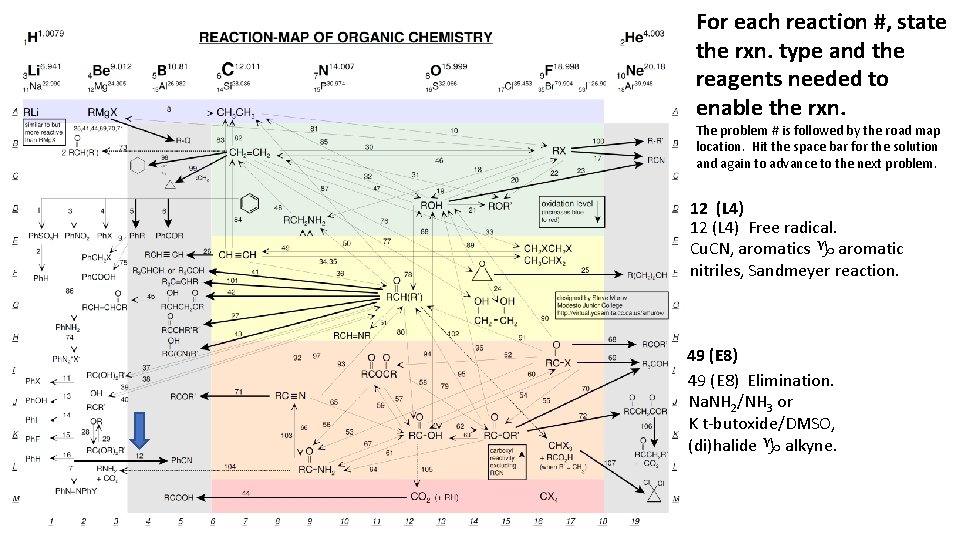
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 12 (L 4) Free radical. Cu. CN, aromatics g aromatic nitriles, Sandmeyer reaction. 49 (E 8) Elimination. Na. NH 2/NH 3 or K t-butoxide/DMSO, (di)halide g alkyne.

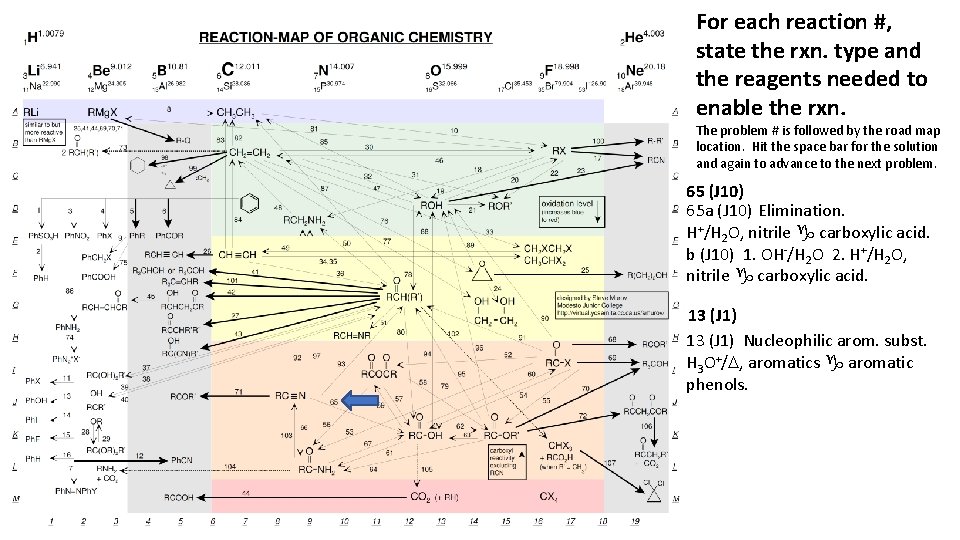
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 65 (J 10) 65 a (J 10) Elimination. H+/H 2 O, nitrile g carboxylic acid. b (J 10) 1. OH-/H 2 O 2. H+/H 2 O, nitrile g carboxylic acid. 13 (J 1) Nucleophilic arom. subst. H 3 O+/D, aromatics g aromatic phenols.

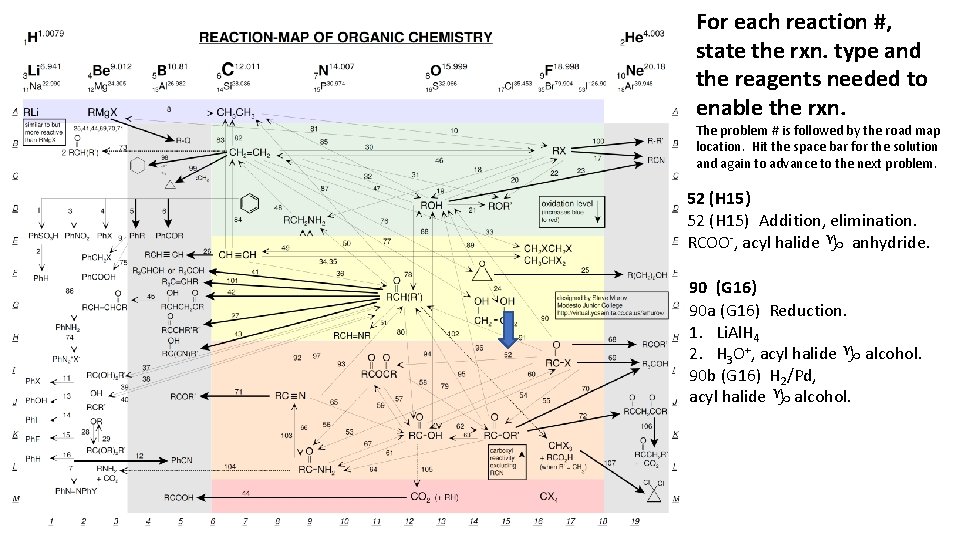
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 52 (H 15) Addition, elimination. RCOO-, acyl halide g anhydride. 90 (G 16) 90 a (G 16) Reduction. 1. Li. Al. H 4 2. H 3 O+, acyl halide g alcohol. 90 b (G 16) H 2/Pd, acyl halide g alcohol.

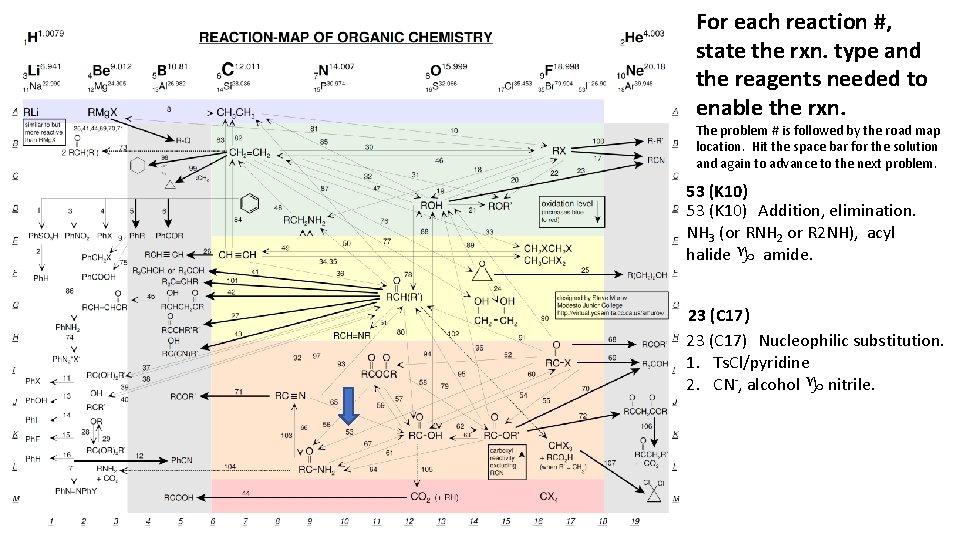
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 53 (K 10) Addition, elimination. NH 3 (or RNH 2 or R 2 NH), acyl halide g amide. 23 (C 17) Nucleophilic substitution. 1. Ts. Cl/pyridine 2. CN-, alcohol g nitrile.

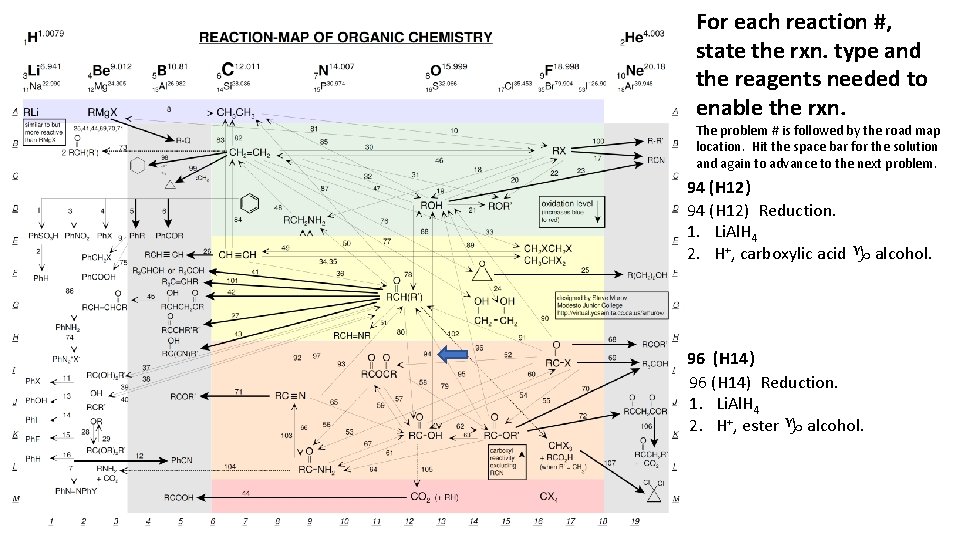
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 94 (H 12) Reduction. 1. Li. Al. H 4 2. H+, carboxylic acid g alcohol. 96 (H 14) Reduction. 1. Li. Al. H 4 2. H+, ester g alcohol.

