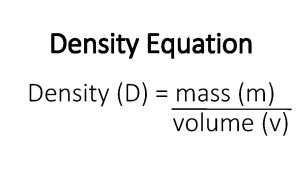Matter Matter Anything that has mass and volume














![• Starting substances [reactants] have different compositions and properties from the new substances • Starting substances [reactants] have different compositions and properties from the new substances](https://slidetodoc.com/presentation_image_h2/ae44dd16e2e9b3bf8b2972933a70ade4/image-41.jpg)
















- Slides: 72

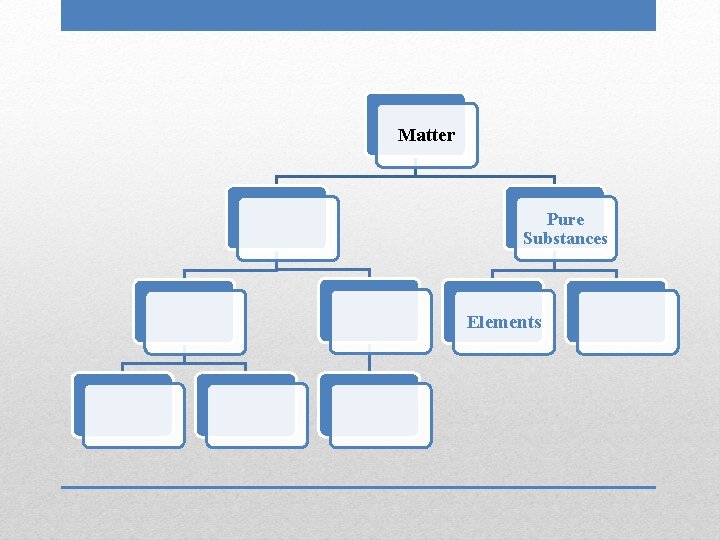
Matter

Matter

• Anything that has mass and volume (occupied space) MATTER Defined

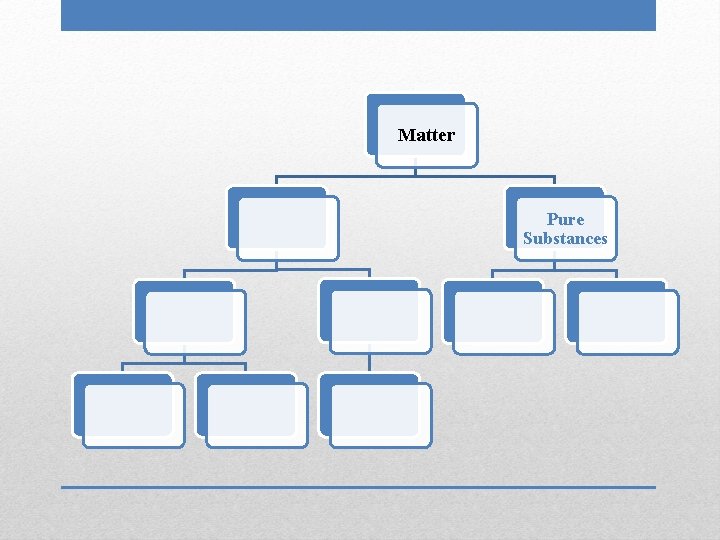
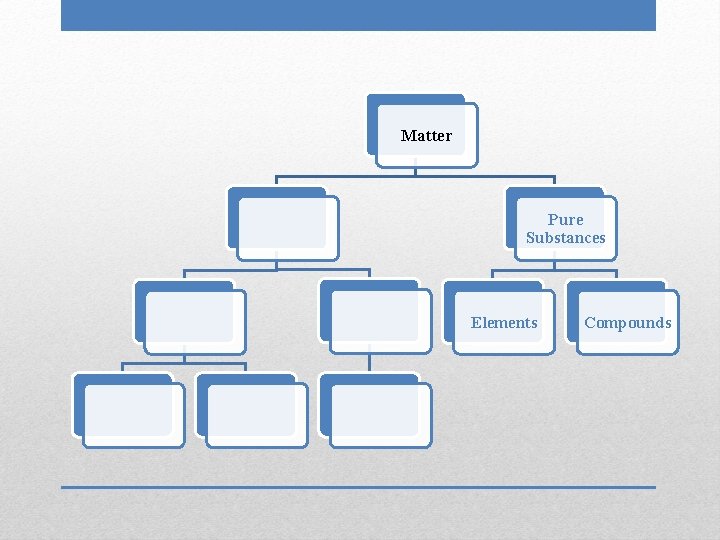
Matter Pure Substances

• Uniform and unchanging composition • Chemical formula can be written for a substance Pure Substances

PROPERTIES OF MATTER Characteristics and behavior

Characteristics that can be observed through the five senses or measured without changing the sample’s composition or identity Physical Properties

Physical properties of pure substances are consistent and unchanging due to their uniform and unchanging compositions Physical Properties

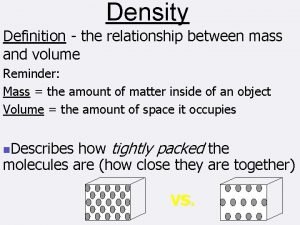
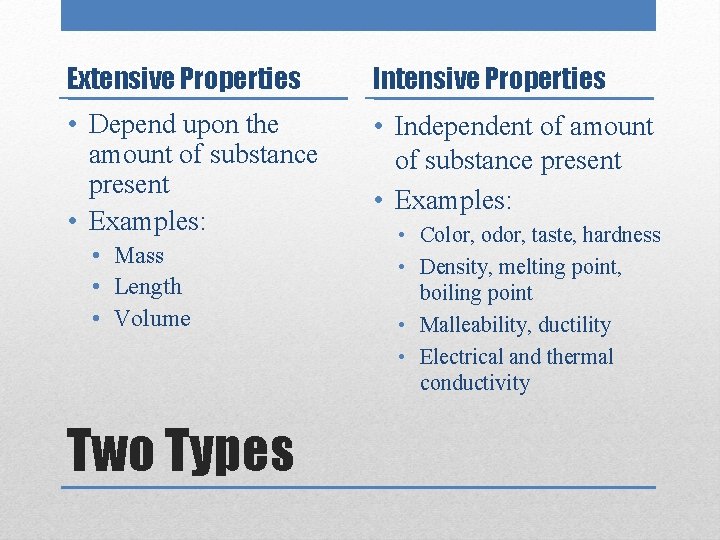
Extensive Properties Intensive Properties • Depend upon the amount of substance present • Examples: • Independent of amount of substance present • Examples: • Mass • Length • Volume Two Types • Color, odor, taste, hardness • Density, melting point, boiling point • Malleability, ductility • Electrical and thermal conductivity

What are some physical properties of water?

