Properties of Matter Matter Anything that has volume













- Slides: 18

Properties of Matter

Matter Anything that has volume and mass

Matter Continued • Element – A pure substance that cannot be broken down into simpler substances by physical or chemical means • Compound – A pure substance composed of two or more elements that are chemically combined

Physical Properties A property of matter that can be observed or measured without changing the identity of the matter.

Examples • • • Color Size Shape Weight Odor Phase

Examples • Boiling point. Water 212 degrees F, 100 C • Freezing point. Water 32 degrees F, 0 C

Density The amount of matter in a given space Density= mass/volume • Mass- The amount of matter that something is made of • Volume- The amount of space something occupies

Density Examples • Which layer has the highest density? • Which layer has the lowest density? Liquid Density (g/m. L) Rubbing Alcohol Lamp Oil Vegetable Oil Water 0. 79 0. 81 0. 92 1 Dawn Dish Soap 1. 06 Light Corn Syrup 1. 33 Honey 1. 42

Density Examples • • Rubbing alcohol Water Copper Mercury 0. 79 g/m. L 1. 0 g/m. L 8. 96 g/m. L 13. 55 g/m. L

Malleability • The ability of a substance to form thin sheets under pressure • Examples: – Gold – Silver – Iron – Aluminum – Copper – Tin

Oxidation The process to chemically combine with oxygen • Examples: ØIron + Oxygen= Iron Oxide (Rust) ØCopper + Carbon Dioxide + Water = Copper Carbonate (Green copper)

Oxidation Examples

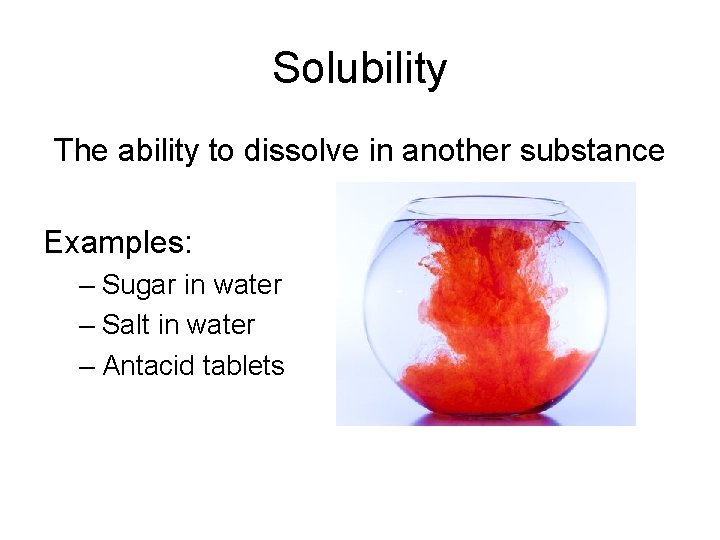
Solubility The ability to dissolve in another substance Examples: – Sugar in water – Salt in water – Antacid tablets

Chemical Properties Describes a substance based on its ability to change into a new substance with different properties.

Examples • Flammable - non flammable • Reacts with water. Does not react with water • Will Oxidize. Will not oxidize

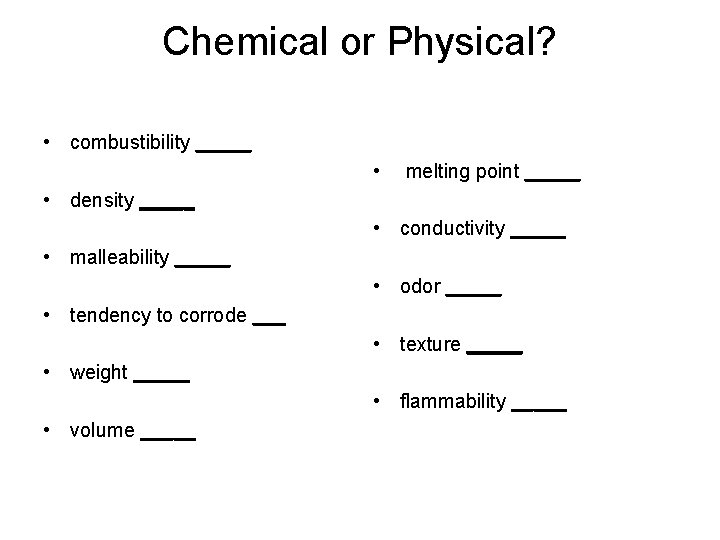
Chemical or Physical? • combustibility _____ • melting point _____ • density _____ • conductivity _____ • malleability _____ • odor _____ • tendency to corrode ___ • texture _____ • weight _____ • flammability _____ • volume _____

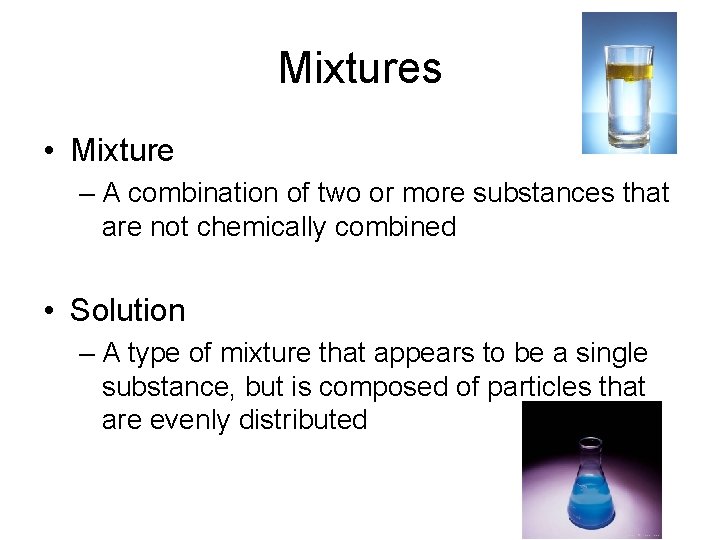
Mixtures • Mixture – A combination of two or more substances that are not chemically combined • Solution – A type of mixture that appears to be a single substance, but is composed of particles that are evenly distributed

Synthetic A man-made substance that usually imitates a naturally occurring substance
 Compare and contrast template
Compare and contrast template Anything with mass and volume
Anything with mass and volume Anything that has mass and volume
Anything that has mass and volume Anything that occupies space
Anything that occupies space Mass vs weight
Mass vs weight Matter anything that
Matter anything that Anything that has mass and takes up space is
Anything that has mass and takes up space is All matter has and takes up
All matter has and takes up 7 diatomic elements
7 diatomic elements Matter is anything that has and occupies
Matter is anything that has and occupies Whats anything that has mass and takes up space
Whats anything that has mass and takes up space Matter is anything that occupies space
Matter is anything that occupies space Buoyancy defintion
Buoyancy defintion Properties of matter hardness
Properties of matter hardness Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block nhĩ thất độ 2 mobitz 1
Block nhĩ thất độ 2 mobitz 1 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart