Mass Relationships in Chemical Reactions Chapter 3 Mass




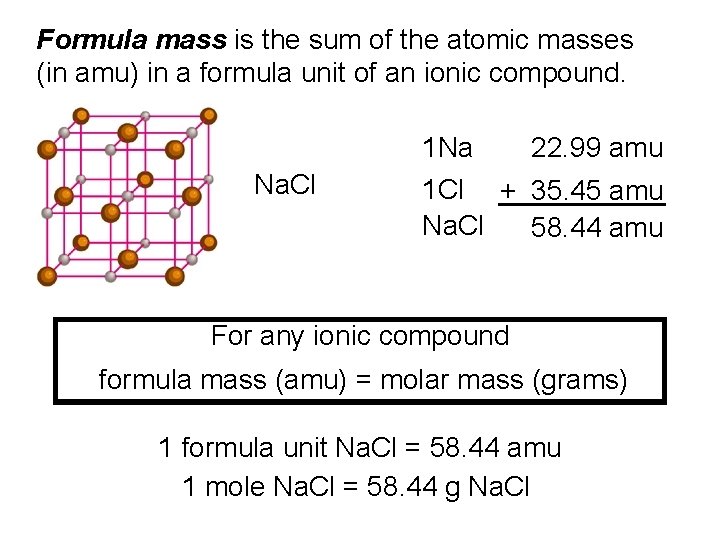
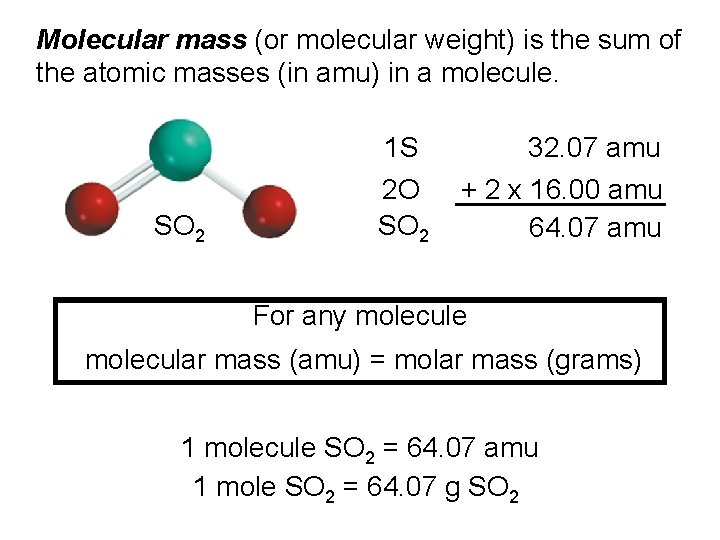

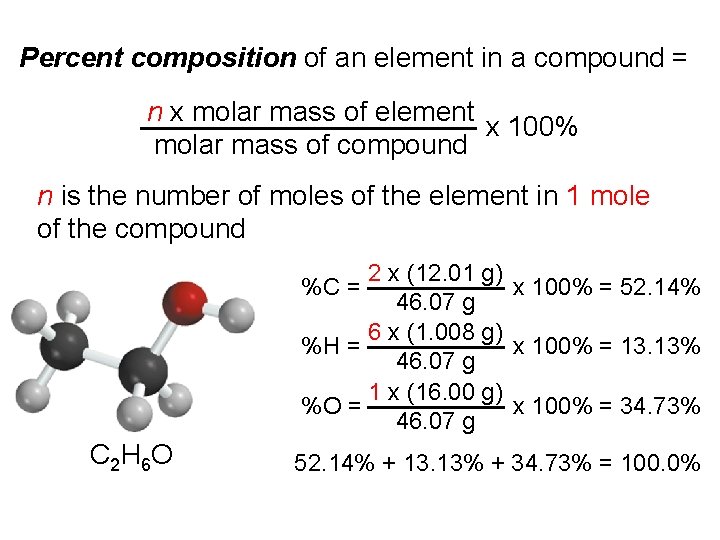

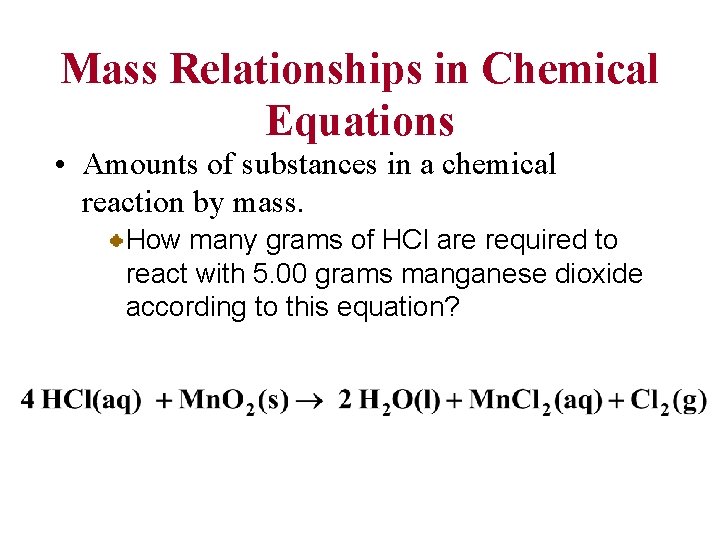
















- Slides: 95

Mass Relationships in Chemical Reactions Chapter 3

Mass and Moles of a Substance • Chemistry requires a method for determining the numbers of molecules in a given mass of a substance. This allows the chemist to carry out “recipes” for compounds based on the relative numbers of atoms involved. The calculation involving the quantities of reactants and products in a chemical equation is called stoichiometry.

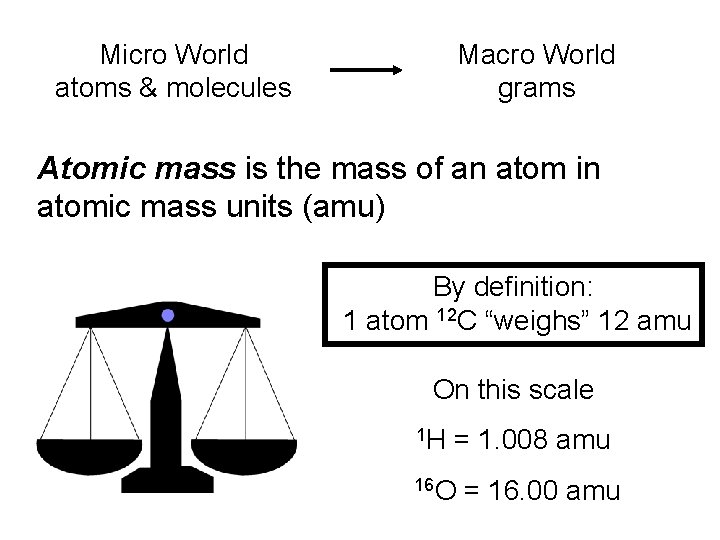
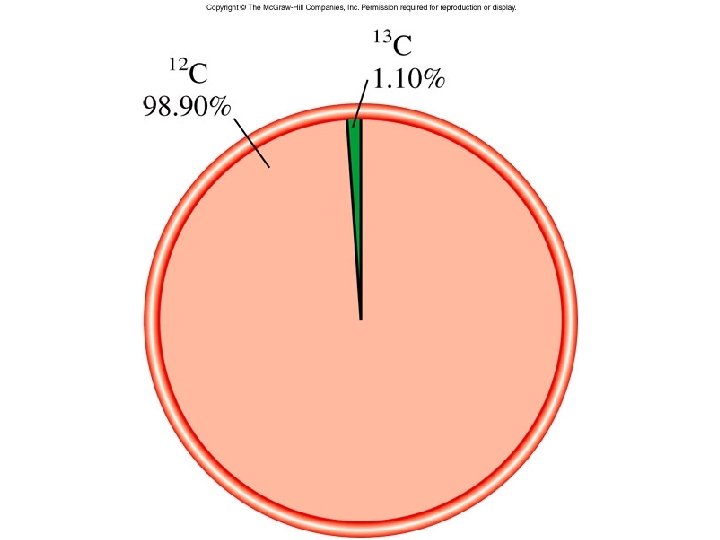
Micro World atoms & molecules Macro World grams Atomic mass is the mass of an atom in atomic mass units (amu) By definition: 1 atom 12 C “weighs” 12 amu On this scale 1 H = 1. 008 amu 16 O = 16. 00 amu

Molecular Weight and Formula Weight • The molecular weight of a substance is the sum of the atomic weights of all the atoms in a molecule of the substance. For, example, a molecule of H 20 contains 2 hydrogen atoms (at 1. 0 amu each) and 1 oxygen atom (16. 0 amu), giving a molecular weight of 18. 0 amu.

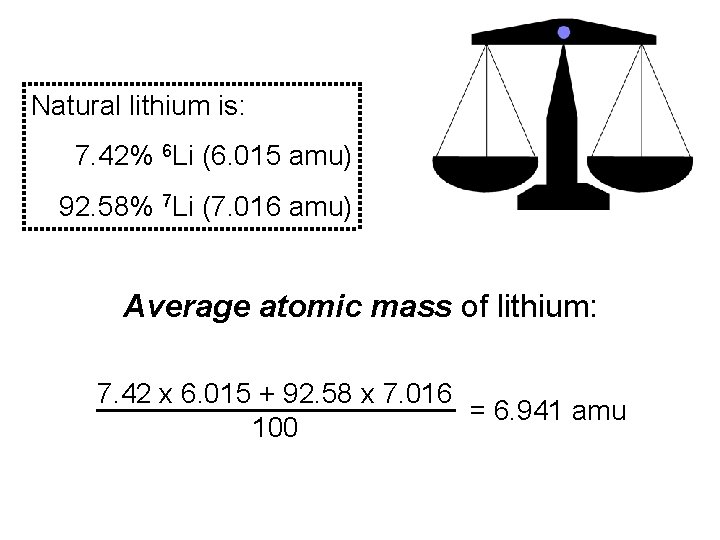
Natural lithium is: 7. 42% 6 Li (6. 015 amu) 92. 58% 7 Li (7. 016 amu) Average atomic mass of lithium: 7. 42 x 6. 015 + 92. 58 x 7. 016 = 6. 941 amu 100


Pg 78 b

Average atomic mass (6. 941)

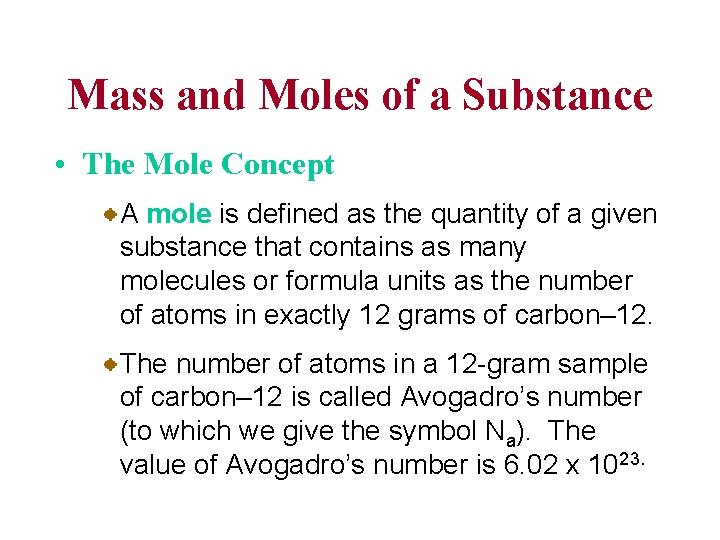
Mass and Moles of a Substance • The Mole Concept A mole is defined as the quantity of a given substance that contains as many molecules or formula units as the number of atoms in exactly 12 grams of carbon– 12. The number of atoms in a 12 -gram sample of carbon– 12 is called Avogadro’s number (to which we give the symbol Na). The value of Avogadro’s number is 6. 02 x 1023.

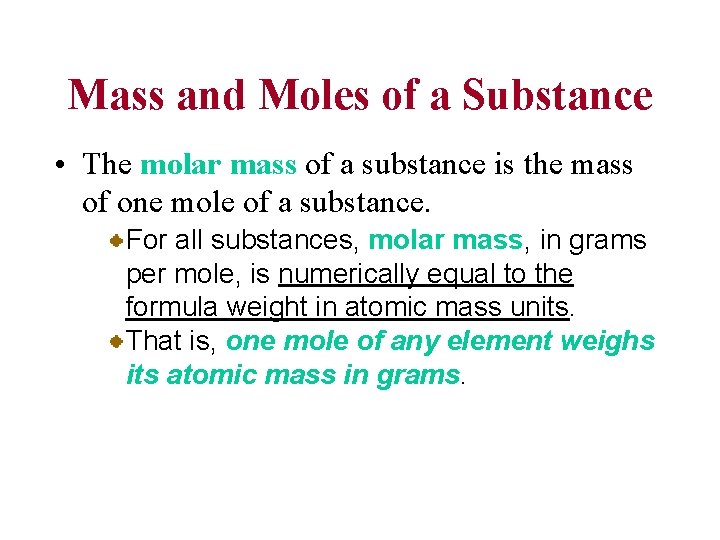
The mole (mol) is the amount of a substance that contains as many elementary entities as there atoms in exactly 12. 00 grams of 12 C 1 mol = NA = 6. 0221367 x 1023 Avogadro’s number (NA)

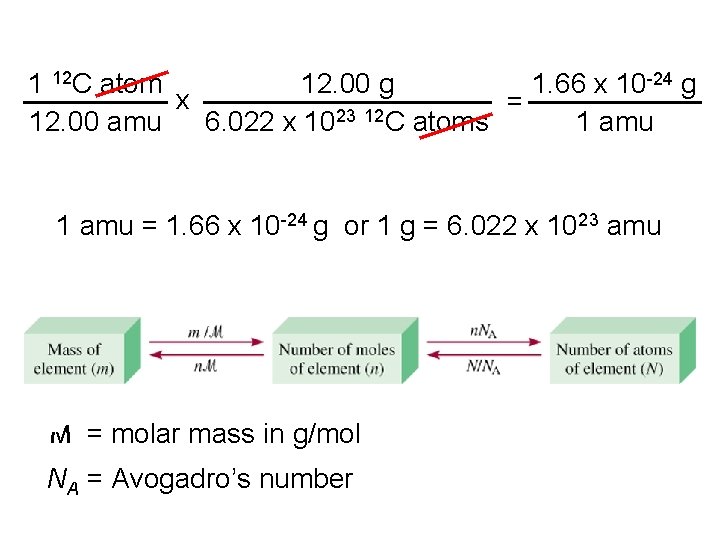
eggs Molar mass is the mass of 1 mole of shoes in grams marbles atoms 1 mole 12 C atoms = 6. 022 x 1023 atoms = 12. 00 g 1 12 C atom = 12. 00 amu 1 mole 12 C atoms = 12. 00 g 12 C 1 mole lithium atoms = 6. 941 g of Li For any element atomic mass (amu) = molar mass (grams)

One Mole of: S C Hg Cu Fe

Mass and Moles of a Substance • The molar mass of a substance is the mass of one mole of a substance. For all substances, molar mass, in grams per mole, is numerically equal to the formula weight in atomic mass units. That is, one mole of any element weighs its atomic mass in grams.

1 12 C atom 12. 00 g 1. 66 x 10 -24 g x = 23 12 12. 00 amu 6. 022 x 10 C atoms 1 amu = 1. 66 x 10 -24 g or 1 g = 6. 022 x 1023 amu M = molar mass in g/mol NA = Avogadro’s number


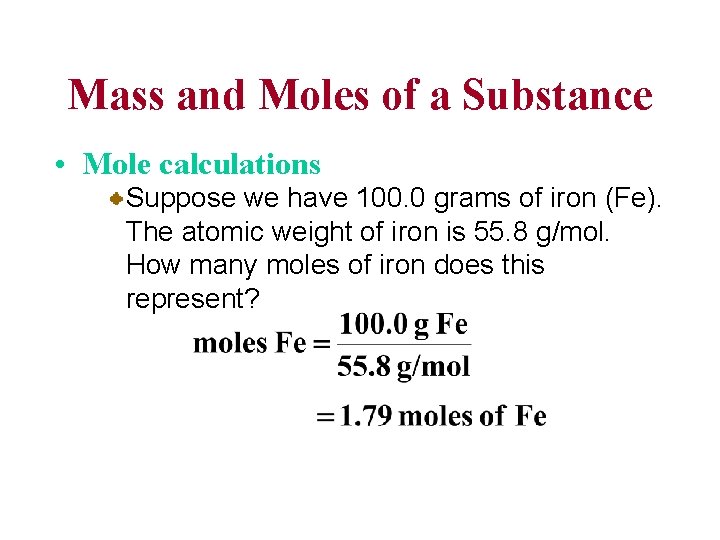
Mass and Moles of a Substance • Mole calculations Suppose we have 100. 0 grams of iron (Fe). The atomic weight of iron is 55. 8 g/mol. How many moles of iron does this represent?

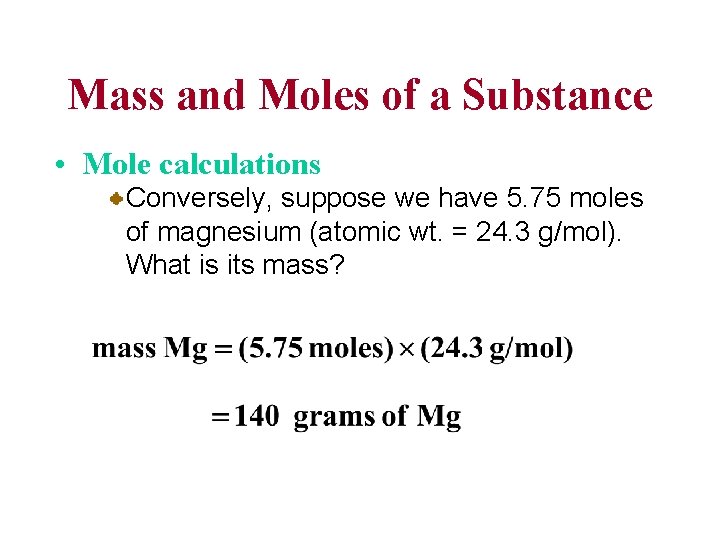
Mass and Moles of a Substance • Mole calculations Conversely, suppose we have 5. 75 moles of magnesium (atomic wt. = 24. 3 g/mol). What is its mass?