The ability of a substance to combine with or change into one or more other substances Includes the inability to change into other substances Chemical Properties

Evident when substance comes in contact with another substance or when thermal or electrical energy is applied Chemical Properties

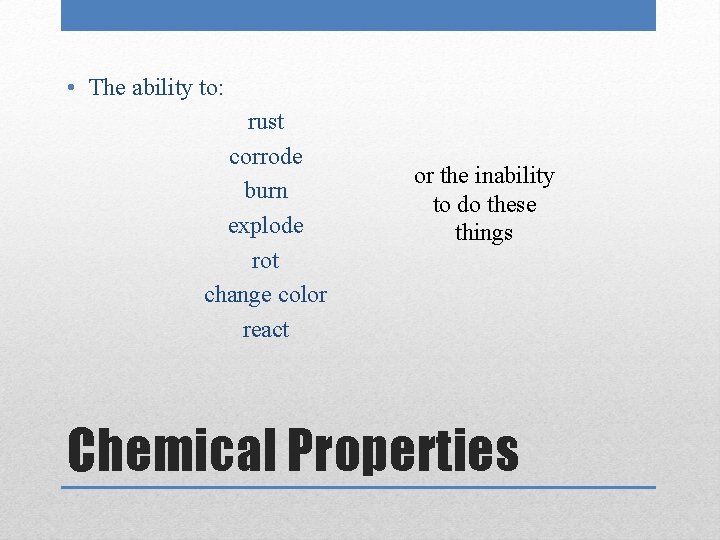
• The ability to: rust corrode burn explode rot change color react or the inability to do these things Chemical Properties

Theability water to The ability ofofiron ofto rust decompose when magnesium combined to into burn within hydrogen andofoxygen the presence oxygen Examples

Substances have unique sets of physical and chemical properties Properties are helpful in identifying unknown substances Properties

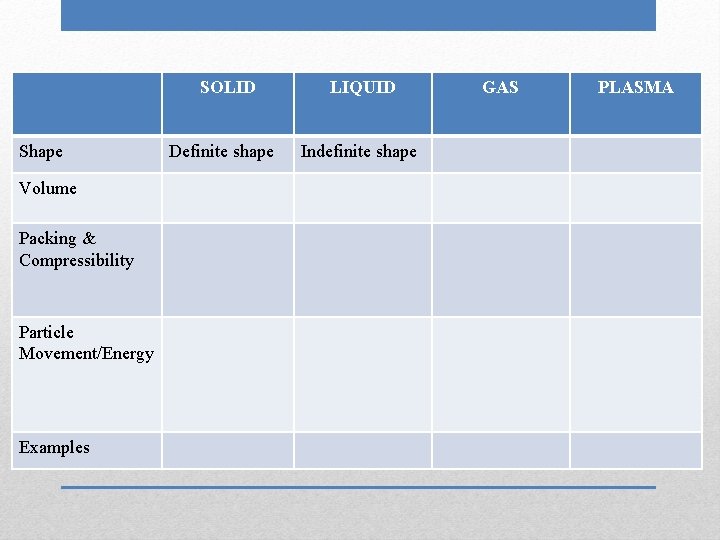
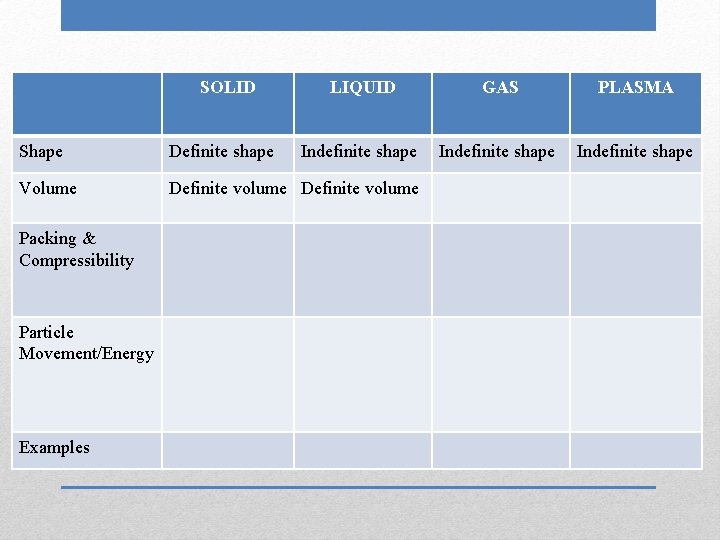
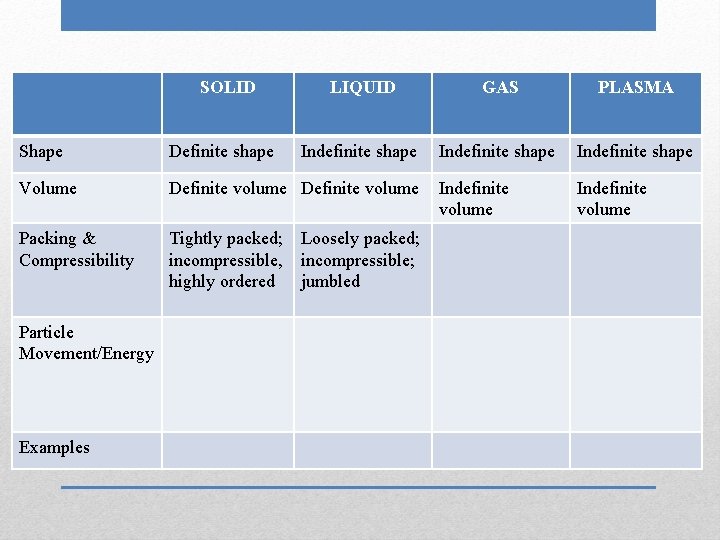
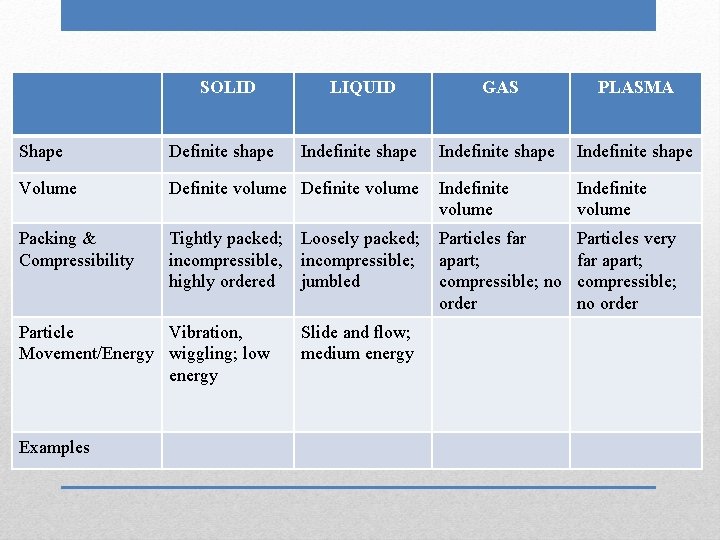
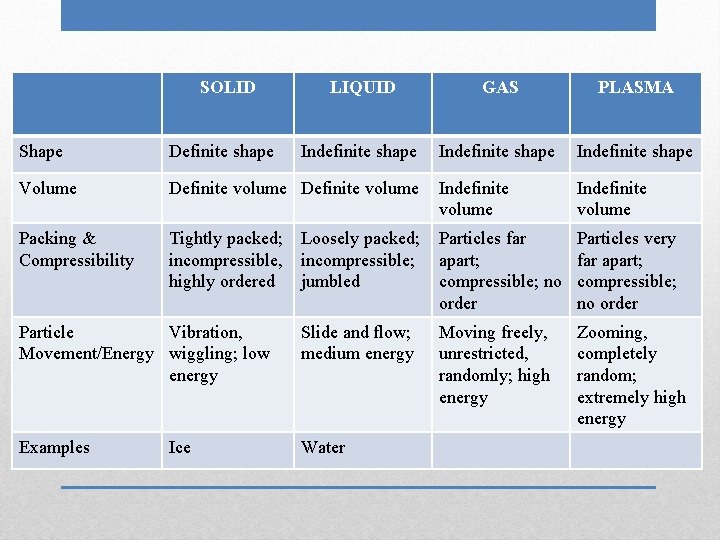
SOLID LIQUID GAS Shape Volume Packing & Compressibility Particle Movement/Energy Examples Phases (States) of Matter PLASMA