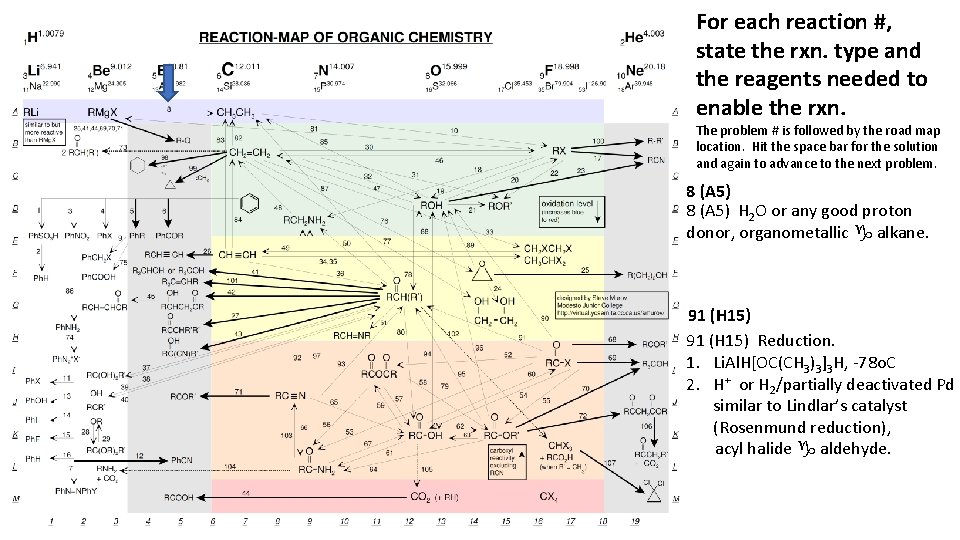
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 8 (A 5) H 2 O or any good proton donor, organometallic g alkane. 91 (H 15) Reduction. 1. Li. Al. H[OC(CH 3)3]3 H, -78 o. C 2. H+ or H 2/partially deactivated Pd similar to Lindlar’s catalyst (Rosenmund reduction), acyl halide g aldehyde.

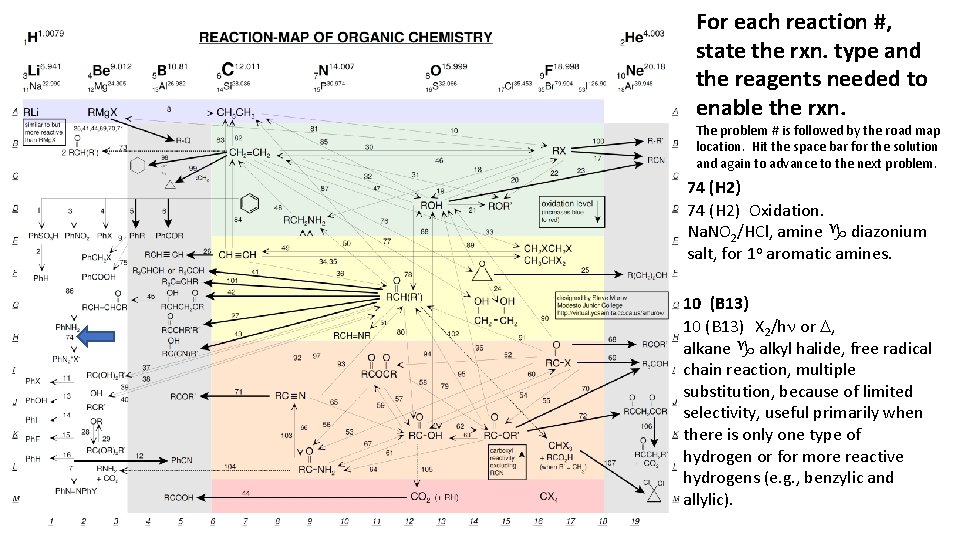
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 74 (H 2) Oxidation. Na. NO 2/HCl, amine g diazonium salt, for 1 o aromatic amines. 10 (B 13) X 2/hn or D, alkane g alkyl halide, free radical chain reaction, multiple substitution, because of limited selectivity, useful primarily when there is only one type of hydrogen or for more reactive hydrogens (e. g. , benzylic and allylic).

For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 75 (F 3) 75 a (F 3) Oxidation. KMn. O 4/H 3 O+/D, alkyl benzene with a alkyl hydrogens g a benzoic acid. b (F 3) K 2 Cr 2 O 7/H 3 O+/D, alkyl benzene with a alkyl hydrogens g a benzoic acid. 54 (J 15) 54 a (J 15) Addition, elimination. H+/H 2 O, acyl halide g carboxylic acid. b (J 15) 1. OH-/H 2 O 2. H+/H 2 O, acyl halide g carboxylic acid.