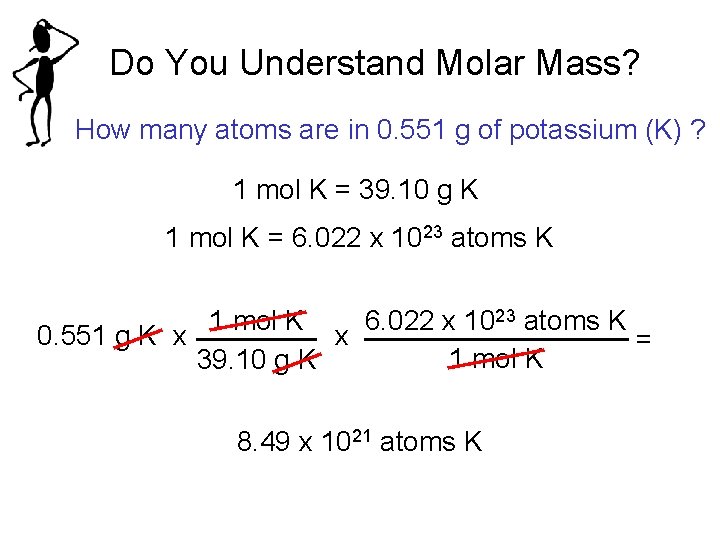
Do You Understand Molar Mass? How many atoms are in 0. 551 g of potassium (K) ? 1 mol K = 39. 10 g K 1 mol K = 6. 022 x 1023 atoms K 1 mol K 6. 022 x 1023 atoms K 0. 551 g K x x = 1 mol K 39. 10 g K 8. 49 x 1021 atoms K

1. What is the mass in grams of 2. 5 mol Na. 2. Calculate the number of atoms in 1. 7 mol B. 3. Calculate the number of atoms in 5. 0 g Al. 4. Calculate the mass of 5, 000 atoms of Au.

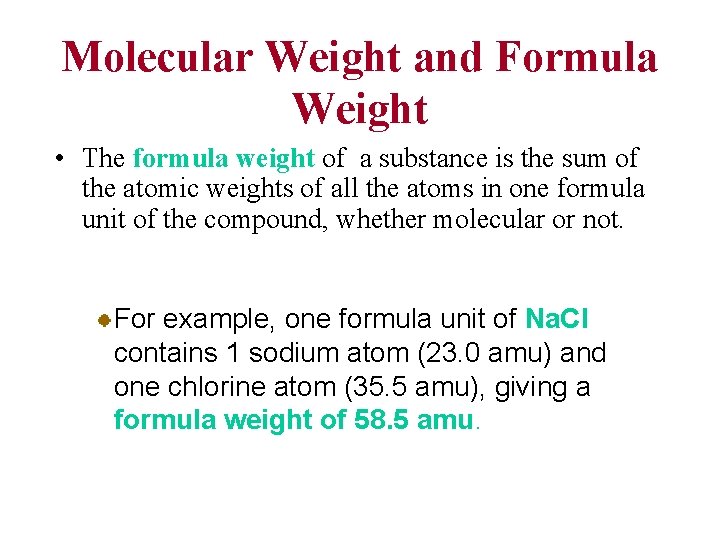
Molecular Weight and Formula Weight • The formula weight of a substance is the sum of the atomic weights of all the atoms in one formula unit of the compound, whether molecular or not. For example, one formula unit of Na. Cl contains 1 sodium atom (23. 0 amu) and one chlorine atom (35. 5 amu), giving a formula weight of 58. 5 amu.
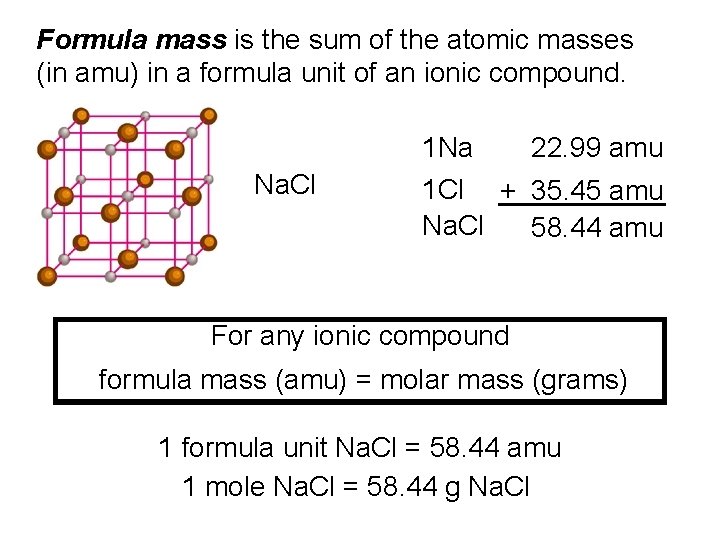
Formula mass is the sum of the atomic masses (in amu) in a formula unit of an ionic compound. 1 Na Na. Cl 22. 99 amu 1 Cl + 35. 45 amu Na. Cl 58. 44 amu For any ionic compound formula mass (amu) = molar mass (grams) 1 formula unit Na. Cl = 58. 44 amu 1 mole Na. Cl = 58. 44 g Na. Cl

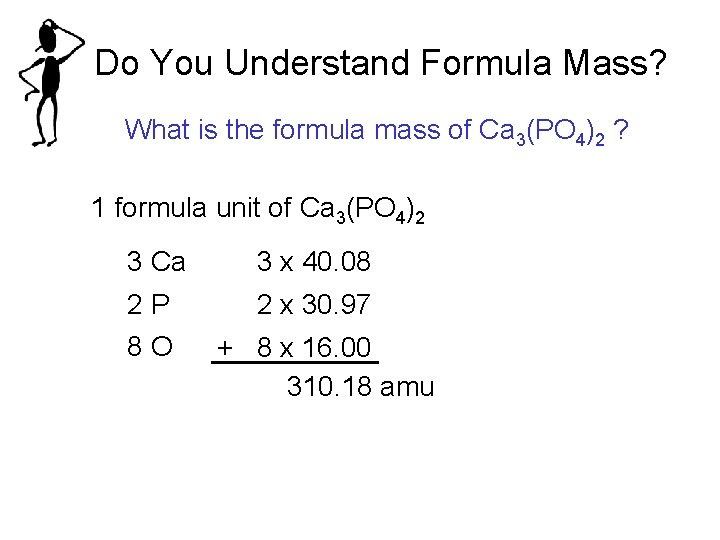
Do You Understand Formula Mass? What is the formula mass of Ca 3(PO 4)2 ? 1 formula unit of Ca 3(PO 4)2 3 Ca 3 x 40. 08 2 P 8 O 2 x 30. 97 + 8 x 16. 00 310. 18 amu
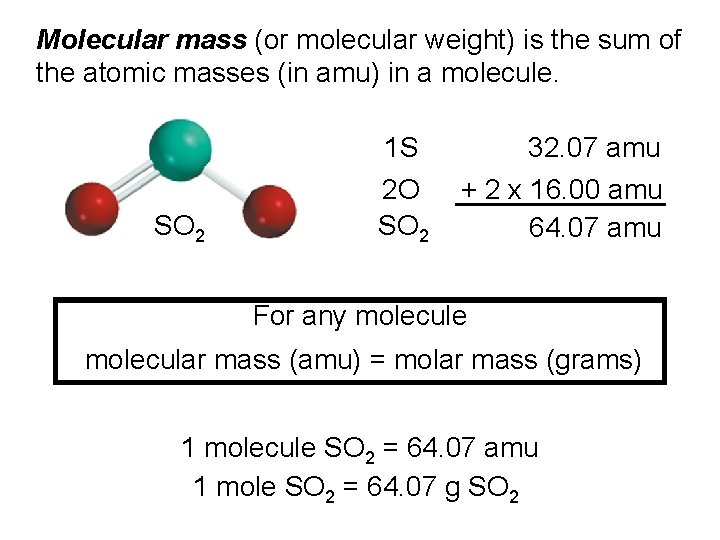
Molecular mass (or molecular weight) is the sum of the atomic masses (in amu) in a molecule. 1 S SO 2 2 O SO 2 32. 07 amu + 2 x 16. 00 amu 64. 07 amu For any molecule molecular mass (amu) = molar mass (grams) 1 molecule SO 2 = 64. 07 amu 1 mole SO 2 = 64. 07 g SO 2

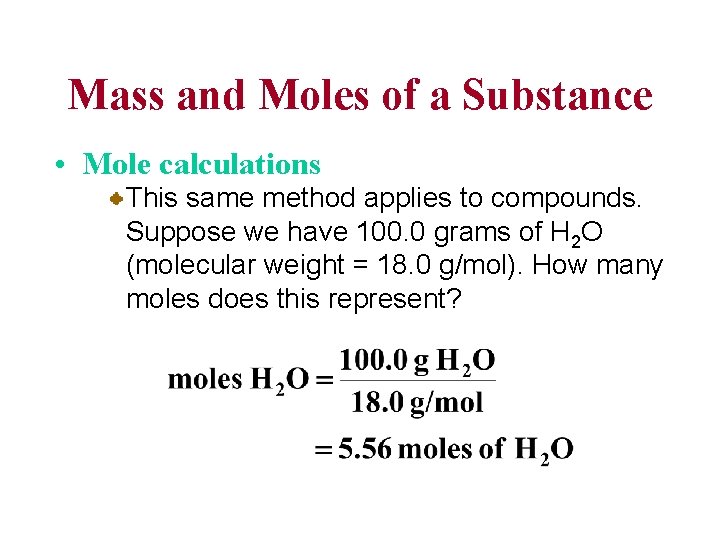
Mass and Moles of a Substance • Mole calculations This same method applies to compounds. Suppose we have 100. 0 grams of H 2 O (molecular weight = 18. 0 g/mol). How many moles does this represent?