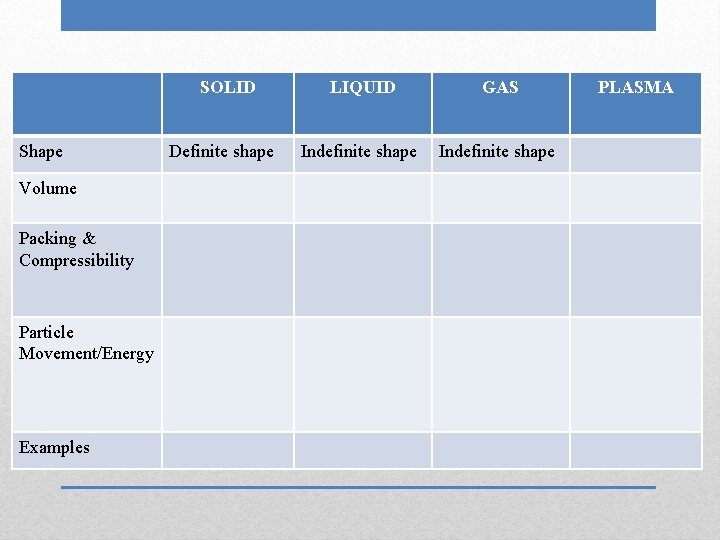
SOLID Shape Volume Packing & Compressibility Particle Movement/Energy Examples Definite shape LIQUID GAS PLASMA

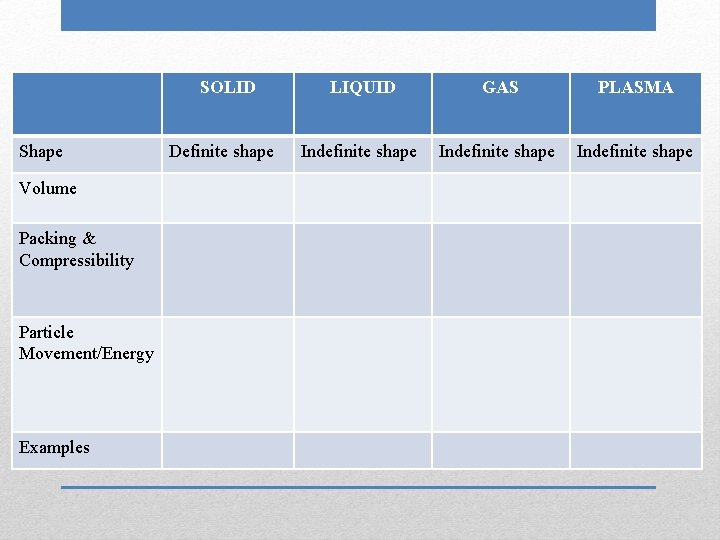
SOLID Shape Volume Packing & Compressibility Particle Movement/Energy Examples Definite shape LIQUID Indefinite shape GAS PLASMA

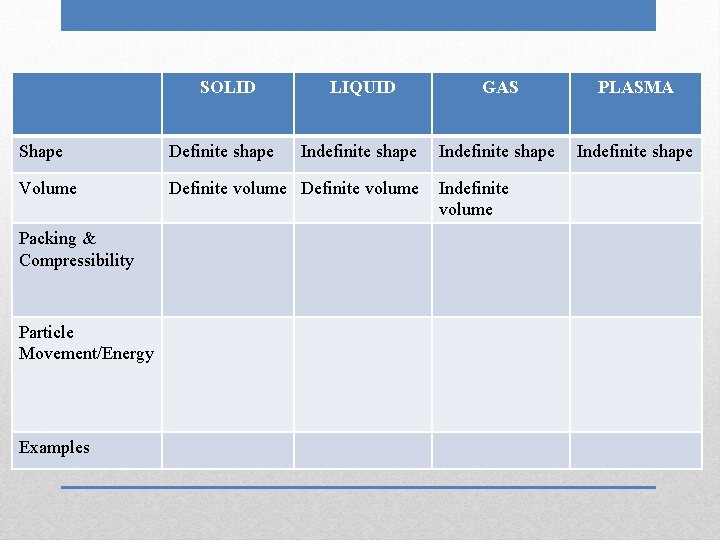
SOLID Shape Volume Packing & Compressibility Particle Movement/Energy Examples Definite shape LIQUID GAS Indefinite shape PLASMA

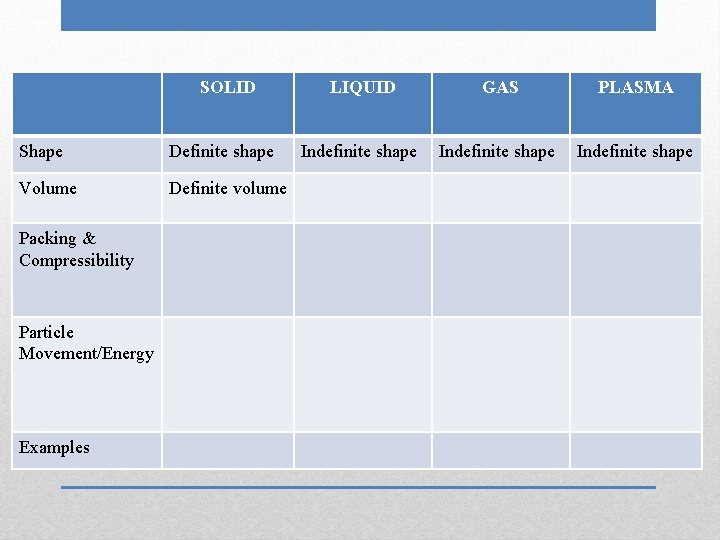
SOLID Shape Volume Packing & Compressibility Particle Movement/Energy Examples Definite shape LIQUID GAS PLASMA Indefinite shape

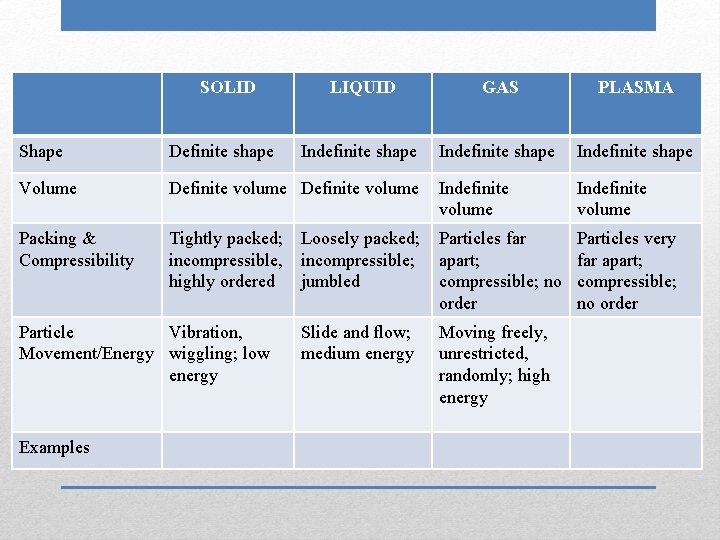
SOLID Shape Definite shape Volume Definite volume Packing & Compressibility Particle Movement/Energy Examples LIQUID GAS PLASMA Indefinite shape

