Matter Anything that has mass and volume Mass










- Slides: 42

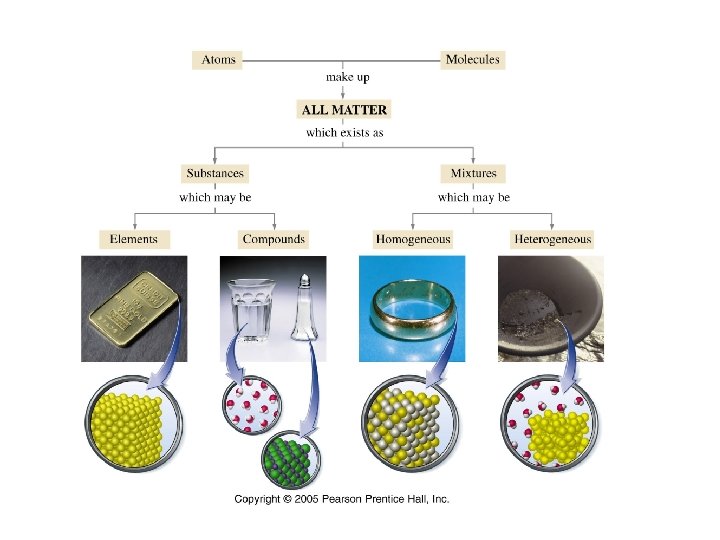
Matter: Anything that has mass and volume Mass – the amount of material in an object (the amount of inertia an object contains. ) Volume – the amount of space an object occupies.


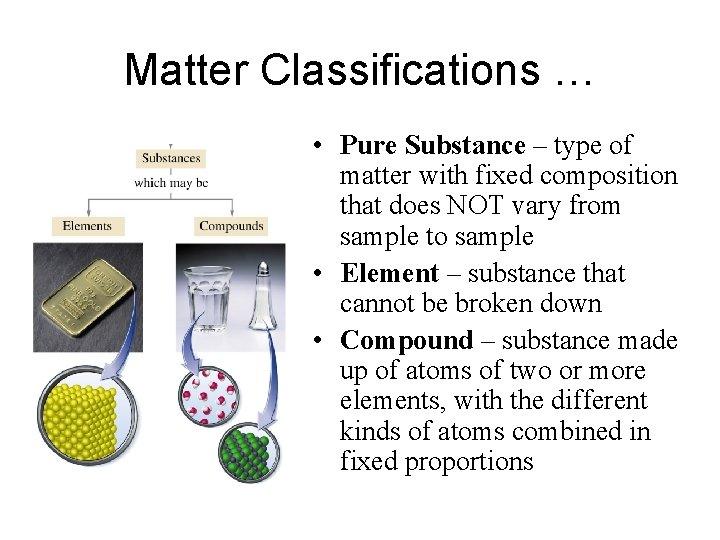
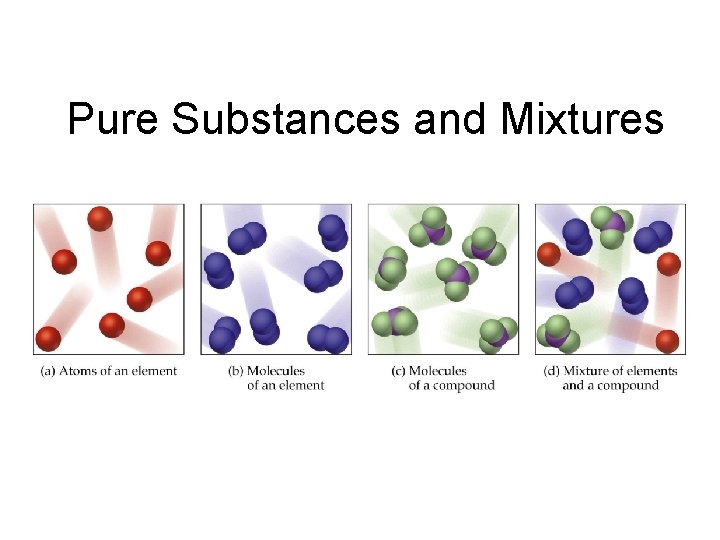
Matter Classifications … • Pure Substance – type of matter with fixed composition that does NOT vary from sample to sample • Element – substance that cannot be broken down • Compound – substance made up of atoms of two or more elements, with the different kinds of atoms combined in fixed proportions