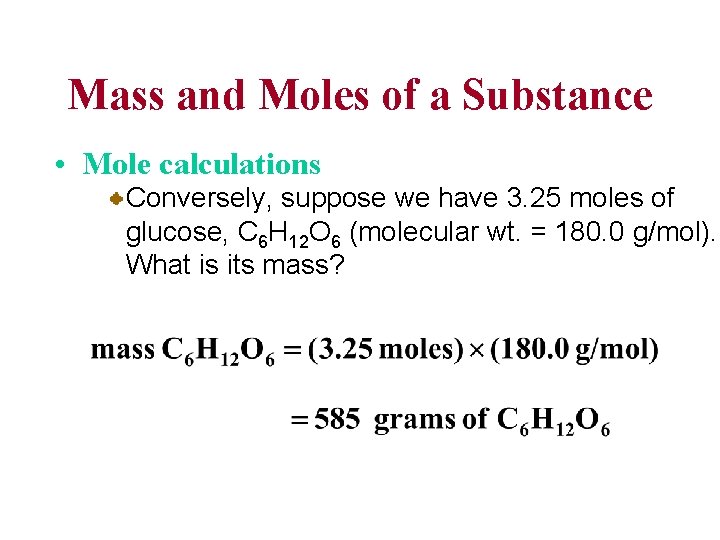
Mass and Moles of a Substance • Mole calculations Conversely, suppose we have 3. 25 moles of glucose, C 6 H 12 O 6 (molecular wt. = 180. 0 g/mol). What is its mass?

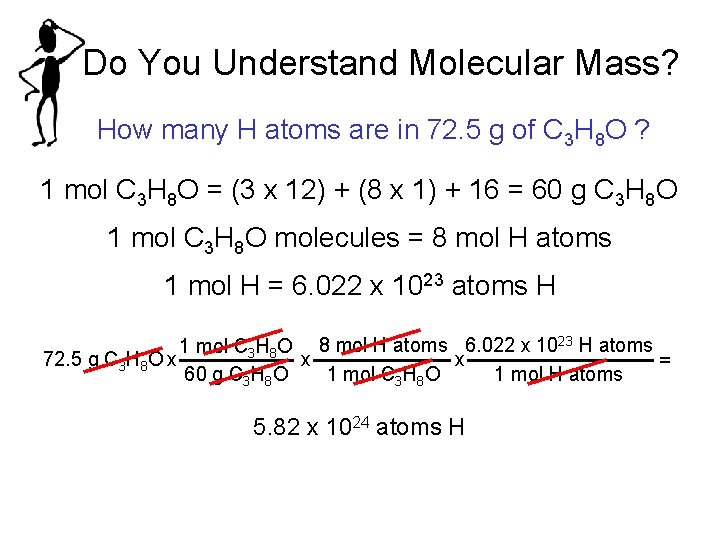
Do You Understand Molecular Mass? How many H atoms are in 72. 5 g of C 3 H 8 O ? 1 mol C 3 H 8 O = (3 x 12) + (8 x 1) + 16 = 60 g C 3 H 8 O 1 mol C 3 H 8 O molecules = 8 mol H atoms 1 mol H = 6. 022 x 1023 atoms H 1 mol C 3 H 8 O 8 mol H atoms 6. 022 x 1023 H atoms 72. 5 g C 3 H 8 O x x x = 1 mol C 3 H 8 O 1 mol H atoms 60 g C 3 H 8 O 5. 82 x 1024 atoms H

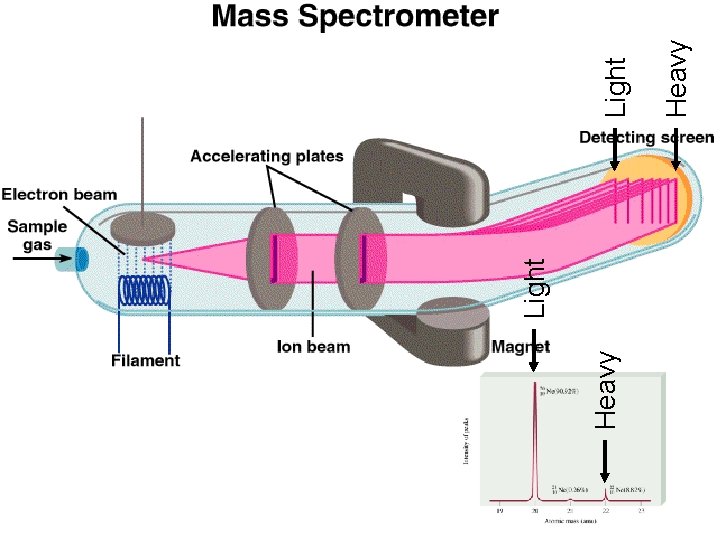
Heavy Light


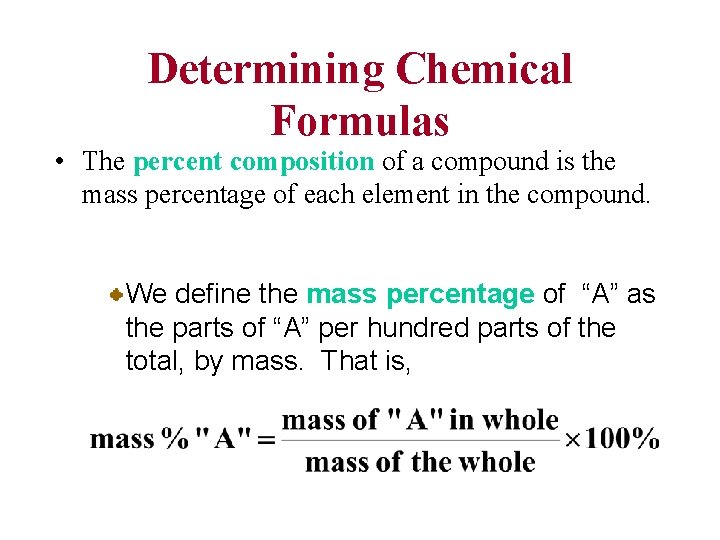
Determining Chemical Formulas • The percent composition of a compound is the mass percentage of each element in the compound. We define the mass percentage of “A” as the parts of “A” per hundred parts of the total, by mass. That is,

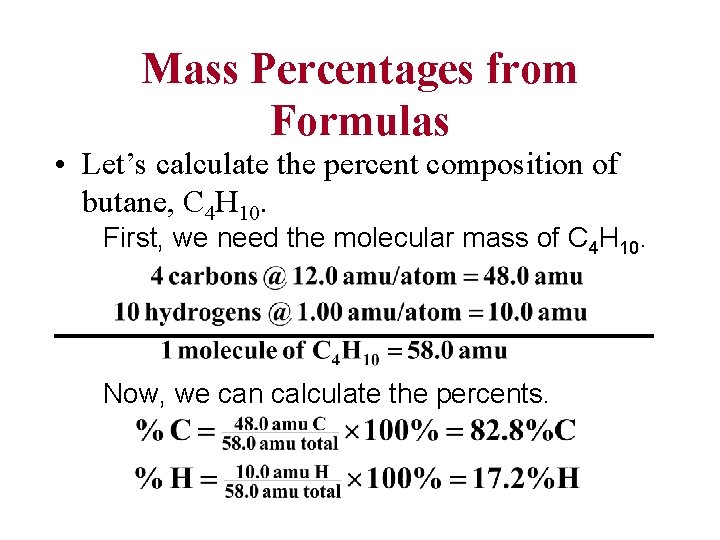
Mass Percentages from Formulas • Let’s calculate the percent composition of butane, C 4 H 10. First, we need the molecular mass of C 4 H 10. Now, we can calculate the percents.
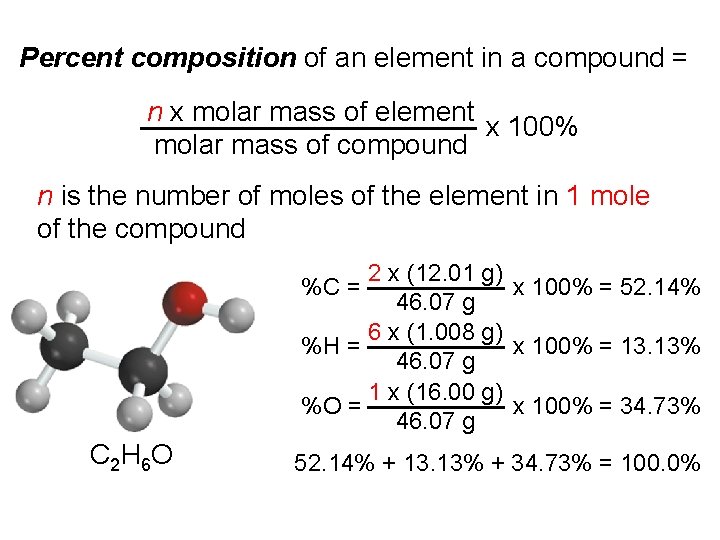
Percent composition of an element in a compound = n x molar mass of element x 100% molar mass of compound n is the number of moles of the element in 1 mole of the compound 2 x (12. 01 g) x 100% = 52. 14% 46. 07 g 6 x (1. 008 g) %H = x 100% = 13. 13% 46. 07 g 1 x (16. 00 g) %O = x 100% = 34. 73% 46. 07 g %C = C 2 H 6 O 52. 14% + 13. 13% + 34. 73% = 100. 0%