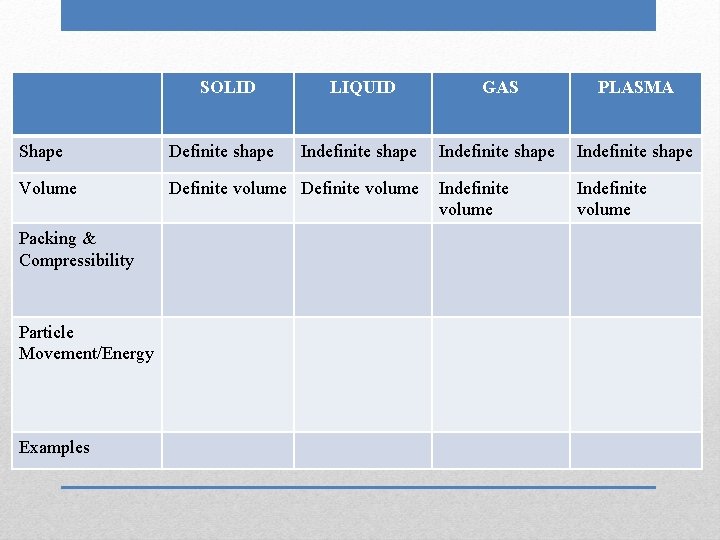
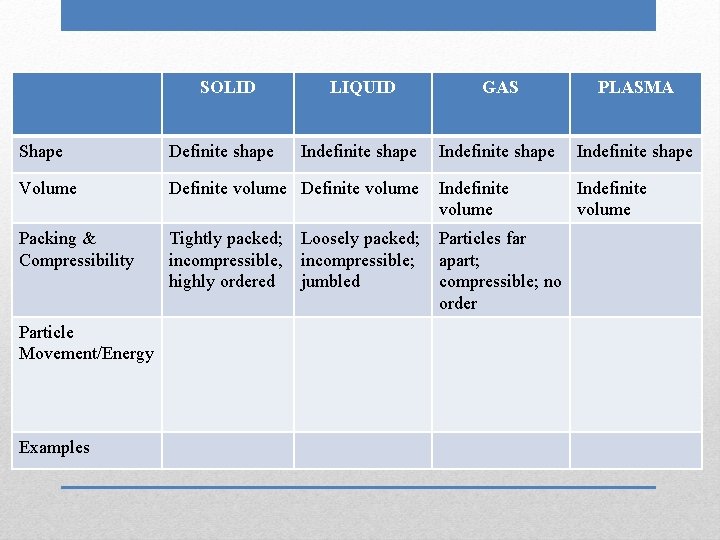
SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Packing & Compressibility Particle Movement/Energy Examples

SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Packing & Compressibility Particle Movement/Energy Examples Indefinite volume

SOLID LIQUID GAS PLASMA Indefinite shape Indefinite volume Shape Definite shape Volume Definite volume Packing & Compressibility Particle Movement/Energy Examples

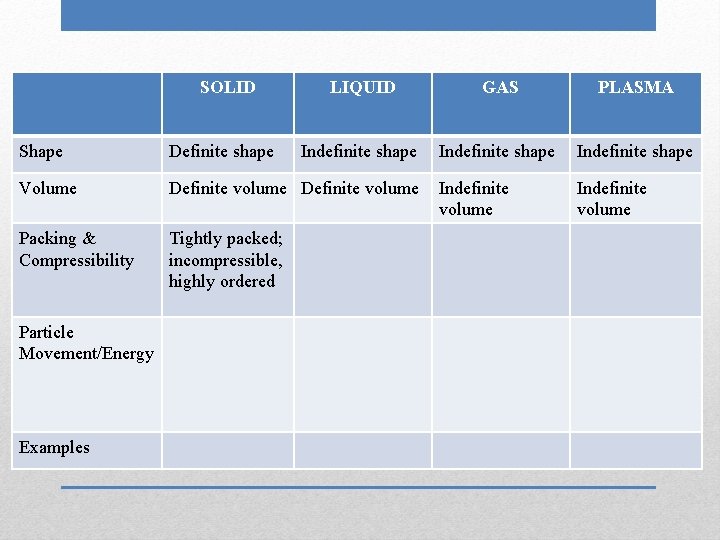
SOLID LIQUID GAS PLASMA Indefinite shape Indefinite volume Shape Definite shape Volume Definite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Particle Movement/Energy Examples

SOLID LIQUID GAS PLASMA Indefinite shape Indefinite volume Shape Definite shape Volume Definite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Particle Movement/Energy Examples Loosely packed; incompressible; jumbled

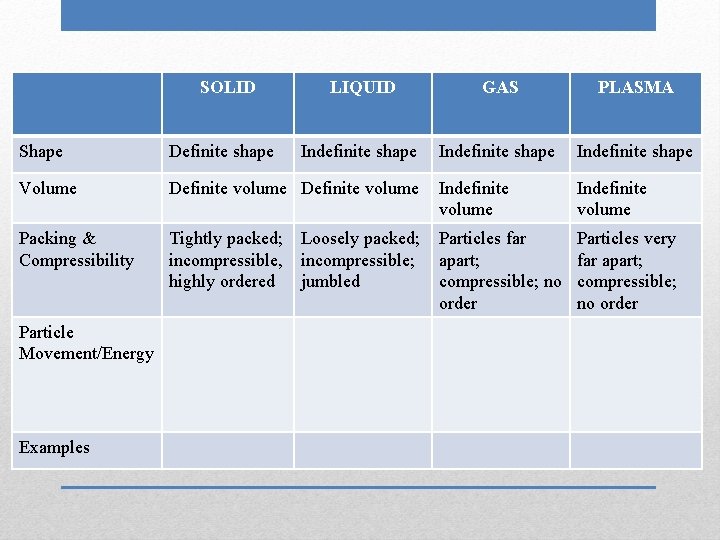
SOLID LIQUID GAS PLASMA Indefinite shape Indefinite volume Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Particles far apart; compressible; no order Particle Movement/Energy Examples Loosely packed; incompressible; jumbled

SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Particles far apart; compressible; no order Particles very far apart; compressible; no order Particle Movement/Energy Examples Loosely packed; incompressible; jumbled

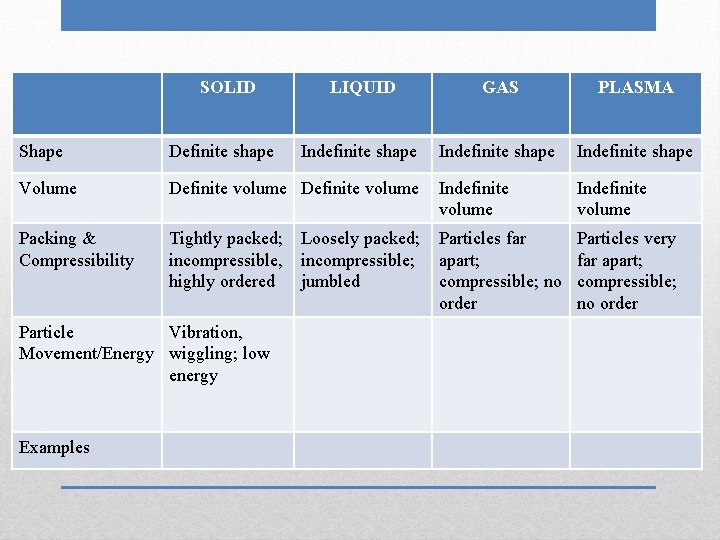
SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Particles far apart; compressible; no order Particles very far apart; compressible; no order Particle Vibration, Movement/Energy wiggling; low energy Examples Loosely packed; incompressible; jumbled

SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Particles far apart; compressible; no order Particles very far apart; compressible; no order Particle Vibration, Movement/Energy wiggling; low energy Examples Loosely packed; incompressible; jumbled Slide and flow; medium energy

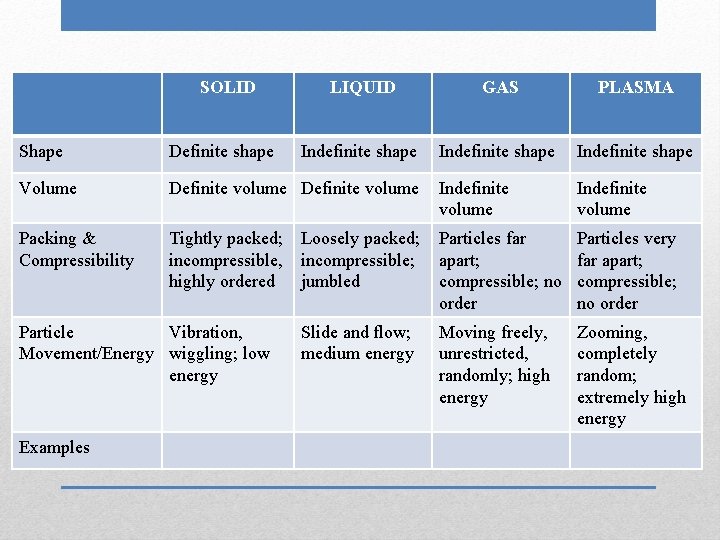
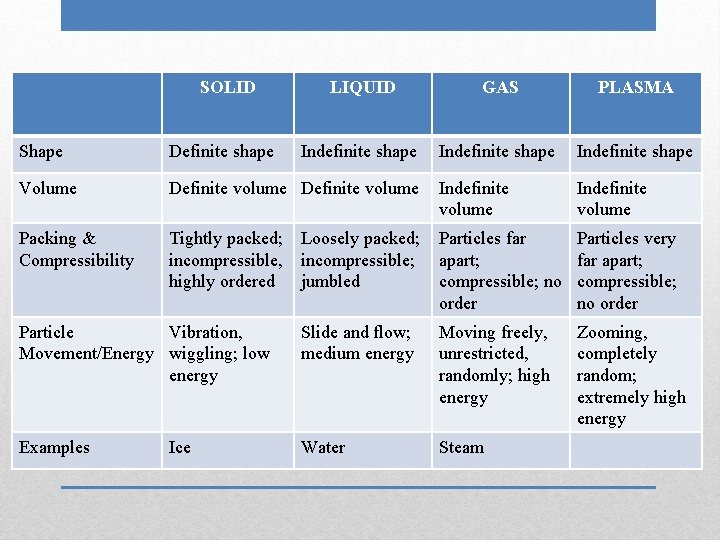
SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Loosely packed; incompressible; jumbled Particles far apart; compressible; no order Particles very far apart; compressible; no order Slide and flow; medium energy Moving freely, unrestricted, randomly; high energy Particle Vibration, Movement/Energy wiggling; low energy Examples

SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Loosely packed; incompressible; jumbled Particles far apart; compressible; no order Particles very far apart; compressible; no order Slide and flow; medium energy Moving freely, unrestricted, randomly; high energy Zooming, completely random; extremely high energy Particle Vibration, Movement/Energy wiggling; low energy Examples

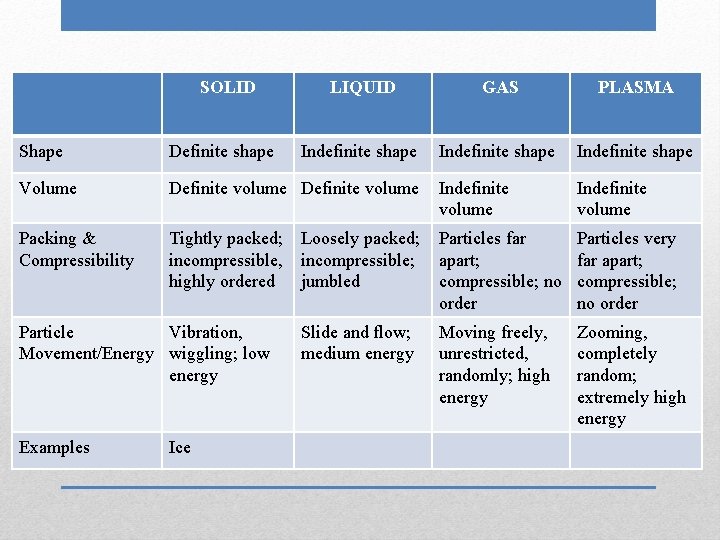
SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Loosely packed; incompressible; jumbled Particles far apart; compressible; no order Particles very far apart; compressible; no order Slide and flow; medium energy Moving freely, unrestricted, randomly; high energy Zooming, completely random; extremely high energy Particle Vibration, Movement/Energy wiggling; low energy Examples Ice

SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Loosely packed; incompressible; jumbled Particles far apart; compressible; no order Particles very far apart; compressible; no order Particle Vibration, Movement/Energy wiggling; low energy Slide and flow; medium energy Moving freely, unrestricted, randomly; high energy Zooming, completely random; extremely high energy Examples Water Ice

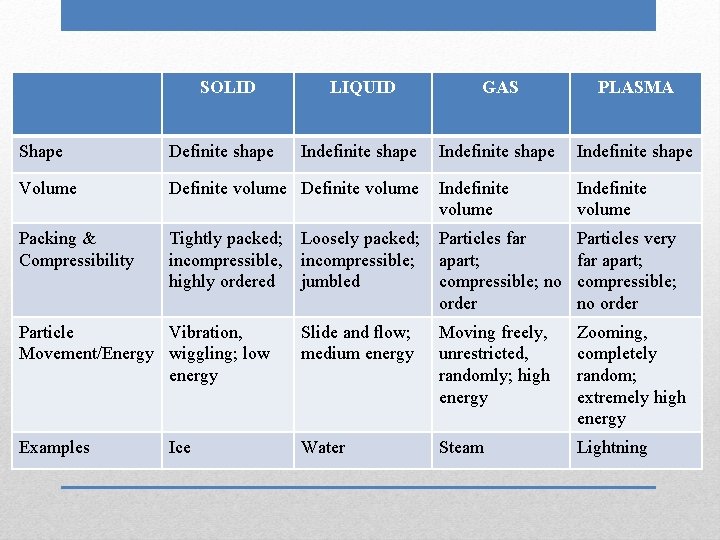
SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Loosely packed; incompressible; jumbled Particles far apart; compressible; no order Particles very far apart; compressible; no order Particle Vibration, Movement/Energy wiggling; low energy Slide and flow; medium energy Moving freely, unrestricted, randomly; high energy Zooming, completely random; extremely high energy Examples Water Steam Ice