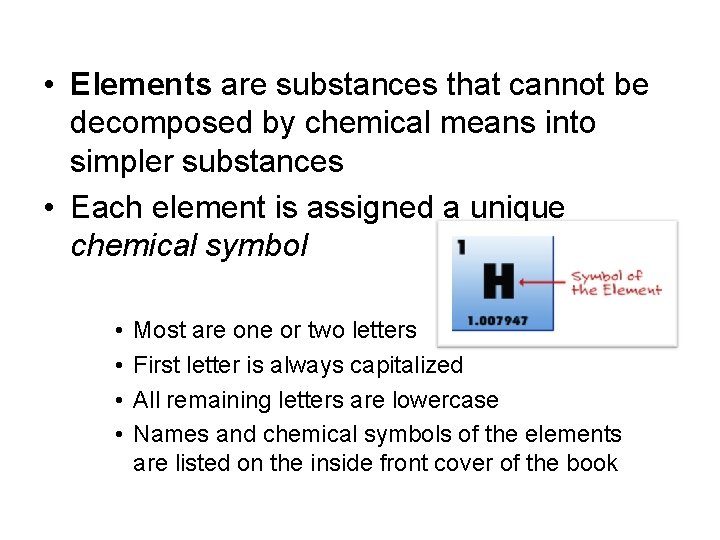
• Elements are substances that cannot be decomposed by chemical means into simpler substances • Each element is assigned a unique chemical symbol • • Most are one or two letters First letter is always capitalized All remaining letters are lowercase Names and chemical symbols of the elements are listed on the inside front cover of the book

• Compounds are substances formed from two or more different elements combined in a fixed proportion by mass • The physical and chemical properties of a compound are, in general, different than the physical and chemical properties of the elements of which it is comprised. • Elements and compounds are examples of pure substances whose composition is the same, regardless of source


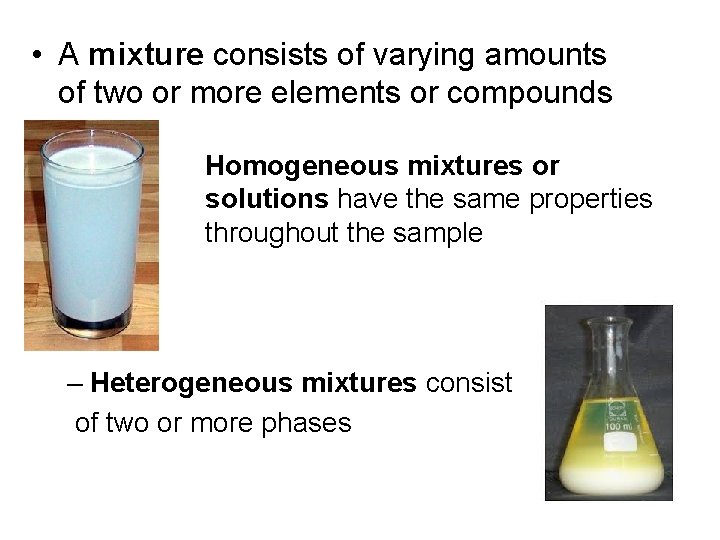
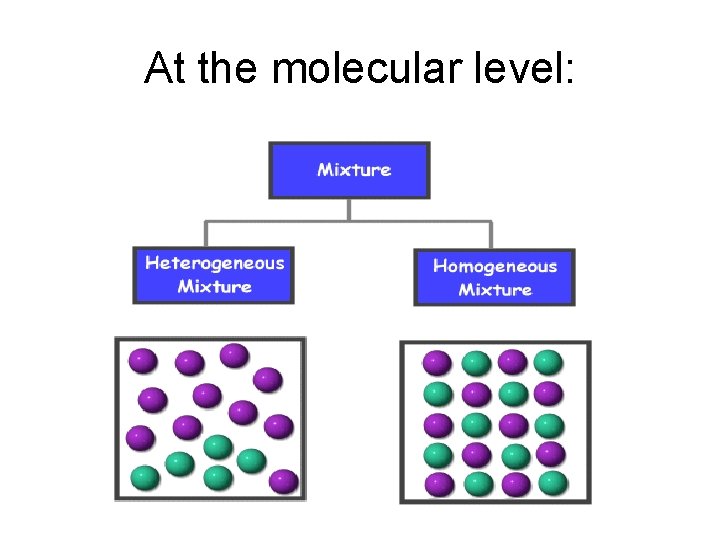
• A mixture consists of varying amounts of two or more elements or compounds Homogeneous mixtures or solutions have the same properties throughout the sample – Heterogeneous mixtures consist of two or more phases

At the molecular level:

Solutions • A solution is a homogeneous mixture in which the two or more components mix freely • The solvent is taken as the component present in the largest amount • A solute is any substance dissolved in the solvent

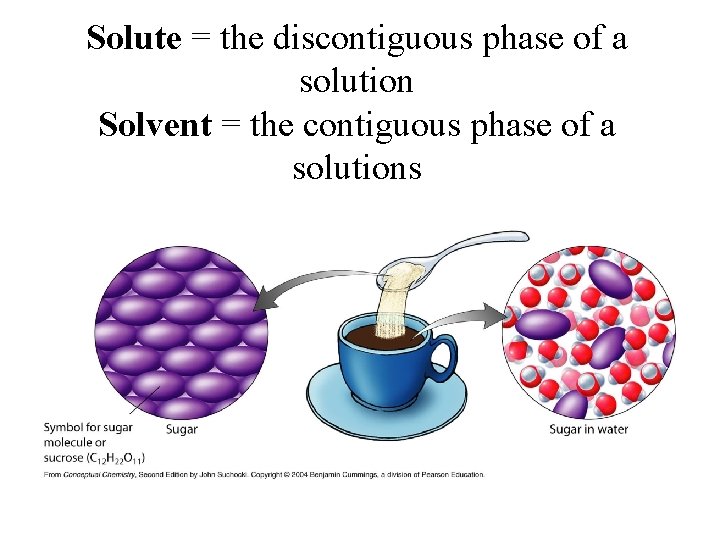
Solute = the discontiguous phase of a solution Solvent = the contiguous phase of a solutions