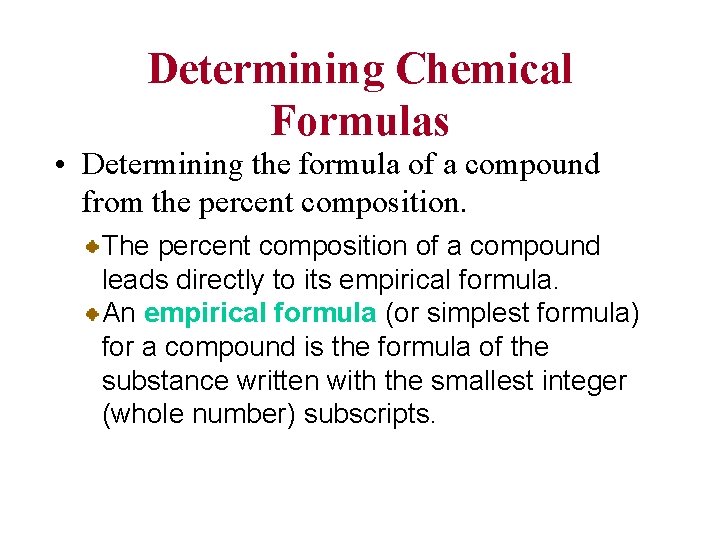
Determining Chemical Formulas • Determining the formula of a compound from the percent composition. The percent composition of a compound leads directly to its empirical formula. An empirical formula (or simplest formula) for a compound is the formula of the substance written with the smallest integer (whole number) subscripts.

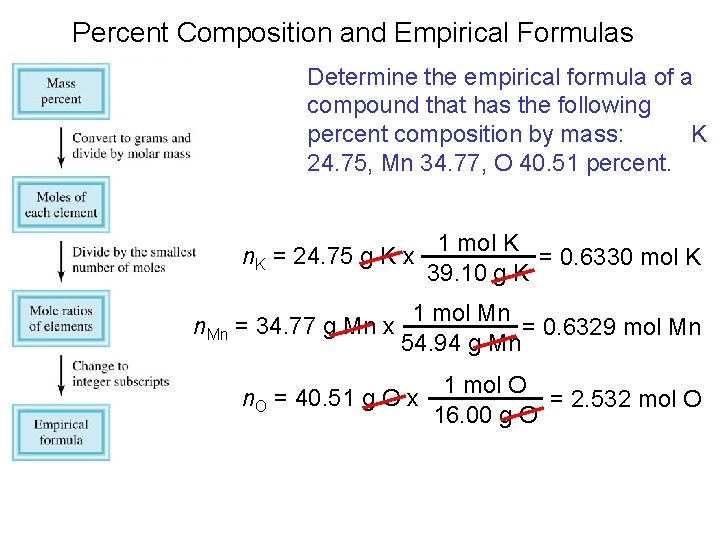
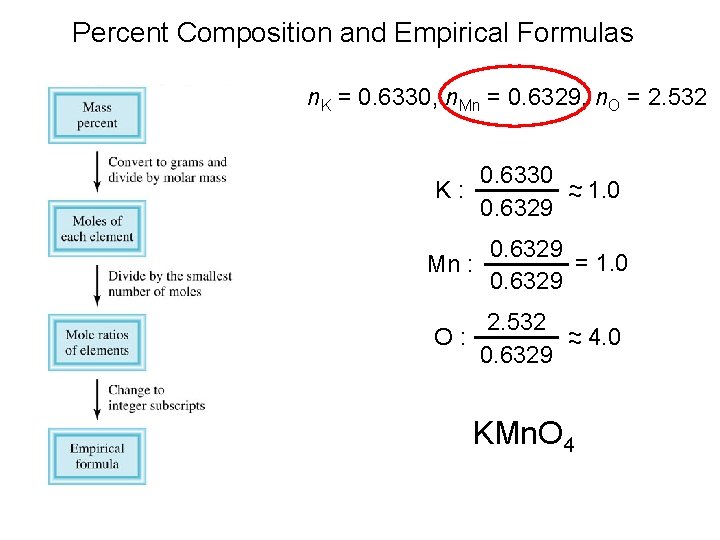
Percent Composition and Empirical Formulas Determine the empirical formula of a compound that has the following percent composition by mass: K 24. 75, Mn 34. 77, O 40. 51 percent. 1 mol K n. K = 24. 75 g K x = 0. 6330 mol K 39. 10 g K n. Mn = 34. 77 g Mn x 1 mol Mn = 0. 6329 mol Mn 54. 94 g Mn n. O = 40. 51 g O x 1 mol O = 2. 532 mol O 16. 00 g O

Percent Composition and Empirical Formulas n. K = 0. 6330, n. Mn = 0. 6329, n. O = 2. 532 0. 6330 ~ K: ~ 1. 0 0. 6329 Mn : 0. 6329 = 1. 0 0. 6329 2. 532 ~ O: ~ 4. 0 0. 6329 KMn. O 4

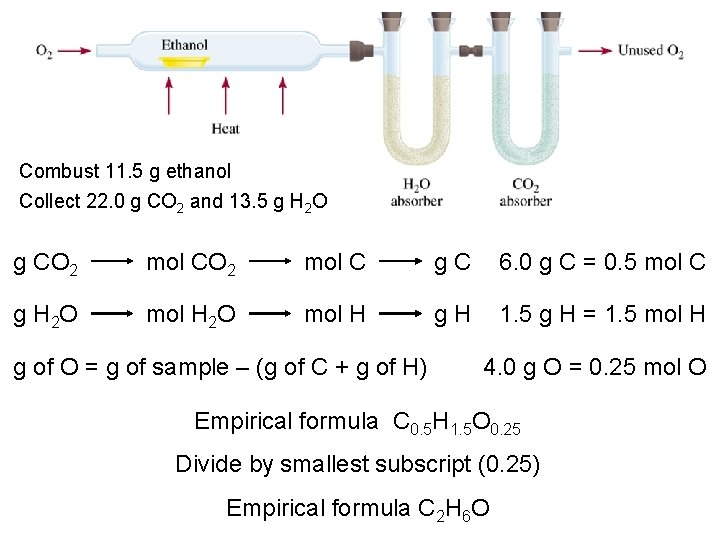
Combust 11. 5 g ethanol Collect 22. 0 g CO 2 and 13. 5 g H 2 O g CO 2 mol C g. C 6. 0 g C = 0. 5 mol C g H 2 O mol H g. H 1. 5 g H = 1. 5 mol H g of O = g of sample – (g of C + g of H) 4. 0 g O = 0. 25 mol O Empirical formula C 0. 5 H 1. 5 O 0. 25 Divide by smallest subscript (0. 25) Empirical formula C 2 H 6 O

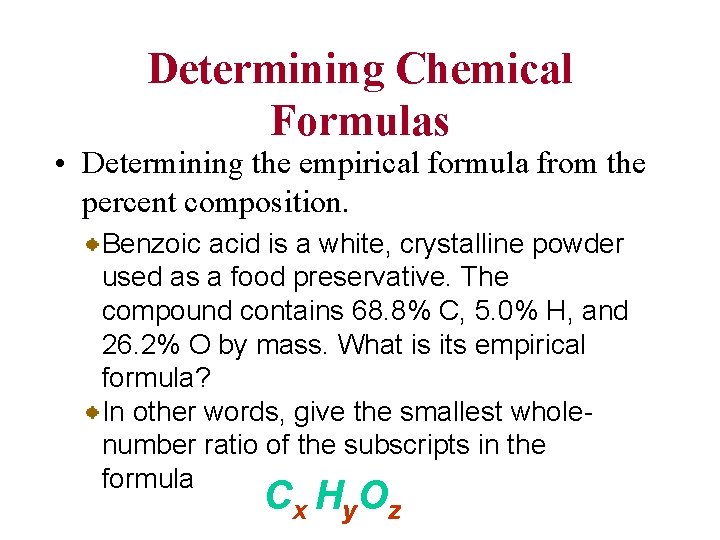
Determining Chemical Formulas • Determining the empirical formula from the percent composition. Benzoic acid is a white, crystalline powder used as a food preservative. The compound contains 68. 8% C, 5. 0% H, and 26. 2% O by mass. What is its empirical formula? In other words, give the smallest wholenumber ratio of the subscripts in the formula C x H y. O z