SOLID LIQUID GAS PLASMA Indefinite shape Shape Definite shape Volume Definite volume Indefinite volume Packing & Compressibility Tightly packed; incompressible, highly ordered Loosely packed; incompressible; jumbled Particles far apart; compressible; no order Particles very far apart; compressible; no order Particle Vibration, Movement/Energy wiggling; low energy Slide and flow; medium energy Moving freely, unrestricted, randomly; high energy Zooming, completely random; extremely high energy Examples Water Steam Lightning Ice

VAPOR VS. GAS Gas: substance that is naturally in gaseous state at room temperature Vapor: gaseous state of substance that is normally solid or liquid at room temperature

Changes which alter a substance without changing its composition or identity Physical Changes

• Cut, break, bend, grind, crumple, split, crush, dissolve, fold • Include phase changes: most substances undergo a change from one state of matter to another as the temperature and pressure conditions change • Melt, freeze, boil, vaporize, condense Physical Changes

Processes involving one or more substances changing into new substances Also referred to as chemical reactions Chemical Changes
![Starting substances reactants have different compositions and properties from the new substances • Starting substances [reactants] have different compositions and properties from the new substances](https://slidetodoc.com/presentation_image_h2/ae44dd16e2e9b3bf8b2972933a70ade4/image-41.jpg)
• Starting substances [reactants] have different compositions and properties from the new substances formed [products] • Represented by chemical equations Reactants → Products Chemical Changes

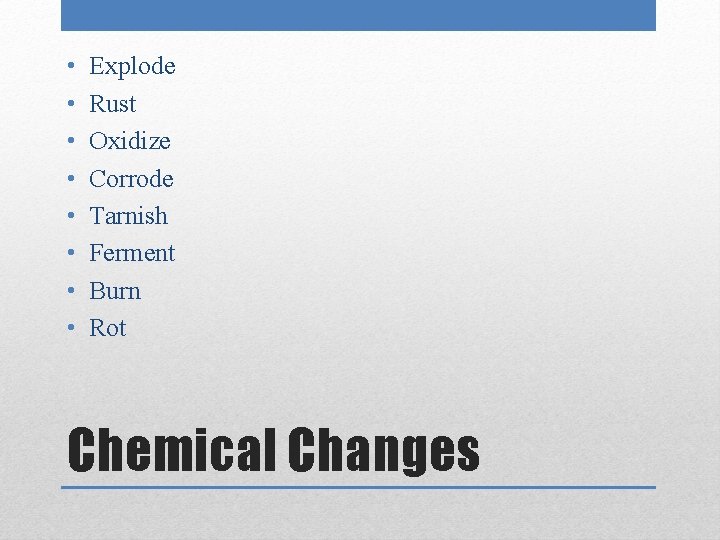
• • Explode Rust Oxidize Corrode Tarnish Ferment Burn Rot Chemical Changes

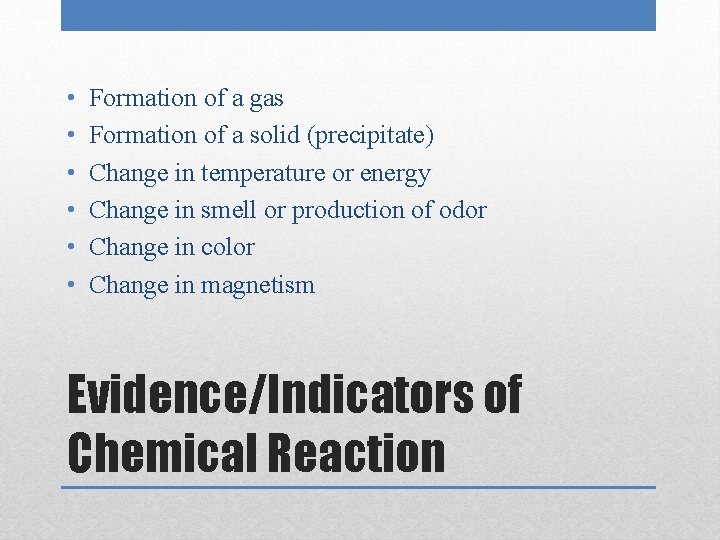
• • • Formation of a gas Formation of a solid (precipitate) Change in temperature or energy Change in smell or production of odor Change in color Change in magnetism Evidence/Indicators of Chemical Reaction

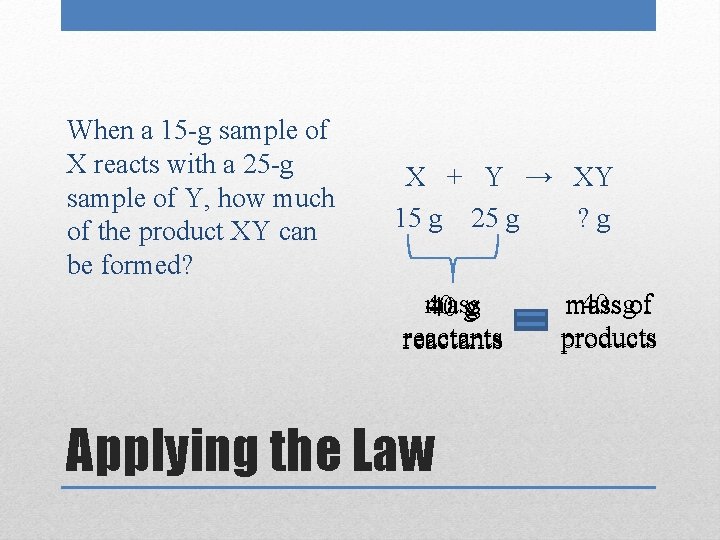
LAW OF CONSERVATION OF MATTER Mass is neither created nor destroyed in any process; it is conserved.

LAW OF CONSERVATION OF MATTER Massreactants = Massproducts

When a 15 -g sample of X reacts with a 25 -g sample of Y, how much of the product XY can be formed? X + Y → XY 15 g 25 g ? g mass 40. 40 gg reactants Applying the Law 40. gof mass products

Matter Pure Substances

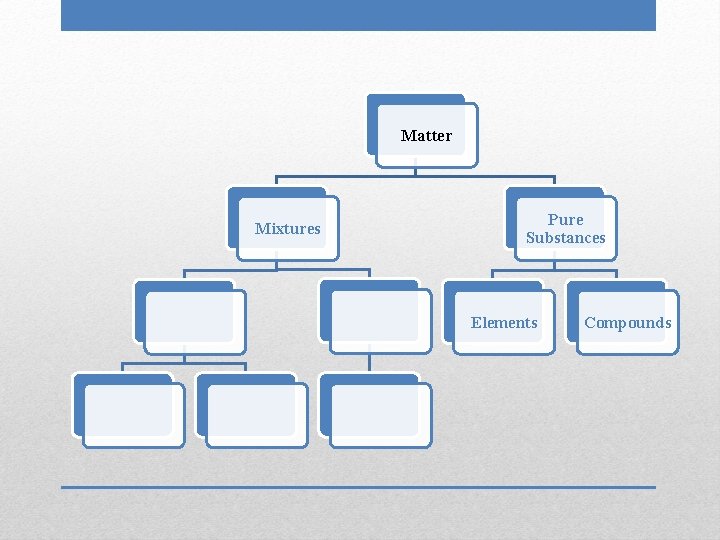
Matter Pure Substances Elements

• Contain only one type of atom: one name, one symbol • Found on Periodic Table • Cannot be separated into simpler substances through physical or chemical means Elements