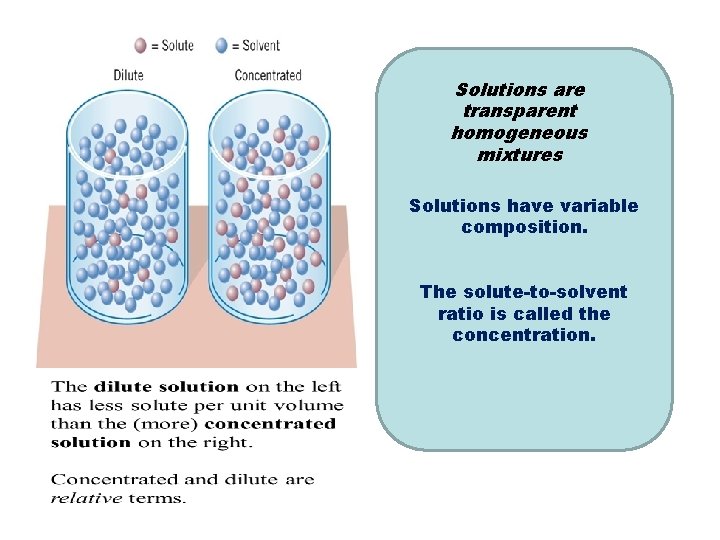
Solutions are Formation of a solution of transparent iodine molecules in ethyl homogeneous alcohol. Ethyl alcohol is the mixtures solvent and iodine the Solutions have variable solute. composition. The solute-to-solvent ratio is called the concentration.

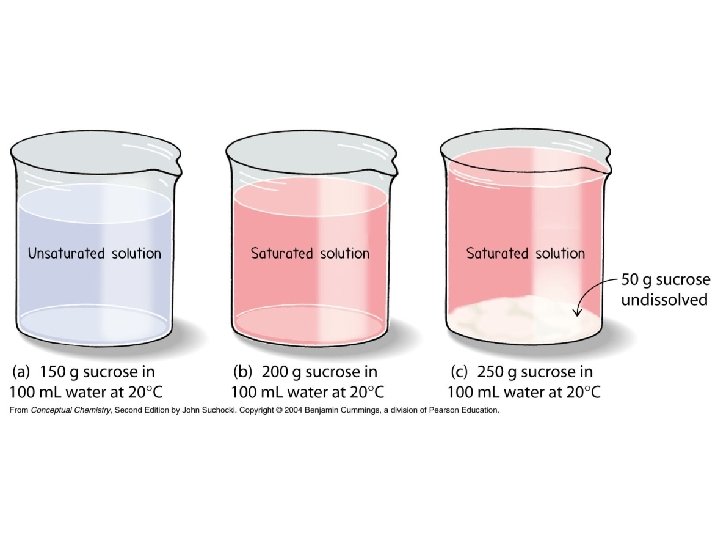
• There is usually a limit to the amount of solute that can dissolve in a given amount of solvent – For example, 36. 0 g Na. Cl is able to dissolve in 100 g of water at 20°C • A solution is said to be saturated when no more solute can be dissolved at the current temperature • Concentration is an intensive, physical property.


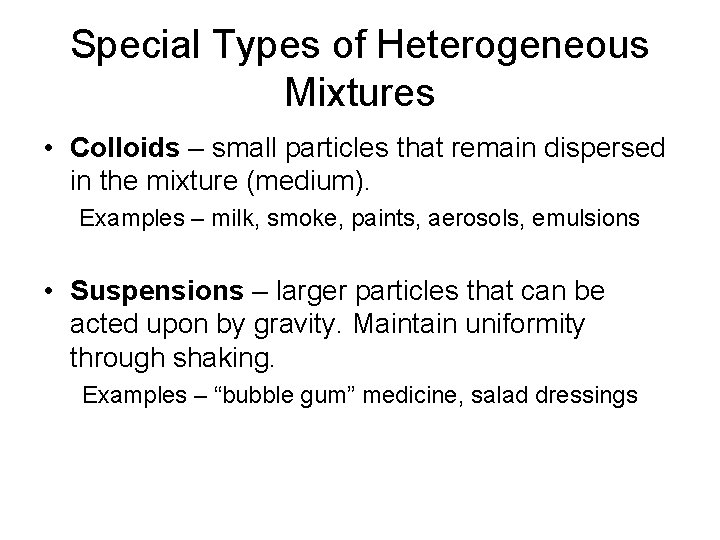
Special Types of Heterogeneous Mixtures • Colloids – small particles that remain dispersed in the mixture (medium). Examples – milk, smoke, paints, aerosols, emulsions • Suspensions – larger particles that can be acted upon by gravity. Maintain uniformity through shaking. Examples – “bubble gum” medicine, salad dressings