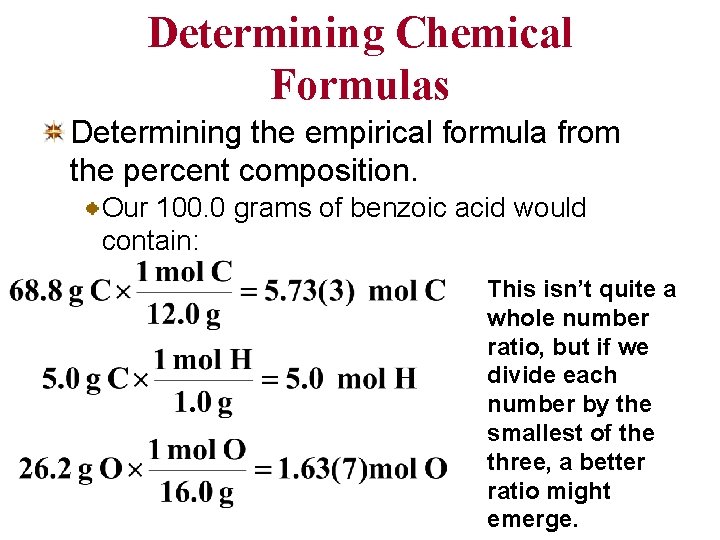
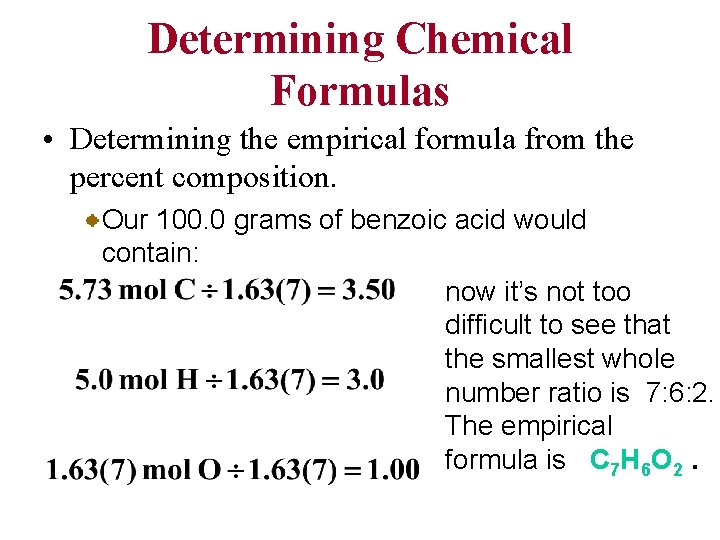
Determining Chemical Formulas Determining the empirical formula from the percent composition. Our 100. 0 grams of benzoic acid would contain: This isn’t quite a whole number ratio, but if we divide each number by the smallest of the three, a better ratio might emerge.

Determining Chemical Formulas • Determining the empirical formula from the percent composition. Our 100. 0 grams of benzoic acid would contain: now it’s not too difficult to see that the smallest whole number ratio is 7: 6: 2. The empirical formula is C 7 H 6 O 2.

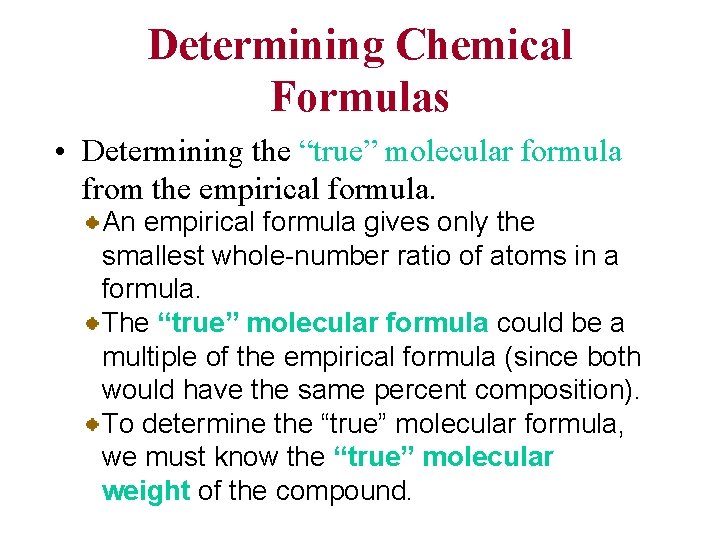
Determining Chemical Formulas • Determining the “true” molecular formula from the empirical formula. An empirical formula gives only the smallest whole-number ratio of atoms in a formula. The “true” molecular formula could be a multiple of the empirical formula (since both would have the same percent composition). To determine the “true” molecular formula, we must know the “true” molecular weight of the compound.

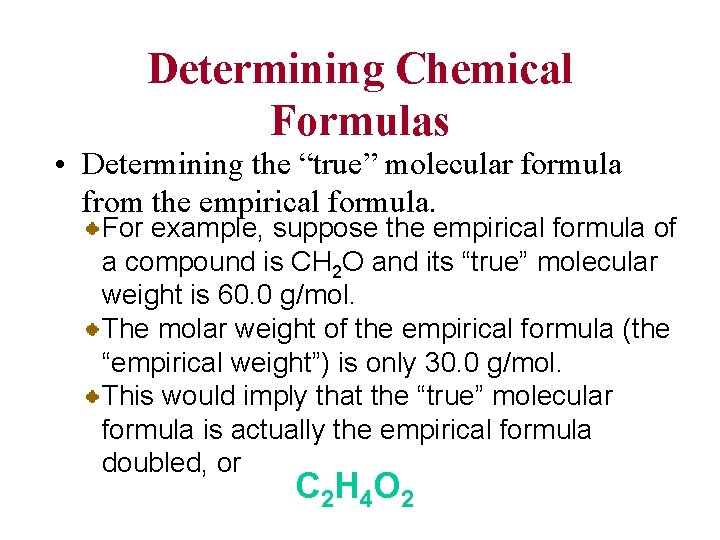
Determining Chemical Formulas • Determining the “true” molecular formula from the empirical formula. For example, suppose the empirical formula of a compound is CH 2 O and its “true” molecular weight is 60. 0 g/mol. The molar weight of the empirical formula (the “empirical weight”) is only 30. 0 g/mol. This would imply that the “true” molecular formula is actually the empirical formula doubled, or C 2 H 4 O 2

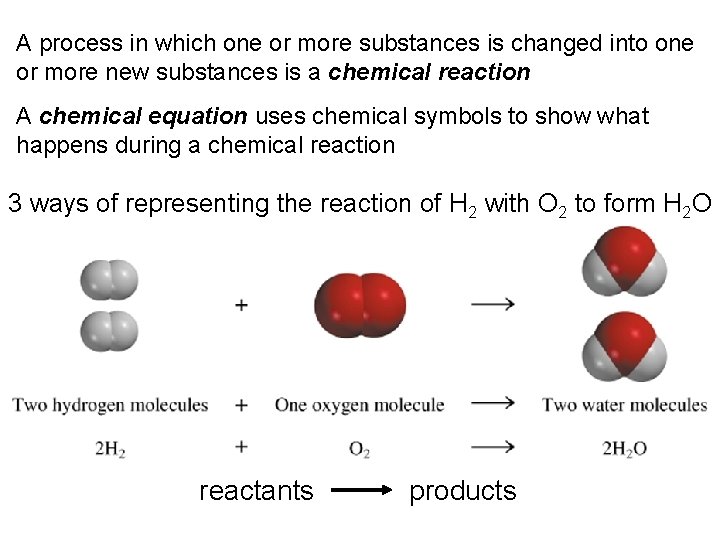
A process in which one or more substances is changed into one or more new substances is a chemical reaction A chemical equation uses chemical symbols to show what happens during a chemical reaction 3 ways of representing the reaction of H 2 with O 2 to form H 2 O reactants products

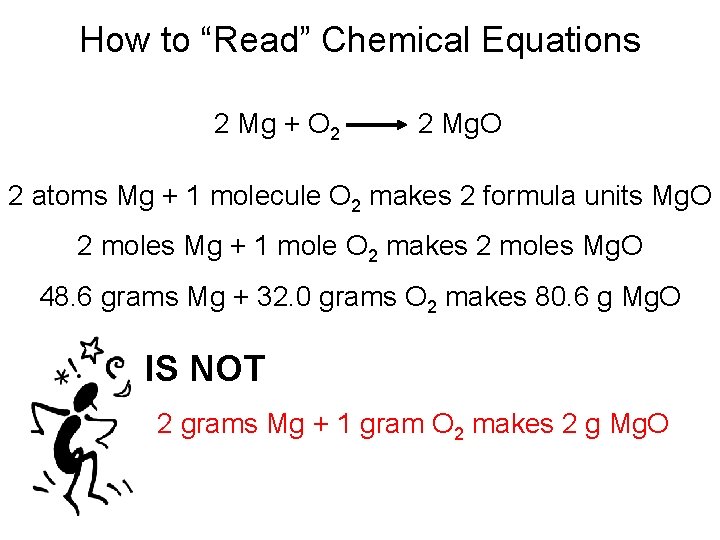
How to “Read” Chemical Equations 2 Mg + O 2 2 Mg. O 2 atoms Mg + 1 molecule O 2 makes 2 formula units Mg. O 2 moles Mg + 1 mole O 2 makes 2 moles Mg. O 48. 6 grams Mg + 32. 0 grams O 2 makes 80. 6 g Mg. O IS NOT 2 grams Mg + 1 gram O 2 makes 2 g Mg. O

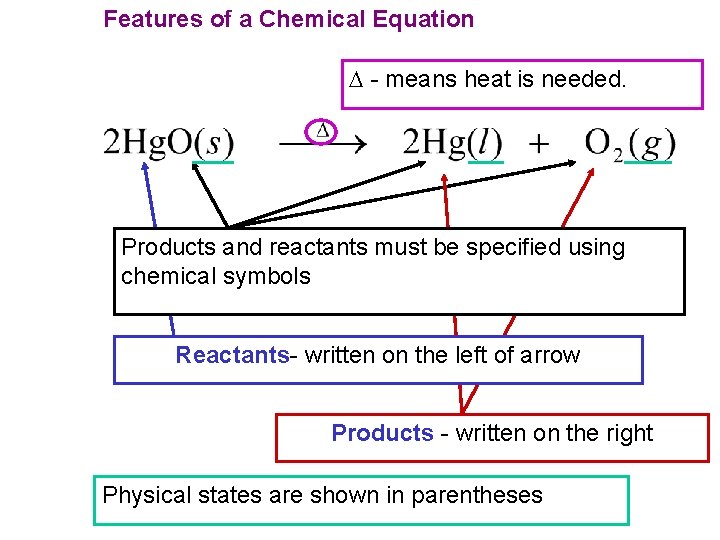
Features of a Chemical Equation - means heat is needed. Products and reactants must be specified using chemical symbols Reactants- written on the left of arrow Products - written on the right Physical states are shown in parentheses