Matter Pure Substances Elements Compounds

• Two or more substances chemically combined: two names/symbols • Can be broken down by chemical means, which requires energy • Properties of compounds are very different from properties of individual elements Compounds

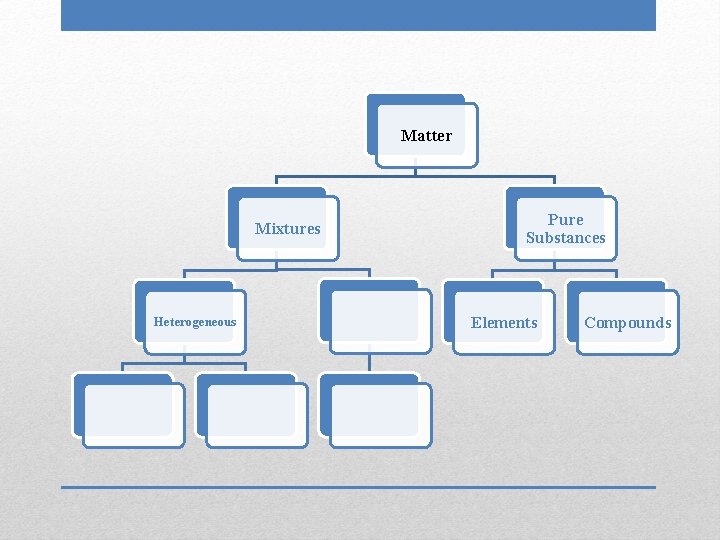
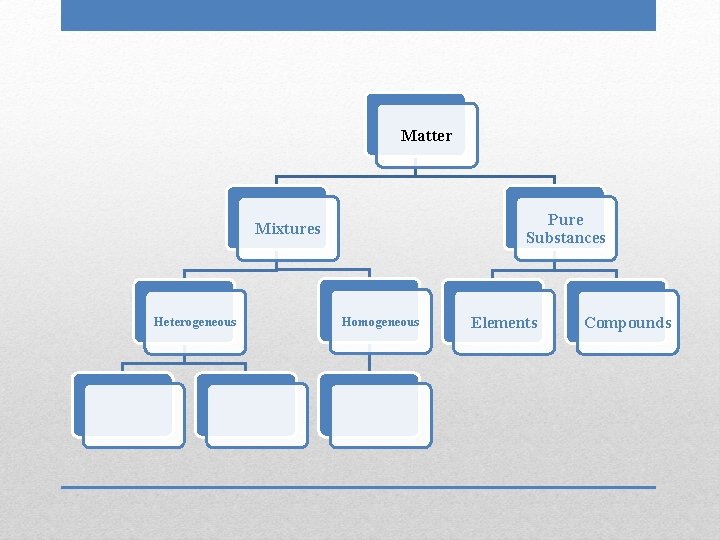
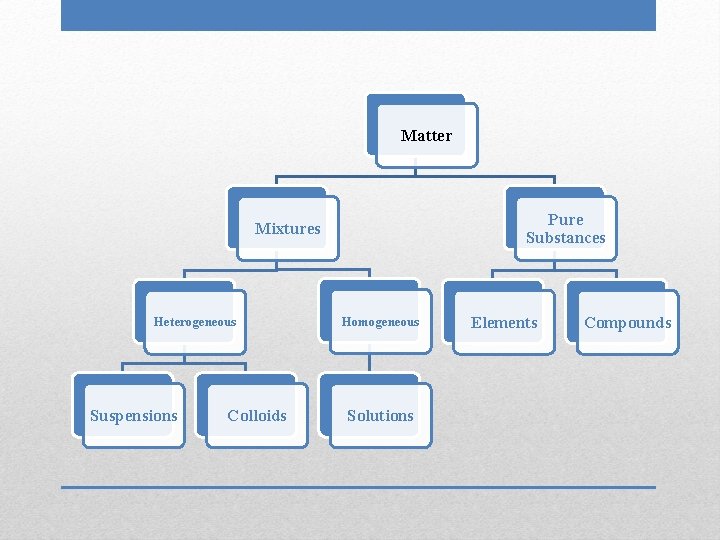
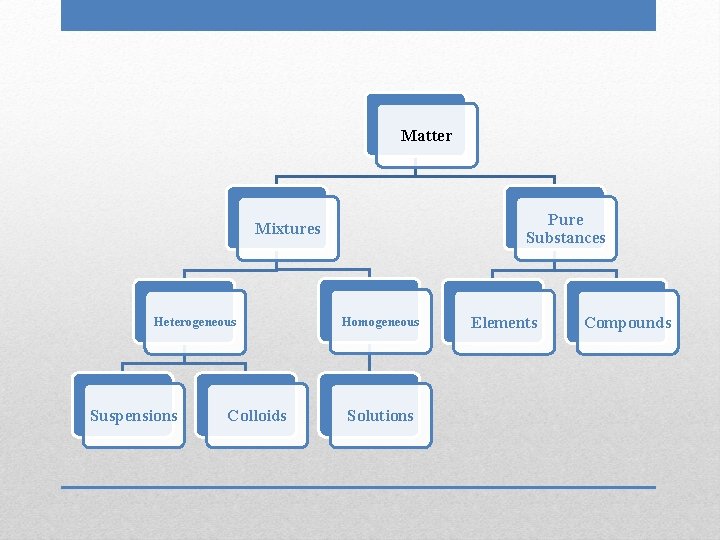
Matter Mixtures Pure Substances Elements Compounds

• • Two or more substances physically combined Substances retain their individual properties Variable composition Can be separated by physical means Mixtures

Matter Mixtures Heterogeneous Pure Substances Elements Compounds

• Not uniform in composition • Different phases; individual substances remain distinct • Tend to have cloudy appearance Heterogeneous Mixtures

Matter Pure Substances Mixtures Heterogeneous Homogeneous Elements Compounds

• Uniform, constant composition • Single phase • Clear, transparent Homogeneous Mixtures

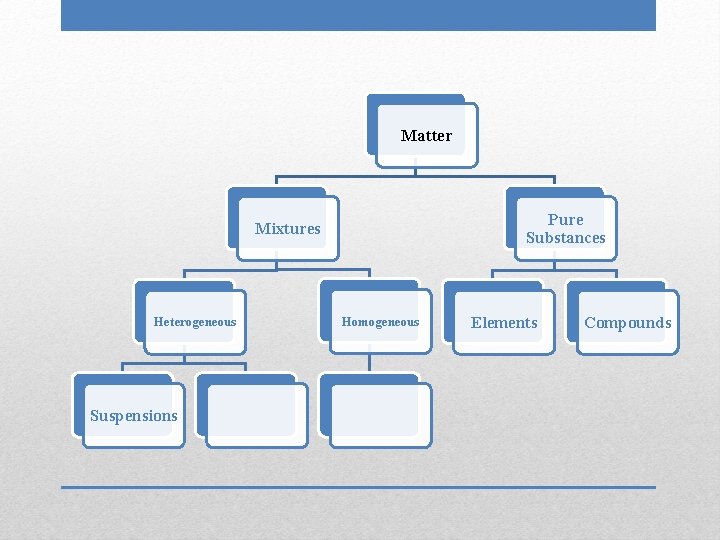
Matter Pure Substances Mixtures Heterogeneous Suspensions Homogeneous Elements Compounds

• Largest particle size • Particles can be filtered out of mixture • Positive Tyndall effect Suspensions

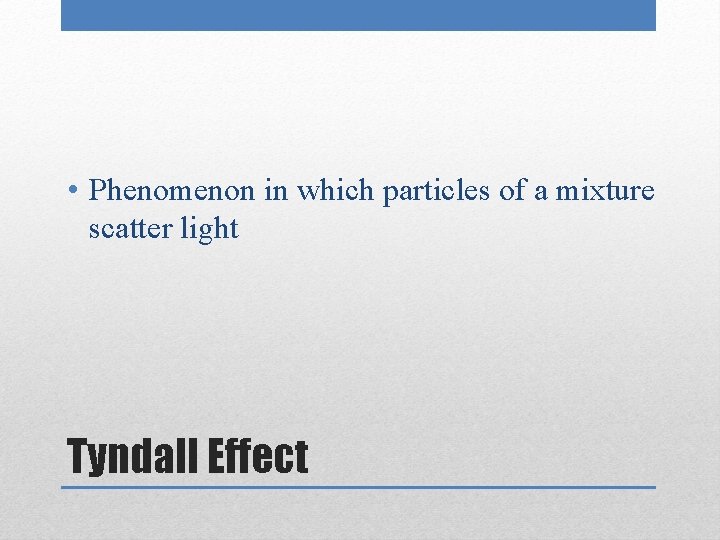
• Phenomenon in which particles of a mixture scatter light Tyndall Effect

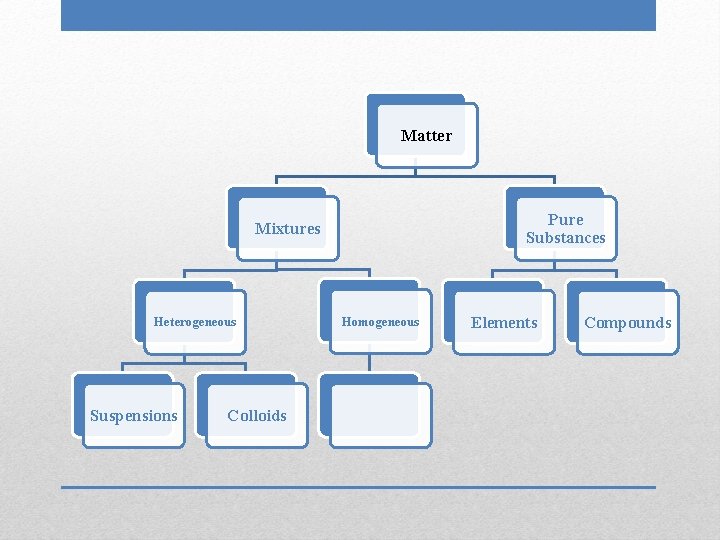
Matter Pure Substances Mixtures Heterogeneous Suspensions Colloids Homogeneous Elements Compounds

• Intermediate particle size • Particles do not filter out • Positive Tyndall effect Colloids

Matter Pure Substances Mixtures Heterogeneous Suspensions Colloids Homogeneous Solutions Elements Compounds

• Smallest particle size • Particles do not filter out • Negative Tyndall effect Solutions

Matter Pure Substances Mixtures Heterogeneous Suspensions Colloids Homogeneous Solutions Elements Compounds

Separation Techniques To separate mixtures into their component substances

• Manual means “by hand” • Use tool, such as tweezers, to remove and separate components of a mixture • Used with suspensions Manual Separation

• Uses a porous barrier to separate solids from liquids. • Example: Filter paper in a funnel to separate sand from water • Used with suspensions Filtration

• Based on the differences in boiling points of substances • Example: When boiling salt water solution, water vaporizes first • Used with solutions, colloids Distillation

• Results in formation of pure solid particles of a substance from a solution • Example: When making rock candy, sugar forms solid crystals from liquid solution • Used with solutions Crystallization

• Based on tendency of components of mixture to travel across surface of another material • Example: Separating ink dyes • Used with solutions Chromatography

• Allows a liquid to be separated quickly from a heavier solid • Example: Pouring water off a mixture with solid materials • Used with suspensions Decantation
 Matter is anything that has mass and volume.
Matter is anything that has mass and volume. Whats anything that has mass and takes up space
Whats anything that has mass and takes up space Matter is anything that has mass and
Matter is anything that has mass and Name the entity that occupies space and has mass
Name the entity that occupies space and has mass Matter vs weight
Matter vs weight The matter is anything that occupies
The matter is anything that occupies It is anything that has mass and occupies space
It is anything that has mass and occupies space It is anything that has mass and occupies space
It is anything that has mass and occupies space Anything that has mass
Anything that has mass Anything that takes up space and has mass is
Anything that takes up space and has mass is Matter is anything that has
Matter is anything that has Matter is anything that
Matter is anything that Anything that takes up space and has mass
Anything that takes up space and has mass Defintion of mass
Defintion of mass All matter has and takes up
All matter has and takes up The matter is anything that occupies
The matter is anything that occupies V=density/mass
V=density/mass Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block av độ 1
Block av độ 1 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Dmv triangle
Dmv triangle Frank has an eraser
Frank has an eraser Matter is defined as anything that
Matter is defined as anything that Matter anything that
Matter anything that Use of density
Use of density Matter is anything that:
Matter is anything that: What is matter
What is matter No matter anything
No matter anything Matter is anything that
Matter is anything that Matter anything that
Matter anything that Matter anything that
Matter anything that Matter anything that
Matter anything that Matter anything that
Matter anything that Matter anything that
Matter anything that Earth has not anything to show more fair figure of speech
Earth has not anything to show more fair figure of speech Density mass volume questions
Density mass volume questions How to find the volume using density and mass
How to find the volume using density and mass D=mass/volume
D=mass/volume Whats the relationship between mass and volume
Whats the relationship between mass and volume What does a burette measure
What does a burette measure Mass, volume and density are all properties of
Mass, volume and density are all properties of Mass and volume to density
Mass and volume to density Big daddy d
Big daddy d Dense materials examples
Dense materials examples Density mass and volume
Density mass and volume Mole mass and mole volume relationships
Mole mass and mole volume relationships Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Atomic
Atomic O
O What makes up the atomic mass
What makes up the atomic mass Mass and matter
Mass and matter Gray matter vs white matter
Gray matter vs white matter Median and lateral apertures
Median and lateral apertures Gray matter and white matter
Gray matter and white matter Telencephalon
Telencephalon Energy conservation quick check
Energy conservation quick check What decreases stroke volume
What decreases stroke volume End diastolic volume meaning
End diastolic volume meaning Solute vs solvent
Solute vs solvent Closing volume vs residual volume
Closing volume vs residual volume Volume kerucut = .....x volume tabung *
Volume kerucut = .....x volume tabung * Write two advantages of large volume parenterals.
Write two advantages of large volume parenterals. Density volume mass triangle
Density volume mass triangle Mass equals density times volume
Mass equals density times volume Mass volume density triangle
Mass volume density triangle Mass of a substance per unit volume
Mass of a substance per unit volume