Matter Energy Key Terms Matter volume Mass Anything











- Slides: 25

Matter & Energy

Key Terms ►Matter volume ►Mass - Anything that has mass and – the amount of matter in an object (how much stuff it’s made of) ►Volume – the amount of space the matter occupies (how big it is)

►Energy ►Work - the ability to do work - using force to move something

Matter and Energy are Conserved ► Law of Conservation of Matter – § matter cannot be created or destroyed § It can be changed from one form to another ► Law of Conservation of Energy – § Energy cannot be created or destroyed § It can be changed from one form to another

Example of Conservation of Mass & Energy ► Generators produce electricity. Are they creating energy? ► What is the generator using to make electricity? ► Is electricity the only form of energy created by the generator?

Example of Conservation of Mass & Energy ► When a gasoline powered generator has burned up all the gas in the tank, has matter that was in the gasoline been destroyed? ► What happened to the matter in the gas? ► If 5 kg of gasoline is burned how much smoke and exhaust is produced?

Kinetic Theory of Matter - 3 Parts All matter is made of tiny particles 2. The particles are always in motion 3. At same temperature, heavier particles move slower than lighter particles 1.

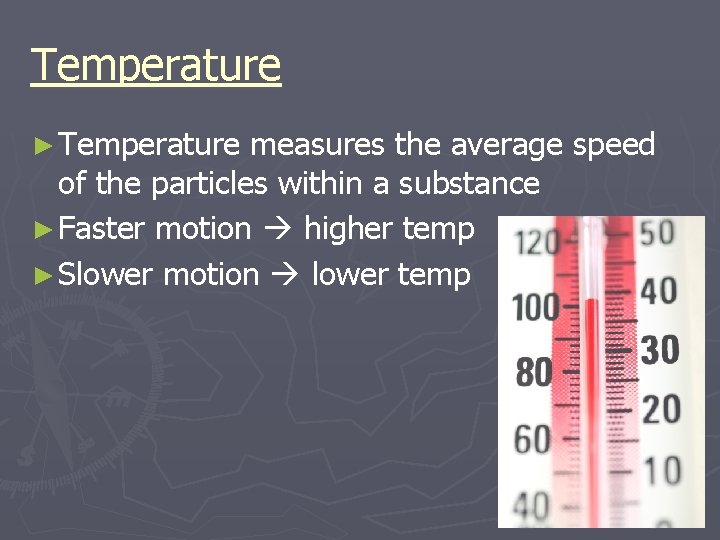
Temperature ► Temperature measures the average speed of the particles within a substance ► Faster motion higher temp ► Slower motion lower temp


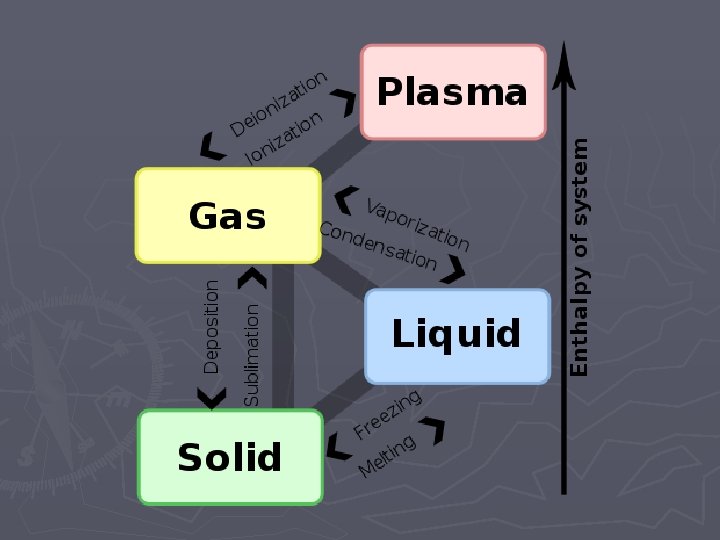
States of Matter ► 1. 2. 3. 4. ► Matter exists in 4 states Solid Liquid Gas Plasma The state of matter depends on the amount of energy

Solid ► Lowest energy state ► Slowest particle motion ► Particles vibrate in place ► Particles packed tight and stuck to each other ► Stuck in one shape and volume

Liquid ► Medium energy state ► Particles move faster than in solid ► Particles packed tight but can slide past each other ► Volume won’t change but shape does

Gas ► High energy state ► Particles move faster than liquid ► Particles are far apart and bouncing off each other ► Volume can change – expand compress

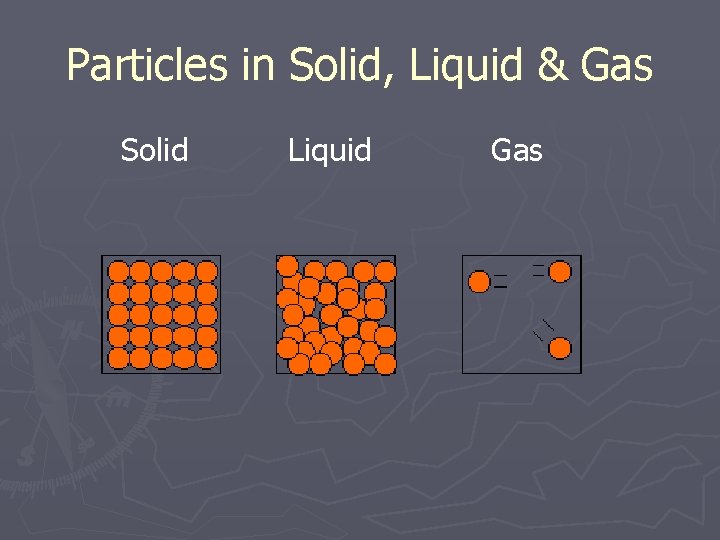
Particles in Solid, Liquid & Gas Solid Liquid Gas

Plasma ► Highest energy state ► Particles move faster than gas ► Particles break apart releasing light ► Lightning, stars

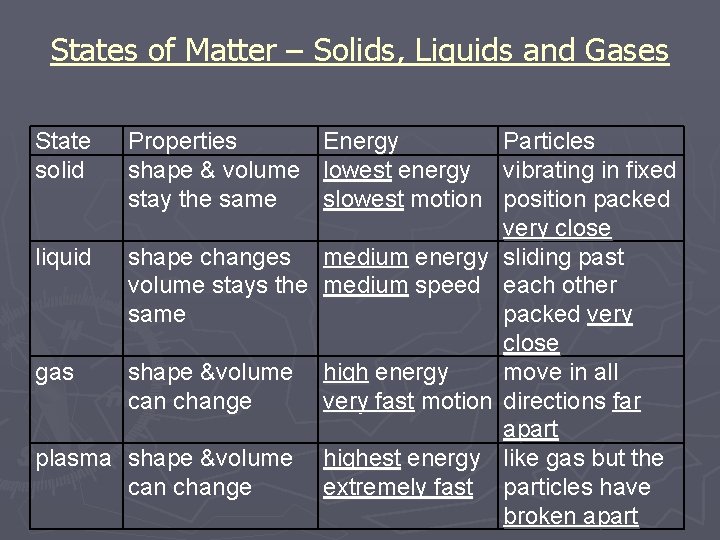
States of Matter – Solids, Liquids and Gases State solid Properties Energy Particles shape & volume lowest energy vibrating in fixed stay the same slowest motion position packed very close liquid shape changes medium energy sliding past volume stays the medium speed each other same packed very close gas shape &volume high energy move in all can change very fast motion directions far apart plasma shape &volume highest energy like gas but the can change extremely fast particles have broken apart

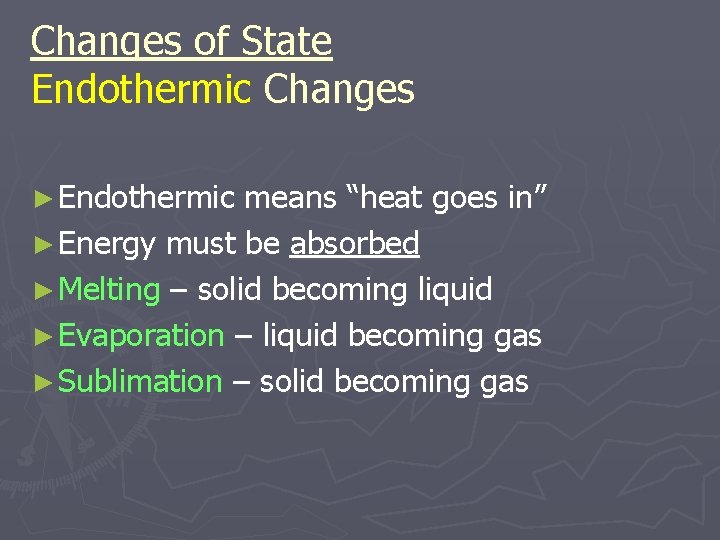
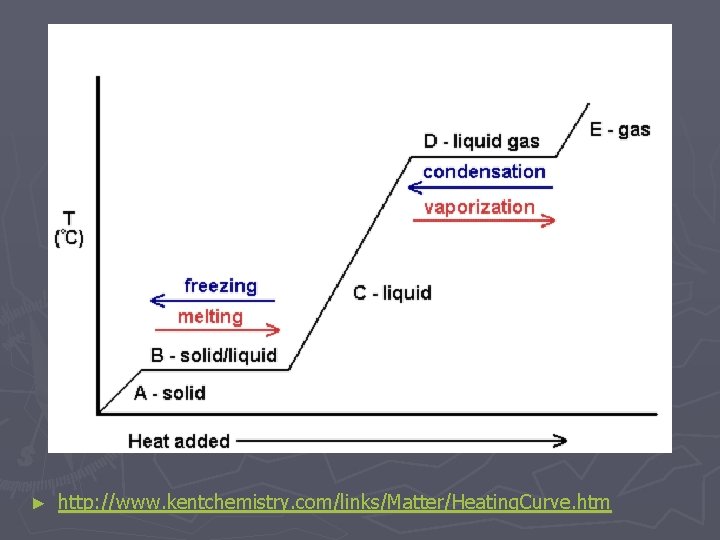
Changes of State Endothermic Changes ► Endothermic means “heat goes in” ► Energy must be absorbed ► Melting – solid becoming liquid ► Evaporation – liquid becoming gas ► Sublimation – solid becoming gas

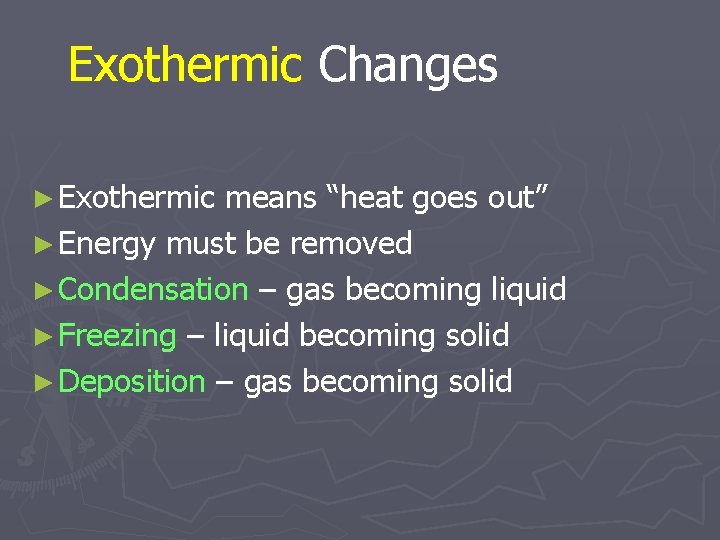
Exothermic Changes ► Exothermic means “heat goes out” ► Energy must be removed ► Condensation – gas becoming liquid ► Freezing – liquid becoming solid ► Deposition – gas becoming solid

Sublimation – Dry Ice ► Dry ice is frozen carbon dioxide ► The freezing point of carbon dioxide is negative 109. 3 degrees Fahrenheit! ► Dry ice changes from solid directly to liquid in normal atmospheric conditions (sublimation) without going through the liquid phase, hence the name, dry ice


Endothermic or Exothermic? ► Gasoline evaporating into a gas ► Ice melting ► Water droplets forming on bathroom mirror ► Hot liquid iron changing into a solid ► Liquid nitrogen changing into nitrogen gas ► Water boiling ► Carbon dioxide gas turning into solid dry ice

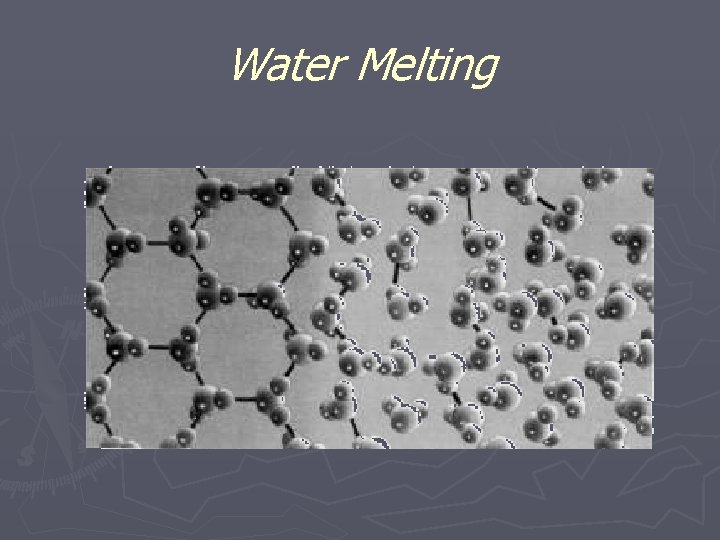
Adding Energy to Matter ► speeds up particle motion OR separates particles causing a change of state ► As ice is heated its particles vibrate faster until it begins melting ► While ice melts its temp stays at 0 o. C until all of it has melted ► Once its melted the particles speed up again (temp rises)

Water Melting

Removing Energy from Matter ► Slows down particles OR brings them closer to each other ► As water vapor cools the particles slow down until they start to condense ► Temp will stay at 100 o. C until all the vapor has become liquid

► http: //www. kentchemistry. com/links/Matter/Heating. Curve. htm