Anything that has mass and volume MATTER Physical













- Slides: 21

Anything that has mass and volume MATTER

Physical Property �A characteristic of a substance that does not involve a chemical change � Examples: �Density �Color �Hardness �Thermal Conduction �State �Ductility �Malleability

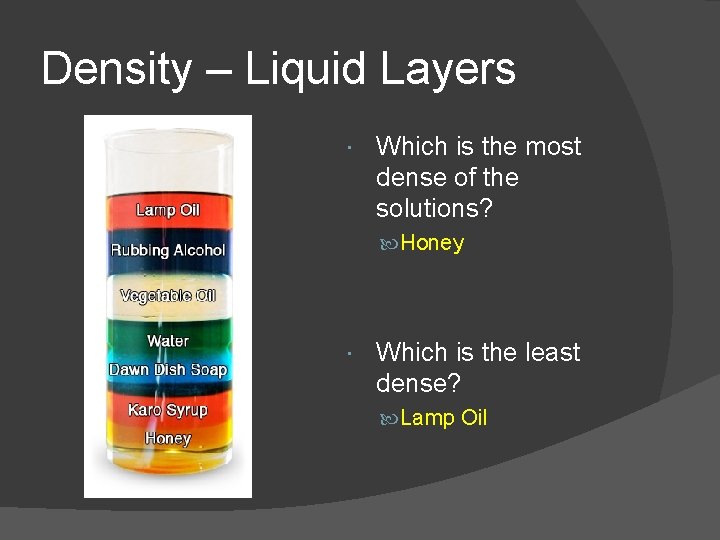
Density – Liquid Layers Which is the most dense of the solutions? Honey Which is the least dense? Lamp Oil

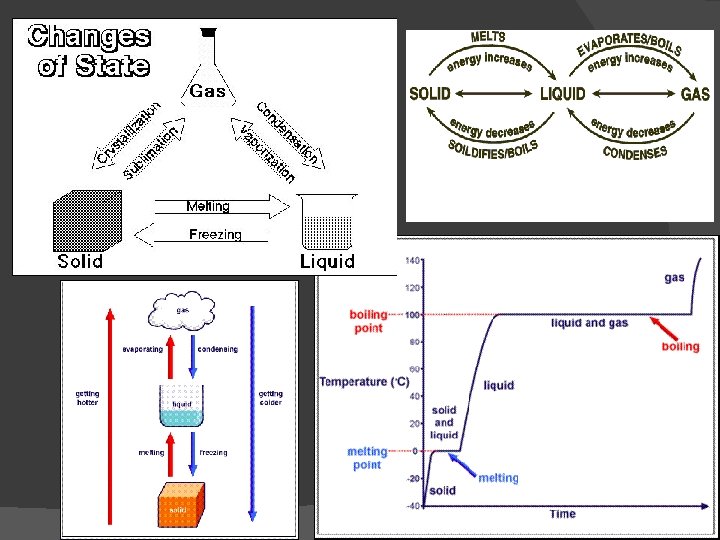
Physical Changes A change of matter from one form to another WITHOUT a change in chemical properties

Chemical Property A property of matter that describes a substance’s ability to participate in chemical reactions. Examples: Flammability Reactivity with Oxygen

Chemical Changes A change that occurs when one or more substances change into entirely new substances with DIFFERENT properties

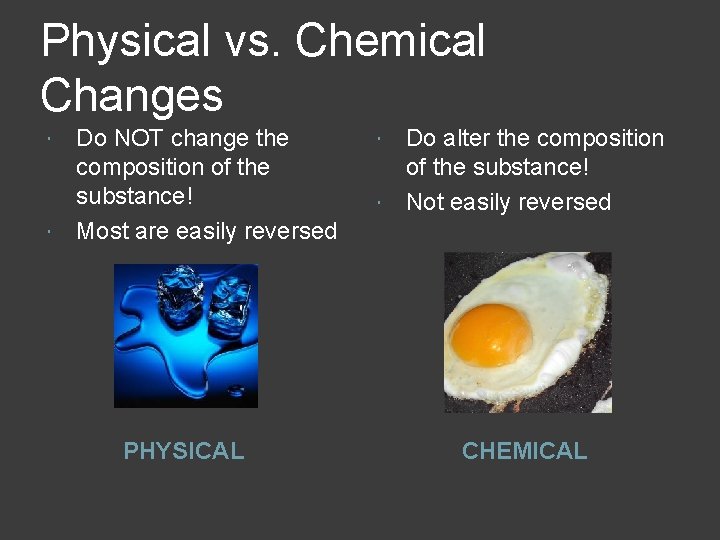
Physical vs. Chemical Changes Do NOT change the composition of the substance! Most are easily reversed PHYSICAL Do alter the composition of the substance! Not easily reversed CHEMICAL