Pure Substances and Mixtures

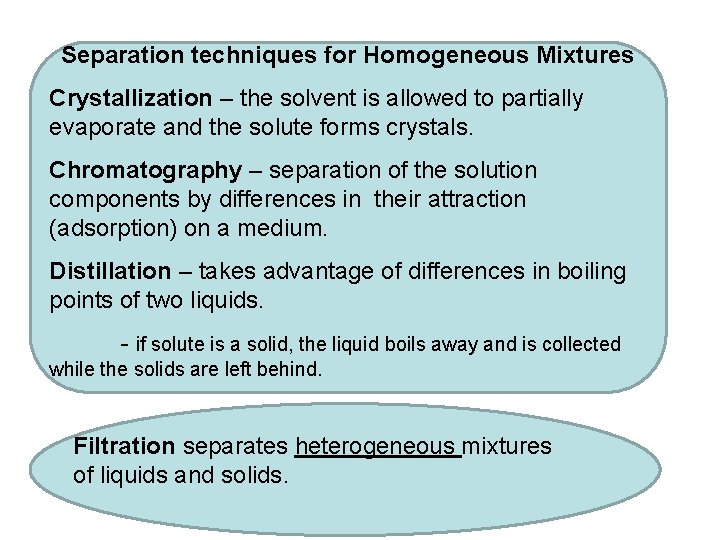
Separation techniques for Homogeneous Mixtures Crystallization – the solvent is allowed to partially evaporate and the solute forms crystals. Chromatography – separation of the solution components by differences in their attraction (adsorption) on a medium. Distillation – takes advantage of differences in boiling points of two liquids. - if solute is a solid, the liquid boils away and is collected while the solids are left behind. Filtration separates heterogeneous mixtures of liquids and solids.

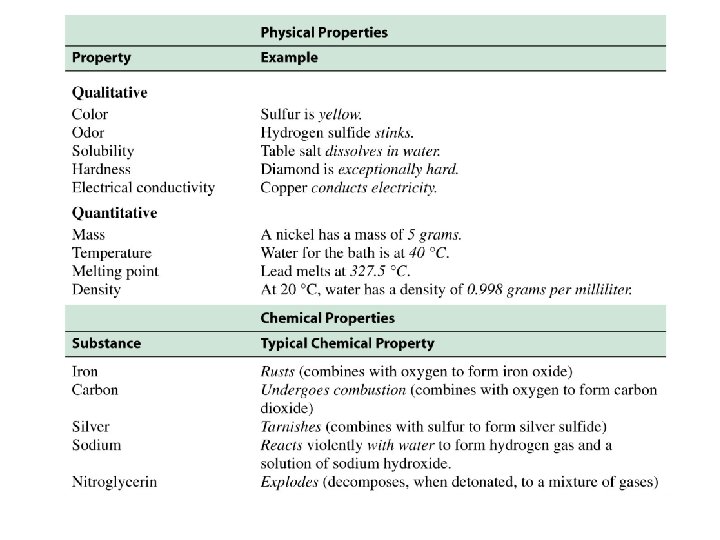
Characteristics or properties of materials distinguish one type of substance from another. This is important because… • Matter is often classified by properties • Properties may also be used to separate one substance from another.

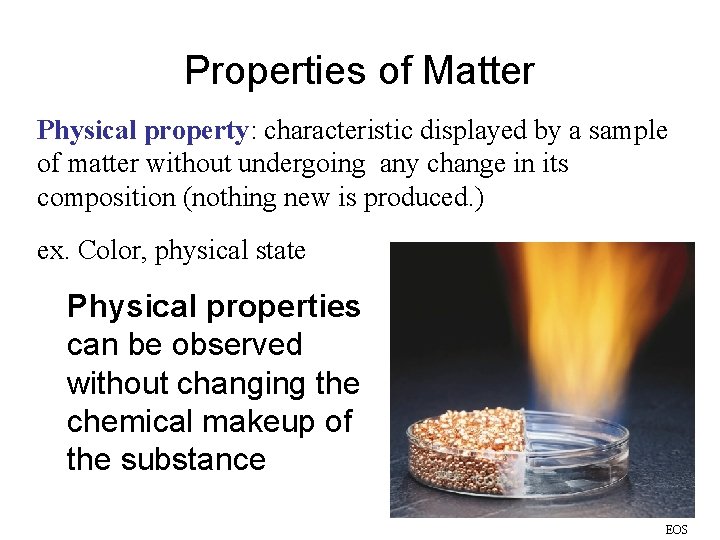
Properties of Matter Physical property: characteristic displayed by a sample of matter without undergoing any change in its composition (nothing new is produced. ) ex. Color, physical state Physical properties can be observed without changing the chemical makeup of the substance EOS

