Matter MATTER is anything that has volume and
















- Slides: 23


Matter MATTER is anything that has volume and mass.

What are the two observable properties of any piece of matter? Answer: volume and mass.

Physical Properties Characteristics of a substance that can be used to identify it. Examples: • Color • Shape • Texture • State of matter • Size • All measurements

Physical Change • A change in one or more physical properties. (changing a physical property does NOT change the substance)

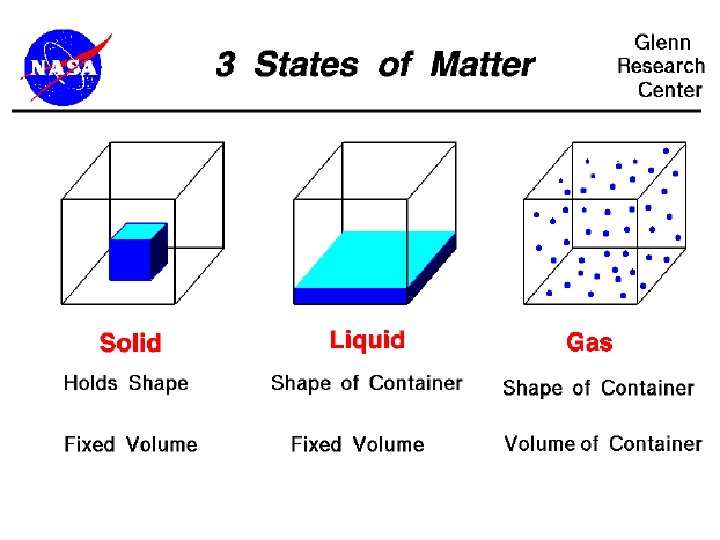
4 States ( Phases ) of Matter • Solid • Liquid • Gas • Plasma

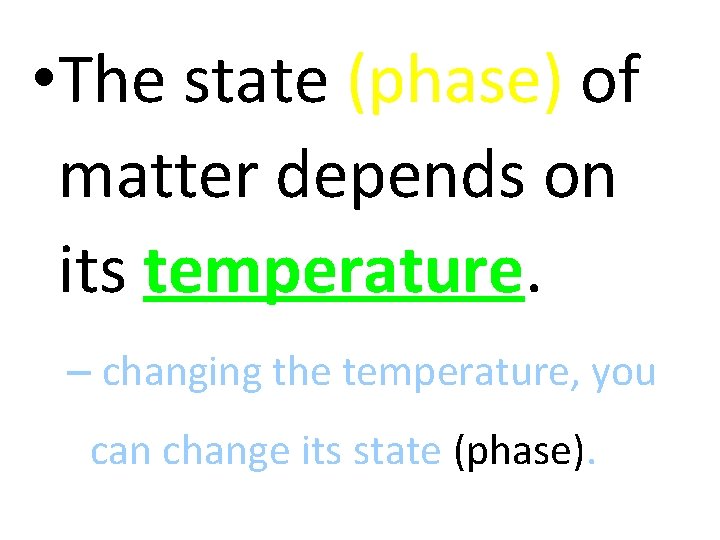
• The state (phase) of matter depends on its temperature. – changing the temperature, you can change its state (phase).

Characteristics of phases • Solid - has a definite shape and volume • Liquid - takes the shape of its container but DOES NOT completely fill it

Characteristics of Matter • Gas - takes the shape of its container and DOES completely fill it • Plasma - hard to contain and very dangerous


Crystalline Solid • Particles arrange themselves in repeating geometric patterns. * crystals


Non-Crystalline Solid • A solid but not made of crystals. * called amorphous solids “having no form”

Kinetic Molecular Theory • The STUFF that makes up matter is in constant motion. * this arrangement and motion helps us determine its state of matter

Arrangement and Motion • Solids - tightly packed; very little motion • Liquids - loosely packed; medium motion • Gases - free and independent; fast motion • Plasma - loosely packed; very high energy level

• Plasma is the most common state of matter in the universe. * 99% of the mass of the Solar System is plasma * least common state of matter on Earth

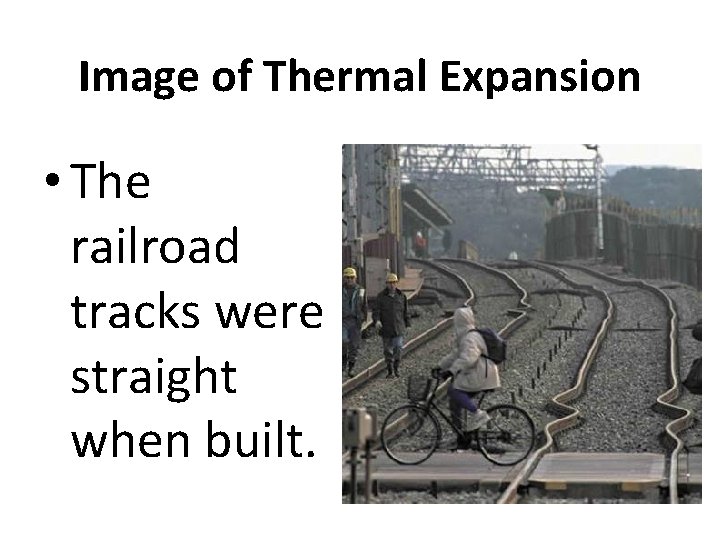
Thermal Expansion • As particles in matter heat up, they move faster and hit against each other with more force. * this causes the matter to expand in all direction * as things cool down they contract

1. ) Hotdogs in the microwave. 2. ) Unopened soda left out in the sun or in your car.

Image of Thermal Expansion • The railroad tracks were straight when built.

• Chemical Properties • Characteristics of a substance that indicates whether it can undergo a certain chemical change. • Burning • Rusting • Rotting

Chemical Change • A change of one substance in a material to a different substance. (changing a chemical property makes a NEW substance)

Law of Conservation of Mass • The mass of all substances before a chemical change EQUALS the mass of all substances after the change. * what goes in must equal what comes out

The End