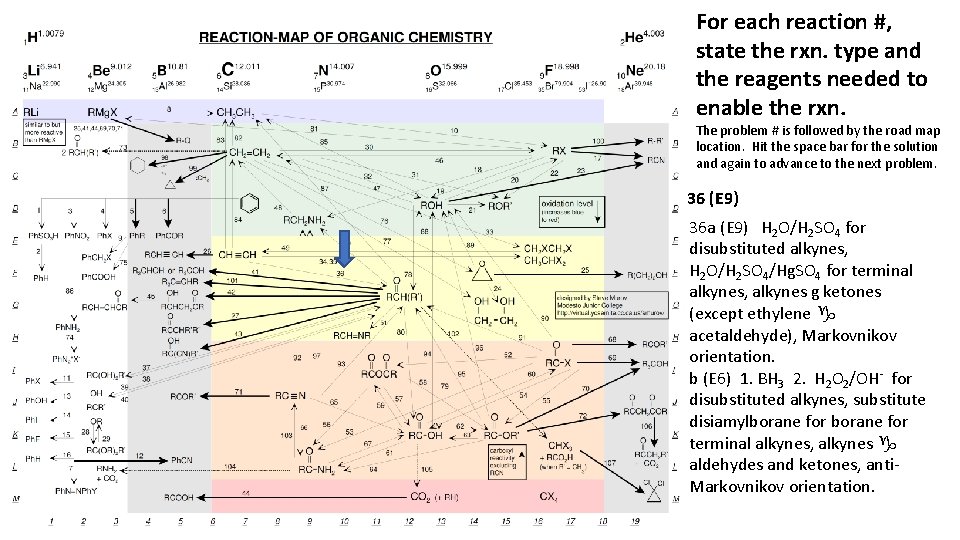
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 36 (E 9) 36 a (E 9) H 2 O/H 2 SO 4 for disubstituted alkynes, H 2 O/H 2 SO 4/Hg. SO 4 for terminal alkynes, alkynes g ketones (except ethylene g acetaldehyde), Markovnikov orientation. b (E 6) 1. BH 3 2. H 2 O 2/OH- for disubstituted alkynes, substitute disiamylborane for terminal alkynes, alkynes g aldehydes and ketones, anti. Markovnikov orientation.

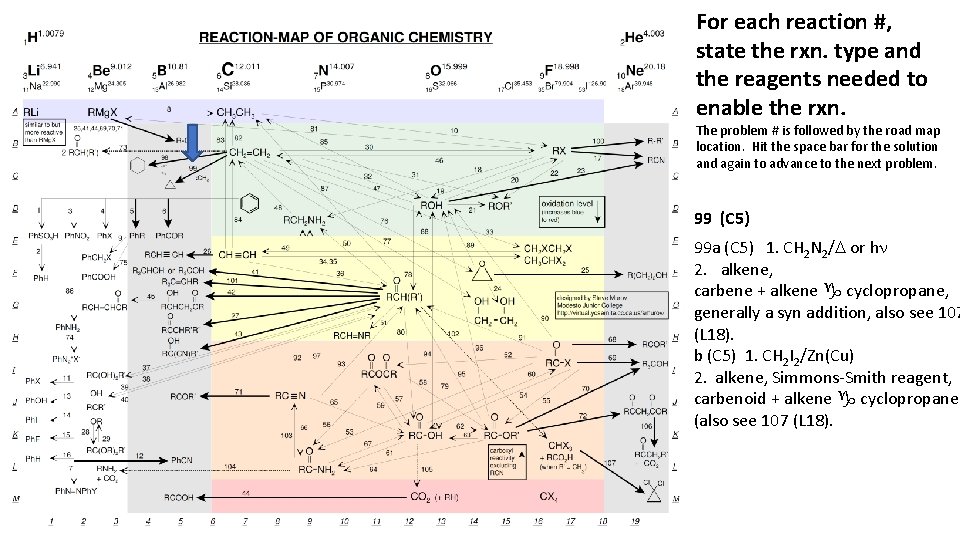
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 99 (C 5) 99 a (C 5) 1. CH 2 N 2/D or hn 2. alkene, carbene + alkene g cyclopropane, generally a syn addition, also see 107 (L 18). b (C 5) 1. CH 2 I 2/Zn(Cu) 2. alkene, Simmons-Smith reagent, carbenoid + alkene g cyclopropane (also see 107 (L 18).

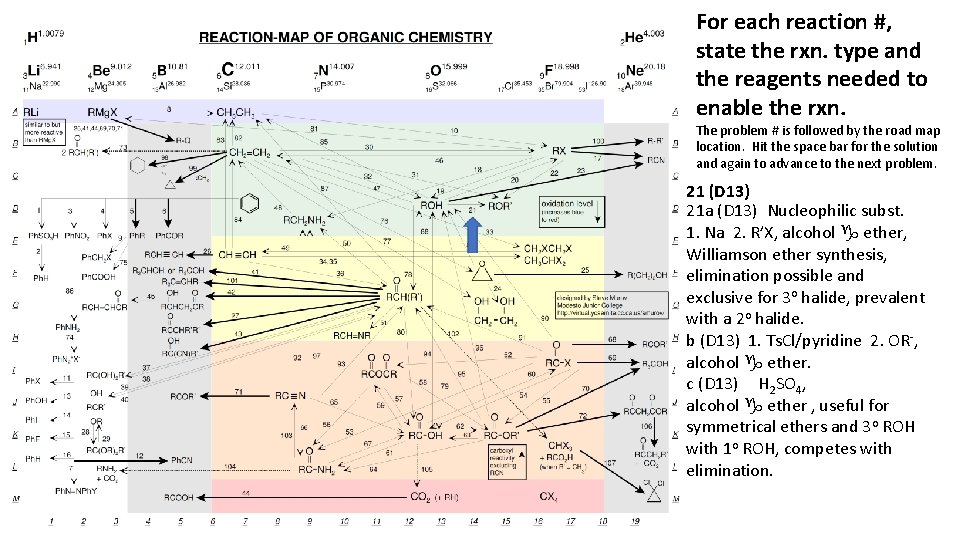
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 21 (D 13) 21 a (D 13) Nucleophilic subst. 1. Na 2. R’X, alcohol g ether, Williamson ether synthesis, elimination possible and exclusive for 3 o halide, prevalent with a 2 o halide. b (D 13) 1. Ts. Cl/pyridine 2. OR-, alcohol g ether. c (D 13) H 2 SO 4, alcohol g ether , useful for symmetrical ethers and 3 o ROH with 1 o ROH, competes with elimination.