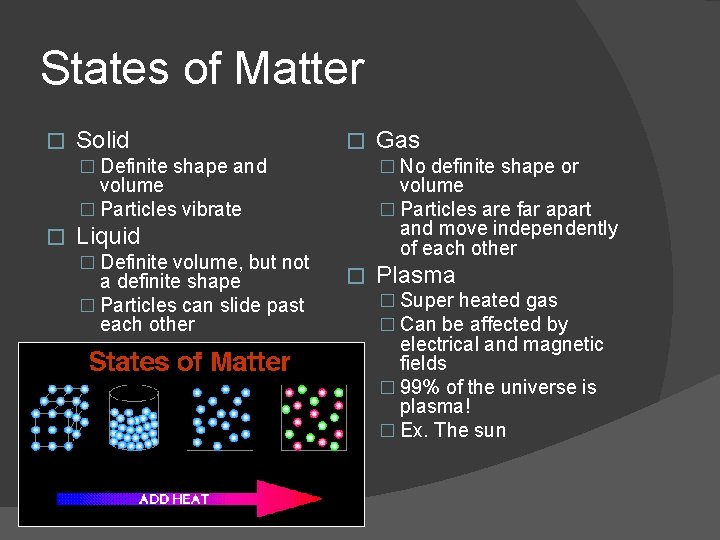
States of Matter � Solid � � Definite shape and � No definite shape or volume � Particles vibrate � volume � Particles are far apart and move independently of each other Liquid � Definite volume, but not a definite shape � Particles can slide past each other Gas � Plasma � Super heated gas � Can be affected by electrical and magnetic fields � 99% of the universe is plasma! � Ex. The sun

Liquids Surface Tension Viscosity The force that acts on The resistance of a the surface of a liquid & that tends to minimize the area of the surface gas or liquid to flow

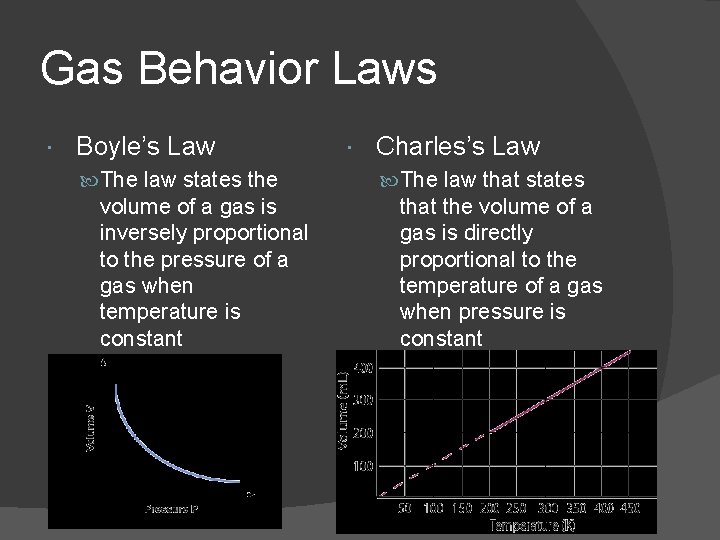
Gas Behavior Laws Boyle’s Law The law states the volume of a gas is inversely proportional to the pressure of a gas when temperature is constant Charles’s Law The law that states that the volume of a gas is directly proportional to the temperature of a gas when pressure is constant


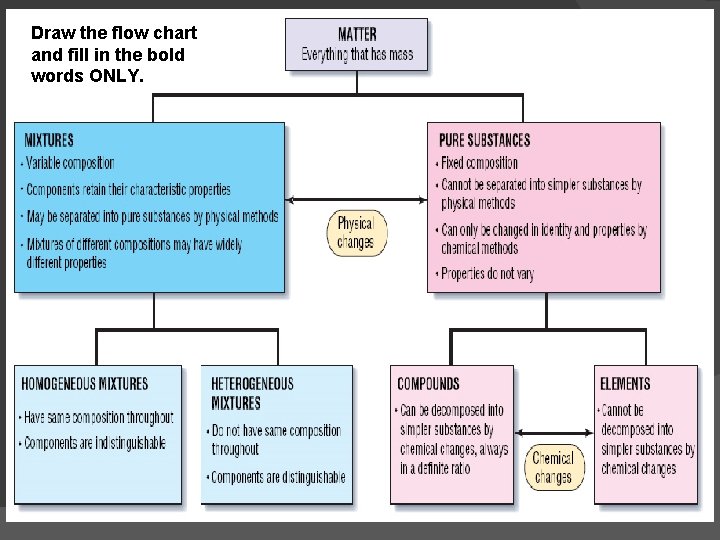
Draw the flow chart and fill in the bold words ONLY.