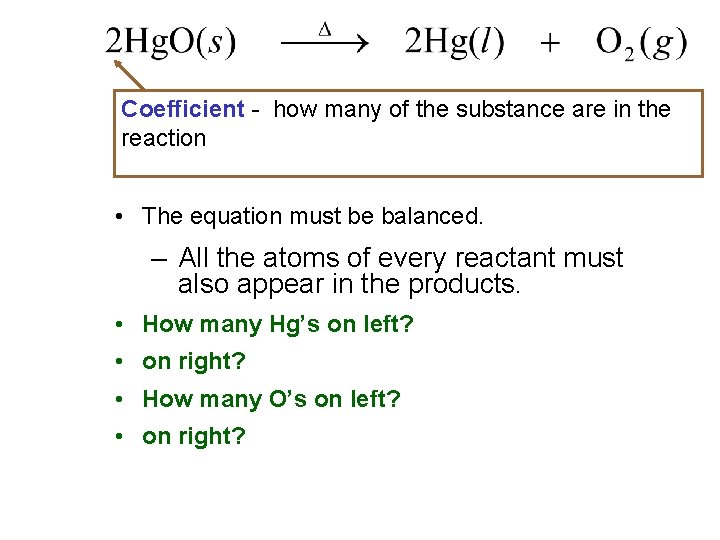
Coefficient - how many of the substance are in the reaction • The equation must be balanced. – All the atoms of every reactant must also appear in the products. • How many Hg’s on left? • on right? • How many O’s on left? • on right?

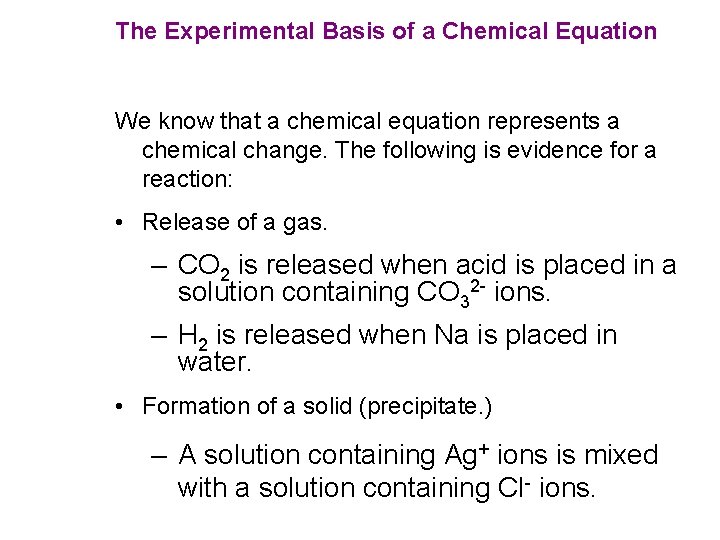
The Experimental Basis of a Chemical Equation We know that a chemical equation represents a chemical change. The following is evidence for a reaction: • Release of a gas. – CO 2 is released when acid is placed in a solution containing CO 32 - ions. – H 2 is released when Na is placed in water. • Formation of a solid (precipitate. ) – A solution containing Ag+ ions is mixed with a solution containing Cl- ions.

• Heat is produced or absorbed (temperature changes) – Acid and base are mixed together • The color changes • Light is absorbed or emitted • Changes in the way the substances behave in an electrical or magnetic field • Changes in electrical properties.

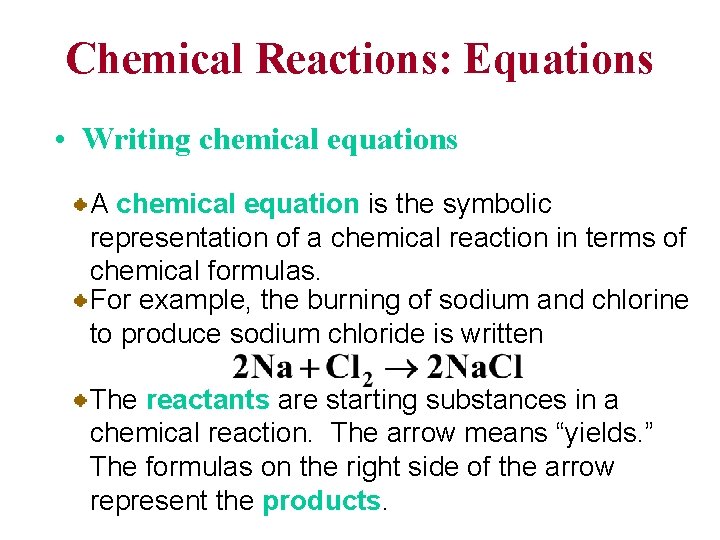
Chemical Reactions: Equations • Writing chemical equations A chemical equation is the symbolic representation of a chemical reaction in terms of chemical formulas. For example, the burning of sodium and chlorine to produce sodium chloride is written The reactants are starting substances in a chemical reaction. The arrow means “yields. ” The formulas on the right side of the arrow represent the products.

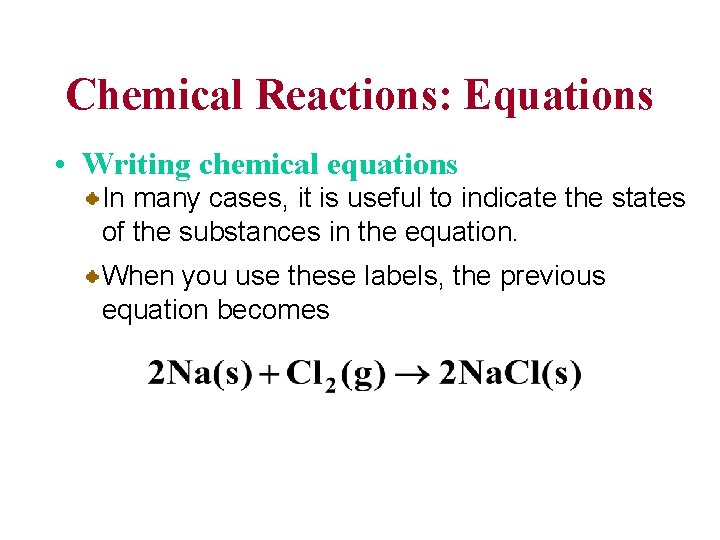
Chemical Reactions: Equations • Writing chemical equations In many cases, it is useful to indicate the states of the substances in the equation. When you use these labels, the previous equation becomes

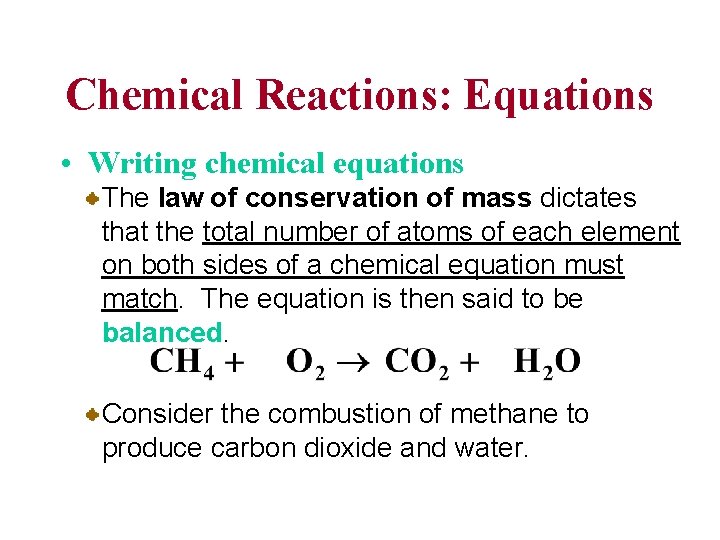
Chemical Reactions: Equations • Writing chemical equations The law of conservation of mass dictates that the total number of atoms of each element on both sides of a chemical equation must match. The equation is then said to be balanced. Consider the combustion of methane to produce carbon dioxide and water.