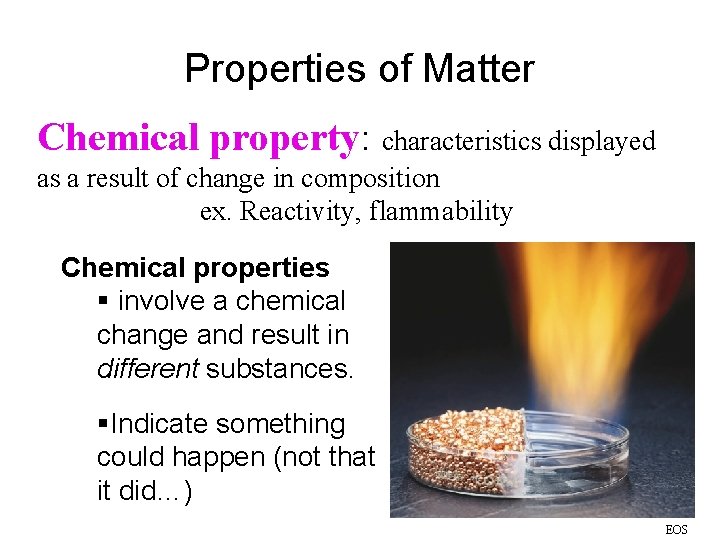
Properties of Matter Chemical property: characteristics displayed as a result of change in composition ex. Reactivity, flammability Chemical properties § involve a chemical change and result in different substances. §Indicate something could happen (not that it did…) EOS

EOS

• Properties can also be described as intensive or extensive – Intensive properties are independent of sample size • Examples: sample color and melting point – Extensive properties depend on sample size • Examples: sample volume and mass • In general, intensive properties are more useful in identifying a substance

Temperature is an intensive, physical property.

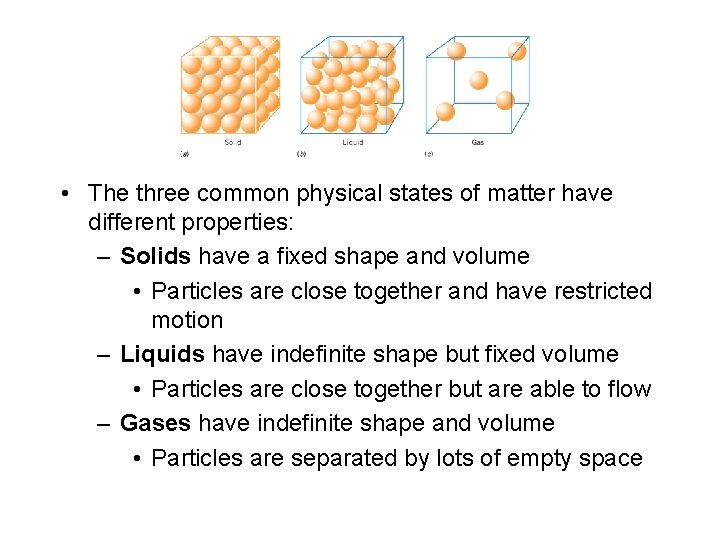
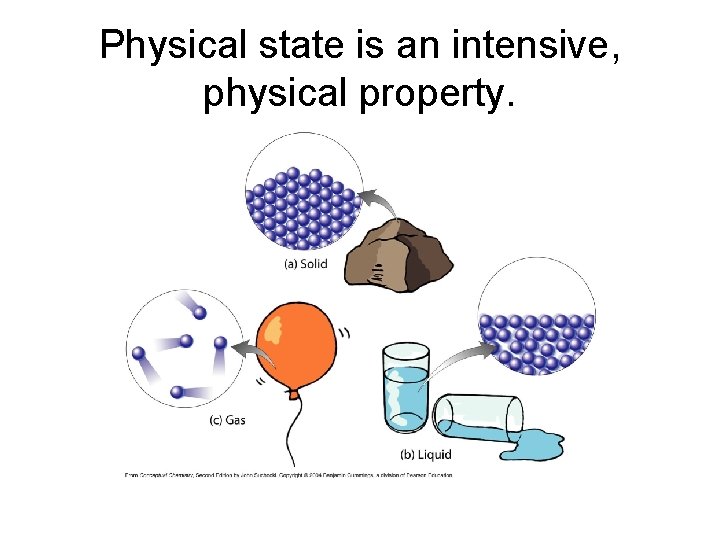
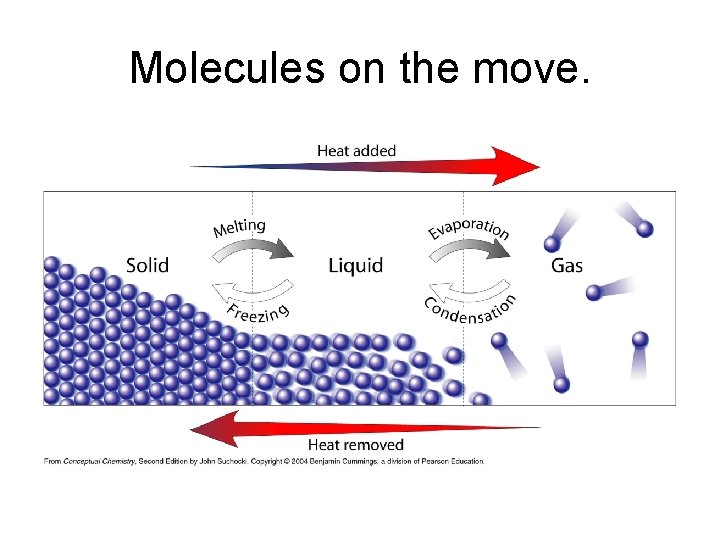
• The three common physical states of matter have different properties: – Solids have a fixed shape and volume • Particles are close together and have restricted motion – Liquids have indefinite shape but fixed volume • Particles are close together but are able to flow – Gases have indefinite shape and volume • Particles are separated by lots of empty space

Physical state is an intensive, physical property.