Mixtures A combination of 2 or more substances that are not chemically combined. Can be separated by PHYSICAL METHODS 2 Types: Heterogeneous Homogeneous

2 Types of Mixtures Heterogeneous Mixture a type of mixture in which the components can be seen typically there are two or more phases present. Ex. Vegetable Soup, cereal & milk, pizza, rocks in sand

2 Types of Mixtures Homogeneous Mixture a type of mixture in which the composition is uniform every part of the solution has the same properties Ex. air, salt water, milk, blood, Kool-Aid

Pure Substances Fixed composition (what they are made of does not vary) CANNOT be separated by physical means 2 Types: Elements Compounds

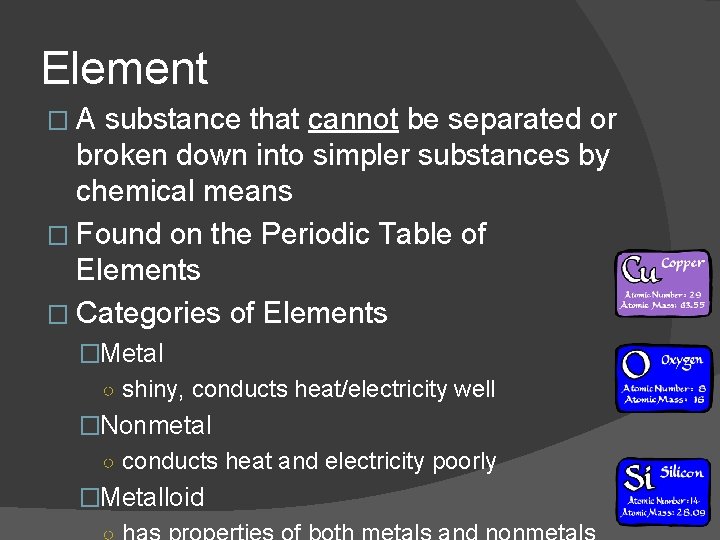
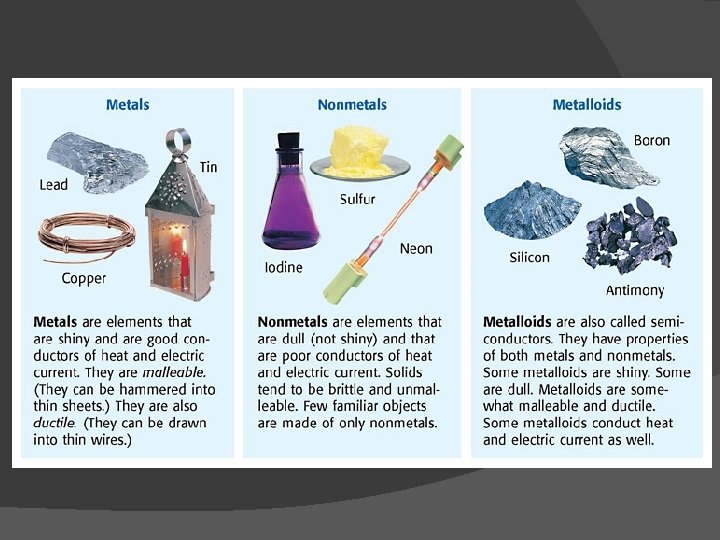
Element �A substance that cannot be separated or broken down into simpler substances by chemical means � Found on the Periodic Table of Elements � Categories of Elements �Metal ○ shiny, conducts heat/electricity well �Nonmetal ○ conducts heat and electricity poorly �Metalloid


Compound A substance made up of atoms of 2 or more different elements joined by chemical bonds (made of elements!) Elements come together in specific ratios to form compounds. Compound Elements Combined Table Salt Sodium and Chlorine Water Hydrogen and Oxygen Vinegar Hydrogen, Carbon, and Oxygen Carbon Dioxide Carbon and Oxygen Baking Soda Sodium, Hydrogen, Carbon, and Oxygen

Forming Sodium Chloride

How can I remember the categories of matter? ? ? http: //listen. grooveshark. com/#/search/songs /? query=chicken%20 noodle%20 soup Chicken Noodle Soup Heterogeneous Mixture Soda Homogeneous Mixture Silver (Ag) Element Crackers contain salt (Na. Cl) Compound