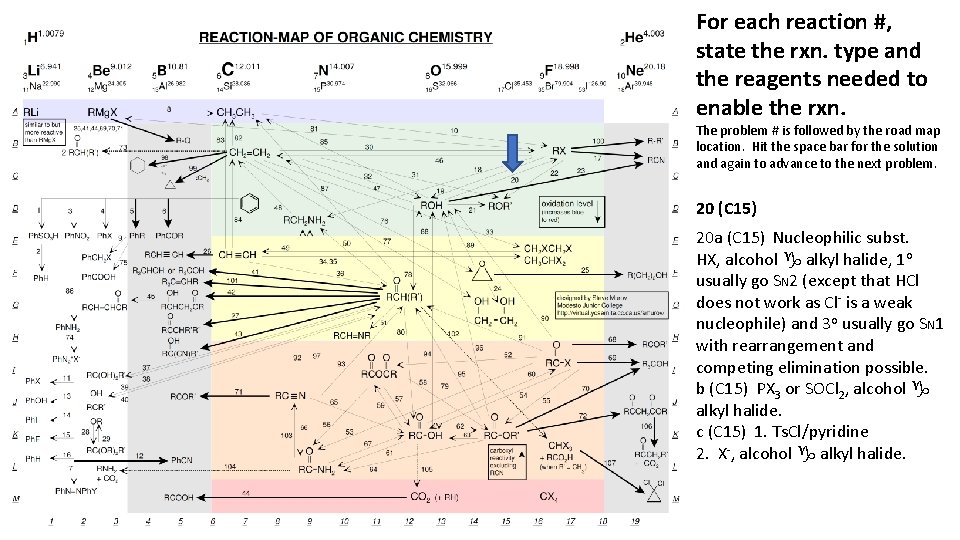
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 20 (C 15) 20 a (C 15) Nucleophilic subst. HX, alcohol g alkyl halide, 1 o usually go SN 2 (except that HCl does not work as Cl- is a weak nucleophile) and 3 o usually go SN 1 with rearrangement and competing elimination possible. b (C 15) PX 3 or SOCl 2, alcohol g alkyl halide. c (C 15) 1. Ts. Cl/pyridine 2. X-, alcohol g alkyl halide.

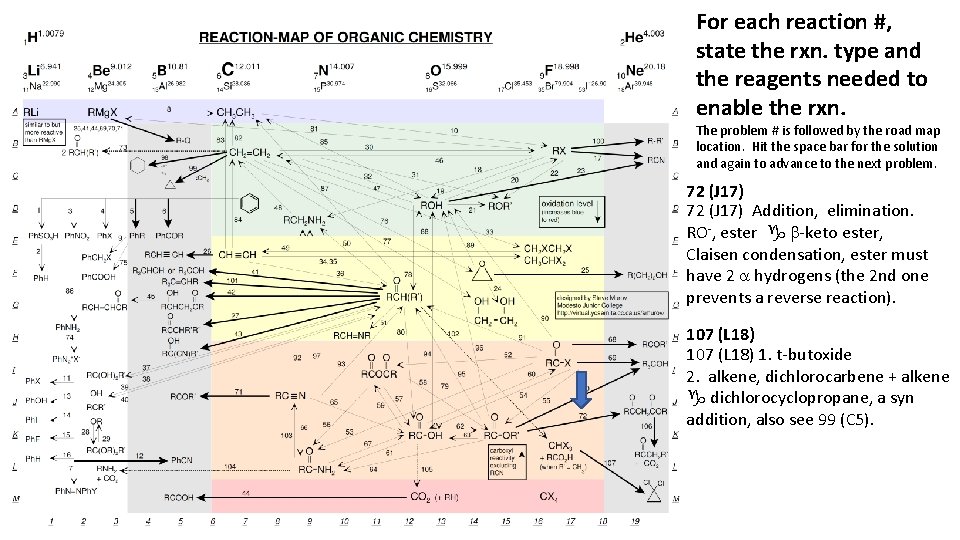
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 72 (J 17) Addition, elimination. RO-, ester g b-keto ester, Claisen condensation, ester must have 2 a hydrogens (the 2 nd one prevents a reverse reaction). 107 (L 18) 1. t-butoxide 2. alkene, dichlorocarbene + alkene g dichlorocyclopropane, a syn addition, also see 99 (C 5).

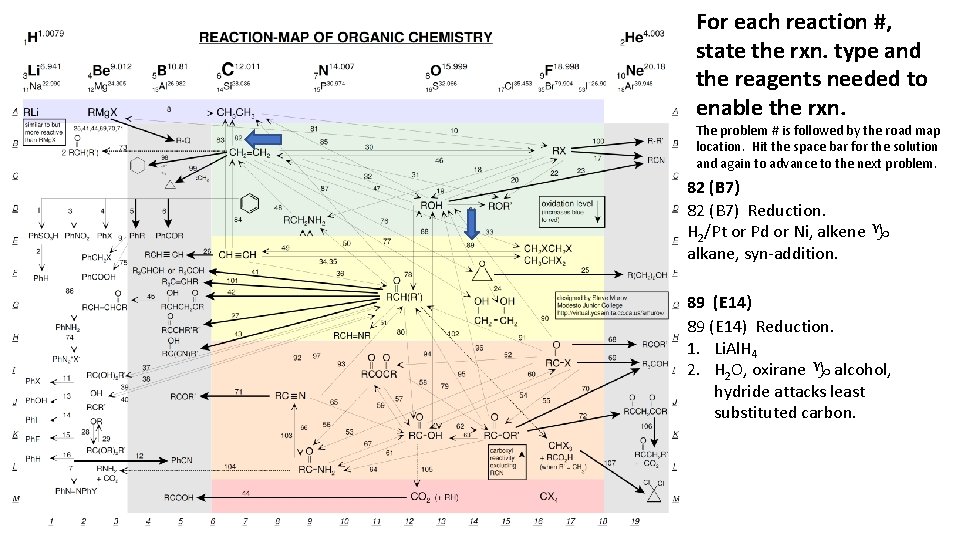
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 82 (B 7) Reduction. H 2/Pt or Pd or Ni, alkene g alkane, syn-addition. 89 (E 14) Reduction. 1. Li. Al. H 4 2. H 2 O, oxirane g alcohol, hydride attacks least substituted carbon.

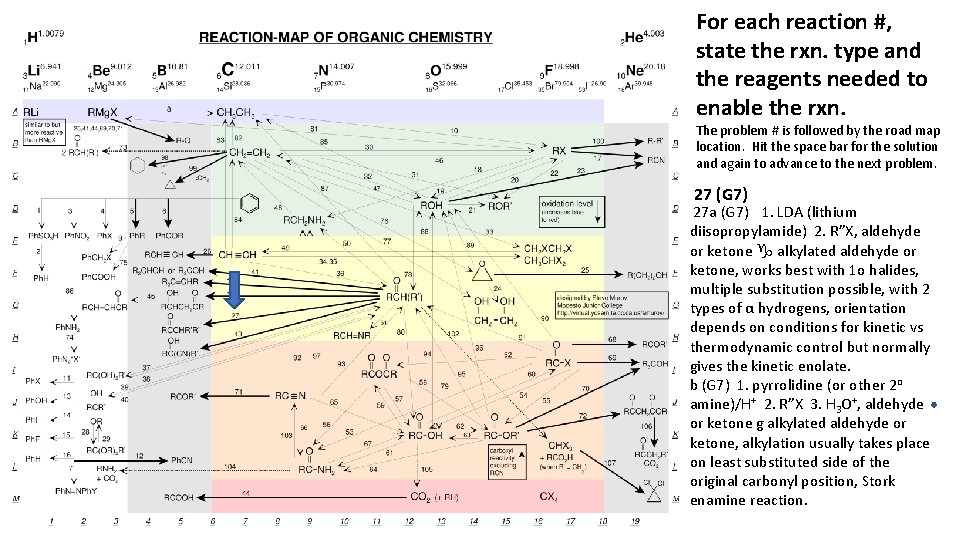
For each reaction #, state the rxn. type and the reagents needed to enable the rxn. The problem # is followed by the road map location. Hit the space bar for the solution and again to advance to the next problem. 27 (G 7) 27 a (G 7) 1. LDA (lithium diisopropylamide) 2. R”X, aldehyde or ketone g alkylated aldehyde or ketone, works best with 1 o halides, multiple substitution possible, with 2 types of α hydrogens, orientation depends on conditions for kinetic vs thermodynamic control but normally gives the kinetic enolate. b (G 7) 1. pyrrolidine (or other 2 o amine)/H+ 2. R”X 3. H 3 O+, aldehyde or ketone g alkylated aldehyde or ketone, alkylation usually takes place on least substituted side of the original carbonyl position, Stork enamine reaction.

Steven Murov, 06/08/12, http: //murov. info/resume. htm , murovs@mjc. edu The article on the Reaction-Map of Organic Chemistry was originally published in the Journal of Chemical Education (Murov, S. J. Chem. Ed. , 2007, 84(7), 1224). The supporting information including the Reaction-Map were published online only. This web site includes only the supporting information and is published with permission from J. Chem. Ed. 2007, 84(7), 1224. Copyright 2007 American Chemical Society. The following web sites are available to J. Chem. Ed. subscribers. http: //pubs. acs. org/doi/pdf/10. 1021/ed 084 p 1224 http: //pubs. acs. org/doi/suppl/10. 1021/ed 084 p 1224/suppl_file/jce 2007 p 1224 w. pdf Abstract: The Reaction -Map of Organic Chemistry has been designed to give organic chemistry students an overview of most of the reactions needed for the organic chemistry course. The chart has been partially organized according to the periodic table on the horizontal axis and according to carbon oxidation level on the vertical axis. In addition the carboxyls are grouped vertically according to decreasing reactivity and carbon - carbon bond forming reactions are emphasized with bold arrows. The chart provides a study aide for students and should help students develop synthetic routes from one functional group to another. The chart should be especially useful for students studying for the final examination for the two semester organic chemistry course. In addition to the chart, three keys are available that organize the reactions according to mechanism, functional group preparations and functional group reactions. Chemistry can be thought of as a search for order in matter and this chart attempts to provide some insight into the order that exists in organic chemistry.