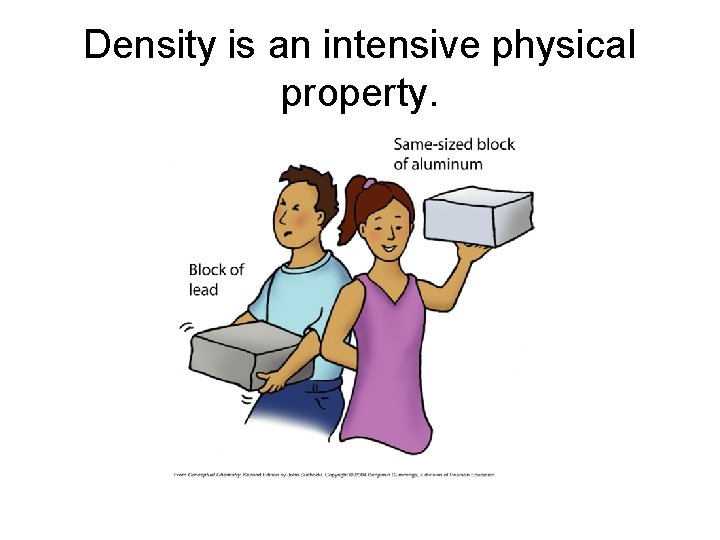
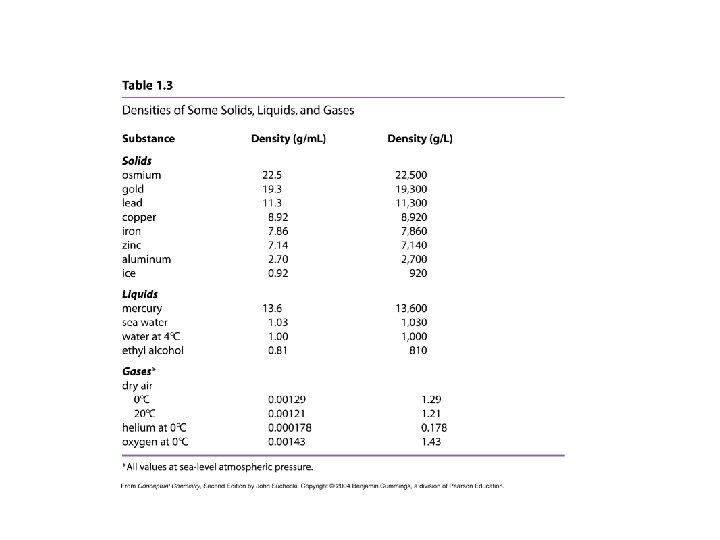
Density is an intensive physical property.


Energy – Not a property of matter but related to changes in matter. Literally means “work within, ” however no object contains work Work (w) is motion against an opposing force that is, to move or displace matter

3 basic types of energy: – Potential (possibility of doing work because of composition or position) – Kinetic (moving objects doing work) – Radiant (energy transferred by electromagnetic waves)

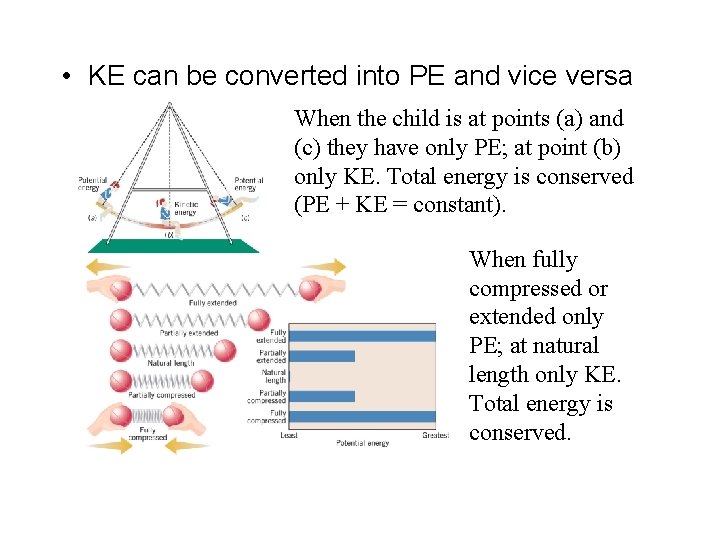
• KE can be converted into PE and vice versa When the child is at points (a) and (c) they have only PE; at point (b) only KE. Total energy is conserved (PE + KE = constant). When fully compressed or extended only PE; at natural length only KE. Total energy is conserved.

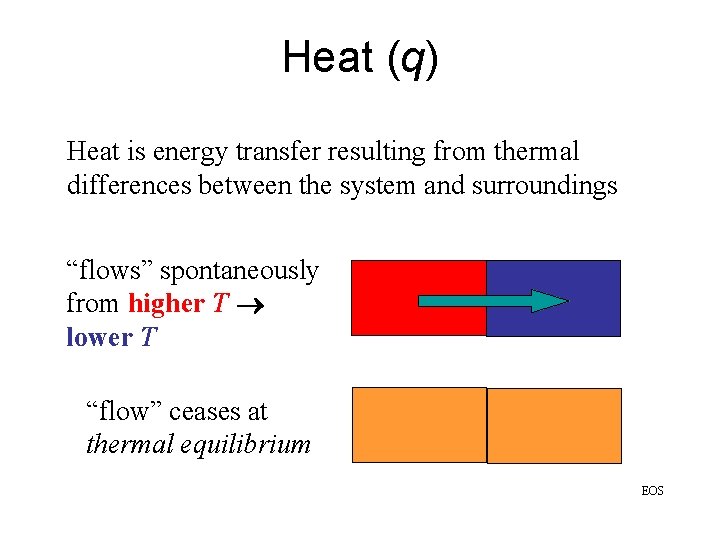
Heat (q) Heat is energy transfer resulting from thermal differences between the system and surroundings “flows” spontaneously from higher T lower T “flow” ceases at thermal equilibrium EOS

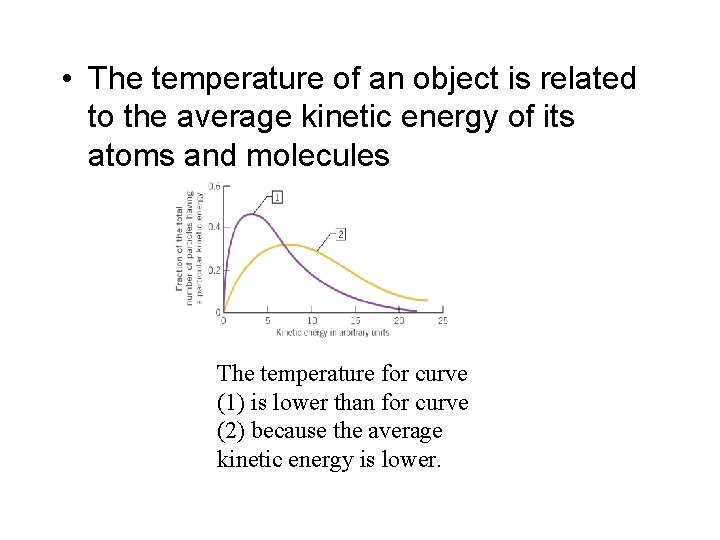
• The energy that is transferred as heat comes from the object’s internal energy • The energy associated with the motion of the object’s molecules is referred to as its molecular kinetic energy • The temperature of an object is related to the average kinetic energy of its atoms and molecules

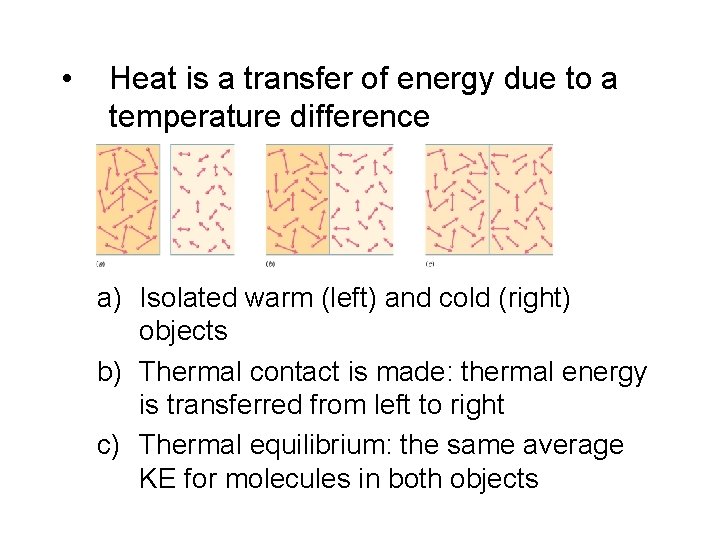
• Heat is a transfer of energy due to a temperature difference a) Isolated warm (left) and cold (right) objects b) Thermal contact is made: thermal energy is transferred from left to right c) Thermal equilibrium: the same average KE for molecules in both objects

• The temperature of an object is related to the average kinetic energy of its atoms and molecules The temperature for curve (1) is lower than for curve (2) because the average kinetic energy is lower.