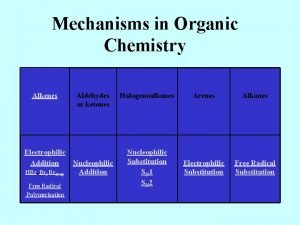
KEYS TO REACTION-MAP OF ORGANIC CHEMISTRY General Organization Left to right, compounds in the colored regions are arranged according to the periodic table. Organolithium and Grignard reagents are under lithium and magnesium but these reagents are used elsewhere on several bold arrows for the synthesis of C-C bonds. Carbon compounds that do not contain other elements (besides hydrogen) are under carbon, carbon - nitrogen (and C, N, O) compounds are under nitrogen, carbon - oxygen compounds are under oxygen and carbon - halogen (and C, O, X) compounds are under fluorine and the halogens. From top to bottom, within groups, the compounds are arranged according to the oxidation level of the compound. The oxidation level of organic compounds is somewhat of a complex concept. Even for propane, the carbons technically have different oxidation states. For the purposes of grouping compounds by oxidation level for this chart, the general guideline used has been that oxidation involves a decrease in the number of bonds to carbon from an atom less electronegative than carbon (most frequently hydrogen) and/or an increase in the number of bonds from carbon to atoms more electronegative than carbon (most frequently N, O, X). Reduction is the reverse. If two carbons change, then the sum of the changes must be considered. When water is added to a double bond, one carbon gains a hydrogen and the other an oxygen and the net oxidation level of the molecule does not change. The increase in carbon oxidation level is indicated by color with red the lowest and blue the highest (or by the degree of darkness in the b/w version).

This organization results in five groups including: 1. alkanes (and organometallics), 2. alkenes (and alkene addition products such as alcohols, ethers and halides), aromatics and amines, 3. alkynes (and alkyne addition products such as carbonyls), 4. carboxyls and 5. carbon dioxide and tetrahalomethane. For the purposes of organizing the numbering of the reactions for this key, the reactions have been grouped according to mechanism of the first step of the reaction. Many reactions fall into more than one group. The addition of hydrogen to p bonds is usually discussed in texts along with electrophilic additions to p bonds but here the hydrogen additions have been placed in the reduction category. Reductions with hydrides such as Li. Al. H 4 are often grouped with nucleophilic additions but here have also been included in the reduction category. The reactions are listed in the order substitution, addition, elimination, addition-elimination, oxidation, reduction, concerted and miscellaneous. To facilitate the finding of reactions from any of the keys that follow, a roadmap grid has been included. For example, the addition of HX to an alkene is represented by reaction 30 which is in grid position B 11. Three keys are have been designed to accompany the map. The keys are arranged according to mechanism, functional group preparations and functional group reactions. Reactions in the three keys contain the reaction numbers and grid locators. Outside of the main region, bold arrows indicate reactions that form C-C bonds. In the color version, products that result from carbon - carbon bond formation are in the grey areas. In the b/w copy, these products are in the area with a graph grid. Dotted arrows represent reactions that result in the breaking of C-C bonds. Also included outside the main region are the reactions of aromatics and miscellaneous reactions.

General Organization Left to right, compounds in the shaded regions are arranged according to the periodic table. Organolithium and Grignard reagents are under lithium and magnesium but these reagents are used elsewhere on several bold arrows for the synthesis of C-C bonds. Carbon compounds that do not contain other elements are under carbon, carbon - nitrogen (and C, N, O) compounds are under nitrogen, carbon - oxygen compounds are under oxygen and carbon - halogen (and C, O, X) compounds are under fluorine and the halogens. From top to bottom, within groups, the compounds are arranged according to the oxidation level of the compound. The oxidation level of organic compounds is somewhat of a complex concept. Even for propane, the carbons technically have different oxidation states. For the purposes of grouping compounds by oxidation level for this chart, the general guideline used has been that oxidation involves a decrease in the number of bonds to carbon from an atom less electronegative than carbon (most frequently hydrogen) and/or an increase in the number of bonds from carbon to atoms more electronegative than carbon (most frequently N, O, X). Reduction is the reverse. If two carbons change, then the sum of the changes must be considered. When water is added to a double bond, one carbon gains a hydrogen and the other an oxygen and the net oxidation level of the molecule does not change. The increase in oxidation level is indicated by the degree of darkness on the next page and in color in the online version. This organization results in five groups including: 1. alkanes (and organometallics), 2. alkenes (and alkene addition products such as alcohols, ethers and halides), aromatics and amines, 3. alkynes (and alkyne addition products such as carbonyls), 4. carboxyls and 5. carbon dioxide and tetrahalomethane. The two crosshatched areas to the left and right of the shaded region contain products of carbon bond forming reactions. These reactions are also emphasized by bold arrows. For the purposes of organizing the numbering of the reactions for this key, the reactions have been grouped according to mechanism of the first step of the reaction. Many reactions fall into more than one group. The addition of hydrogen to π bonds is usually discussed in texts along with electrophilic additions to π bonds but here the hydrogen additions have been placed in the reduction category. Reductions with hydrides such as Li. Al. H 4 are often grouped with nucleophilic additions but here have also been included in the reduction category. The reactions are listed in the order substitution, addition, elimination, addition-elimination, oxidation, reduction, concerted and miscellaneous. To facilitate the finding of reactions from any of the keys that follow, a roadmap grid has been included. For example, the addition of HX to an alkene is represented by reaction 30 which is in grid position B 11.