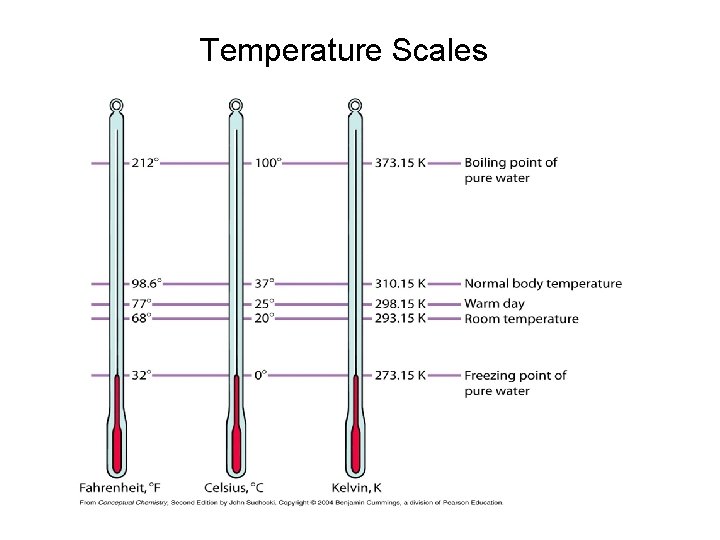
Temperature Scales

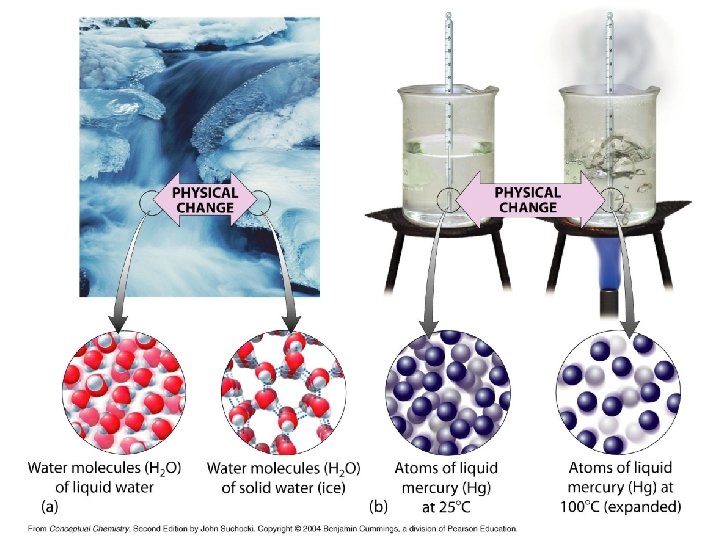
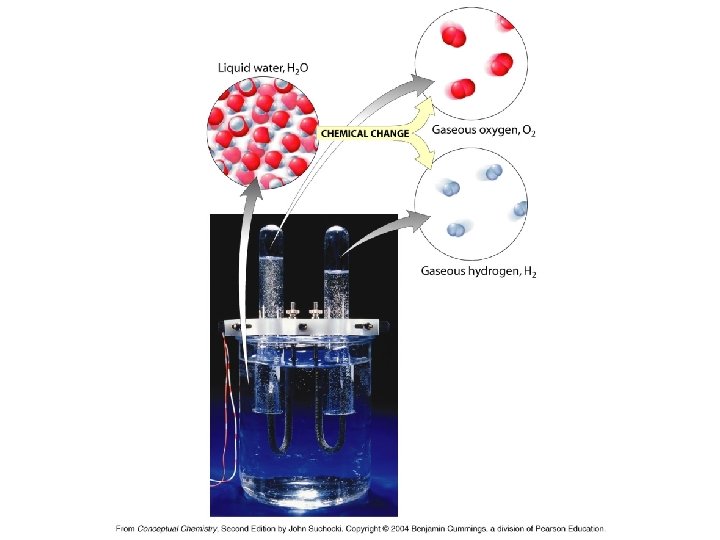
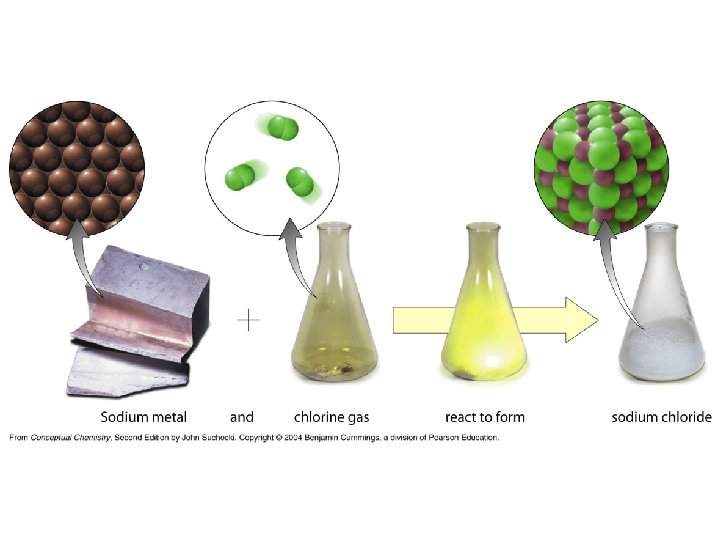
Physical and Chemical Changes Physical Change: changes in appearance but not in composition e. g. , sublimation of ice in the winter Chemical Change: changes resulting in altered composition and/or molecular structure e. g. , spoilage of foods EOS

Indications of Physical Change • The substance HAS NOT CHANGED its identity. • Its physical properties may change. • Changes of State are physical changes


Molecules on the move.

Indications of Chemical Change • Results in a new substance being formed! • Changes in physical properties: • Color change • Formation of a gas • Formation of a precipitate • Production or loss of heat • Production of other types of energy



The Law of Conservation of Mass or Matter In any chemical reaction, matter (or mass) is neither created nor destroyed.