Reaction-Map as a Study Aid At the end of a two semester course in organic chemistry, a student should be able to perform the exercises below. In addition to the exercises below, a student of organic chemistry should be able to demonstrate competency with spectroscopic, stereochemical and multistep synthetic challenges. 1. For each numbered reaction, classify the reaction by mechanism (e. g. , substitution, nucleophilic) and list the reagents, conditions, regioselectivity, stereoselectivity and restrictions associated with the reaction. 2. List all methods of preparing each functional group. 3. List all reactions of each functional group. 4. Write mechanisms for reactions: 3 (D 2), 10 (B 13), 19 (C 13), 32 (C 11), 33 (E 14), 39 (J 3) +28 (K 2), 42 (G 7), 46 (B 13), 47 (C 11), 62 (K 13), 72 (J 17), 106 (K 19). The problems above should be attempted without reference to the keys but the keys can be used to help check for the correctness of answers. For the answers to question #4, reference to an organic chemistry textbook may be required. Color Chart: Key blue - alkanes, organometallics green - alkenes, aromatics, alkyl halides, alcohols, ethers, amines yellow - alkynes, carbonlys (aldehydes, ketones), oxiranes, diols, dihalides, carbonyl derivatives orange - carboxyls (acyl halides, anhydrides, carboxylic acids, esters, amides, nitriles), haloforms red - carbon dioxide, carbon tetrahalides grey areas with bold arrows - C-C forming reactions arrows with dotted lines - s C-C bond cleavage reactions
 Organic chemistry reaction pathways
Organic chemistry reaction pathways Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Travelling is very popular nowadays.
Travelling is very popular nowadays. School nowadays
School nowadays Nowadays learning english is very necessary
Nowadays learning english is very necessary Nowadays present perfect
Nowadays present perfect Nowadays people are more
Nowadays people are more костюм графа дракулы
костюм графа дракулы What is mathematics
What is mathematics Organic compounds
Organic compounds The elementary irreversible organic liquid-phase reaction
The elementary irreversible organic liquid-phase reaction Organic chemistry
Organic chemistry Unit of rate of reaction for first order reaction
Unit of rate of reaction for first order reaction E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Activity formula
Activity formula Founder of organic chemistry
Founder of organic chemistry Canola oil
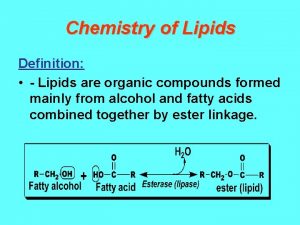
Canola oil Ester organic chemistry
Ester organic chemistry Functional group vs homologous series
Functional group vs homologous series Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Nomenclature of ethers
Nomenclature of ethers Is alkane an organic compound
Is alkane an organic compound What is the leveling effect organic chemistry
What is the leveling effect organic chemistry In iupac naming priority order
In iupac naming priority order Organic chemistry lab report example
Organic chemistry lab report example Alkane organic chemistry
Alkane organic chemistry Organic chemistry grade 10
Organic chemistry grade 10 Organic chemistry
Organic chemistry Organic chemistry chapter 1
Organic chemistry chapter 1 Organic chemistry wade
Organic chemistry wade General formula of alkanes
General formula of alkanes Cracking organic chemistry
Cracking organic chemistry Organic biochemistry
Organic biochemistry Organic chemistry myanmar
Organic chemistry myanmar Electrophilic addition hbr
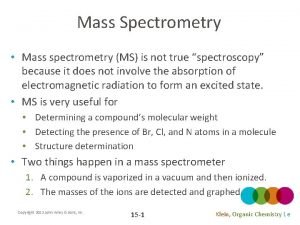
Electrophilic addition hbr Mass spec of chlorine
Mass spec of chlorine Hono organic chemistry
Hono organic chemistry Leaving group ability
Leaving group ability Organic chemistry topic 11
Organic chemistry topic 11 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Alkene alcohol naming
Alkene alcohol naming What is organic chemistry like
What is organic chemistry like Neon organic or inorganic
Neon organic or inorganic Organic chemistry vocabulary
Organic chemistry vocabulary Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry laboratory ch 2540 manual
Organic chemistry laboratory ch 2540 manual A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Saponifiable lipids example
Saponifiable lipids example Ario acidity
Ario acidity Percentage yield practice questions
Percentage yield practice questions Polarimetry organic chemistry
Polarimetry organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Radicals
Radicals Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Organic chemistry
Organic chemistry Organic chemistry chapter 9
Organic chemistry chapter 9 Chemistry ethics case studies
Chemistry ethics case studies Chapter 7 chemistry review
Chapter 7 chemistry review Entane
Entane Analytical chemistry chapter 1
Analytical chemistry chapter 1