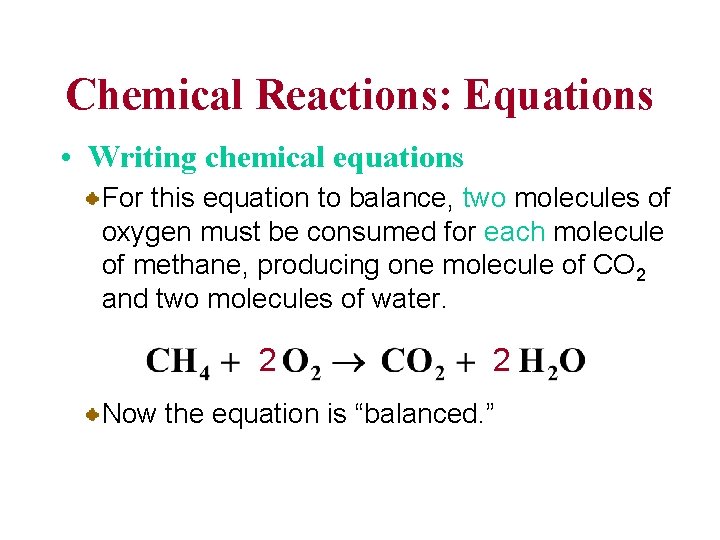
Chemical Reactions: Equations • Writing chemical equations For this equation to balance, two molecules of oxygen must be consumed for each molecule of methane, producing one molecule of CO 2 and two molecules of water. 2 2 Now the equation is “balanced. ”

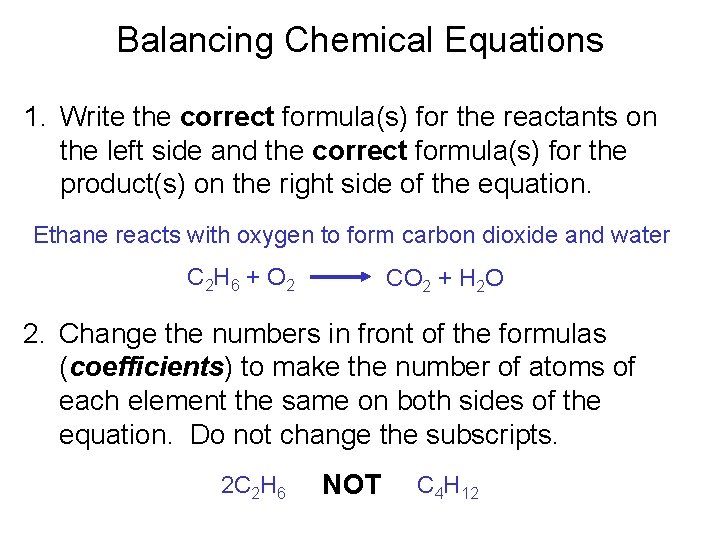
Balancing Chemical Equations 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C 2 H 6 + O 2 CO 2 + H 2 O 2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each element the same on both sides of the equation. Do not change the subscripts. 2 C 2 H 6 NOT C 4 H 12

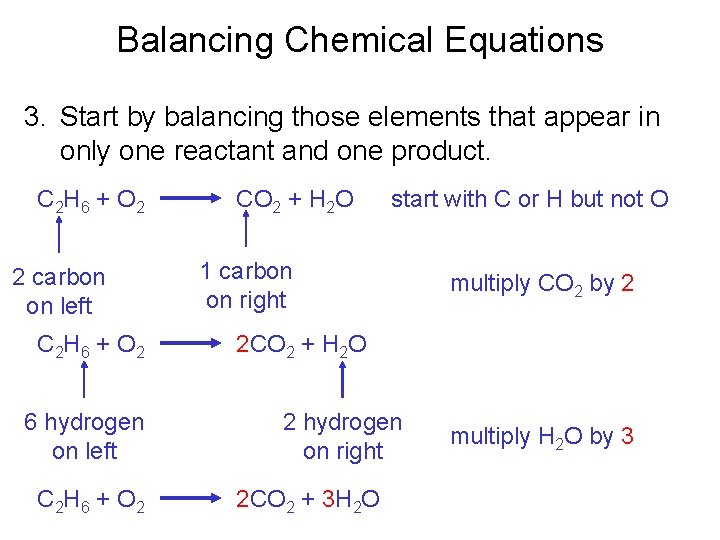
Balancing Chemical Equations 3. Start by balancing those elements that appear in only one reactant and one product. C 2 H 6 + O 2 2 carbon on left C 2 H 6 + O 2 6 hydrogen on left C 2 H 6 + O 2 CO 2 + H 2 O start with C or H but not O 1 carbon on right multiply CO 2 by 2 2 CO 2 + H 2 O 2 hydrogen on right 2 CO 2 + 3 H 2 O multiply H 2 O by 3

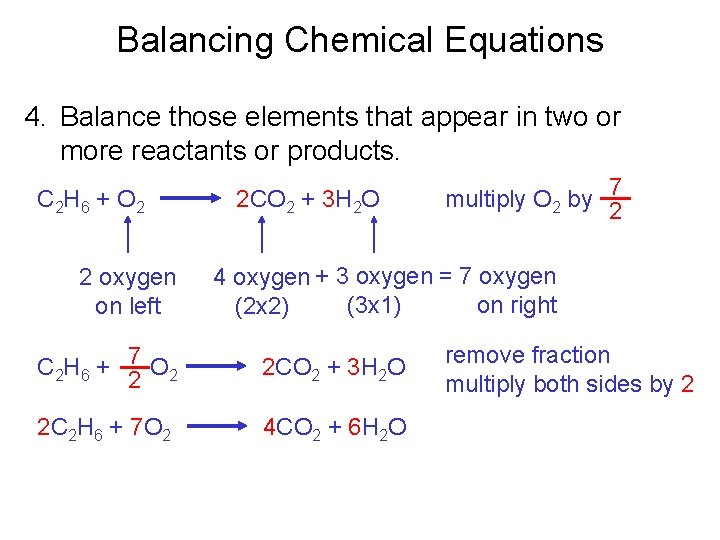
Balancing Chemical Equations 4. Balance those elements that appear in two or more reactants or products. C 2 H 6 + O 2 2 oxygen on left 2 CO 2 + 3 H 2 O multiply O 2 by 7 2 4 oxygen + 3 oxygen = 7 oxygen (3 x 1) on right (2 x 2) C 2 H 6 + 7 O 2 2 2 CO 2 + 3 H 2 O 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O remove fraction multiply both sides by 2

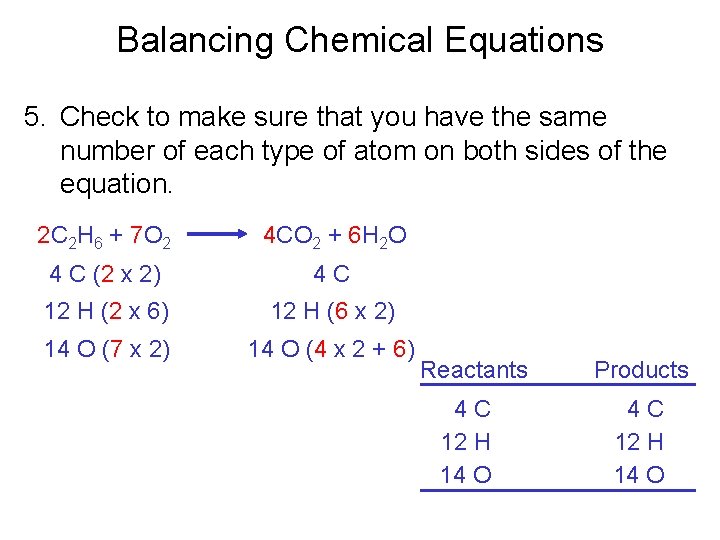
Balancing Chemical Equations 5. Check to make sure that you have the same number of each type of atom on both sides of the equation. 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O 4 C (2 x 2) 4 C 12 H (2 x 6) 12 H (6 x 2) 14 O (7 x 2) 14 O (4 x 2 + 6) Reactants 4 C 12 H 14 O Products 4 C 12 H 14 O

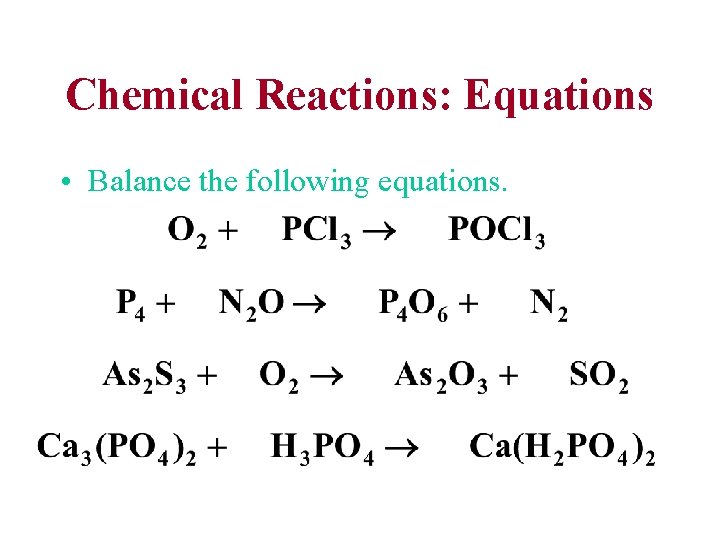
Chemical Reactions: Equations • Balance the following equations.

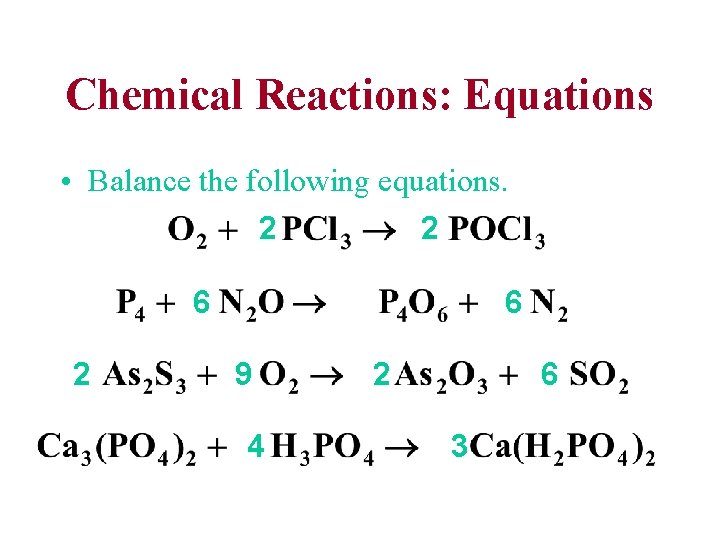
Chemical Reactions: Equations • Balance the following equations. 2 2 6 9 4 2 6 3

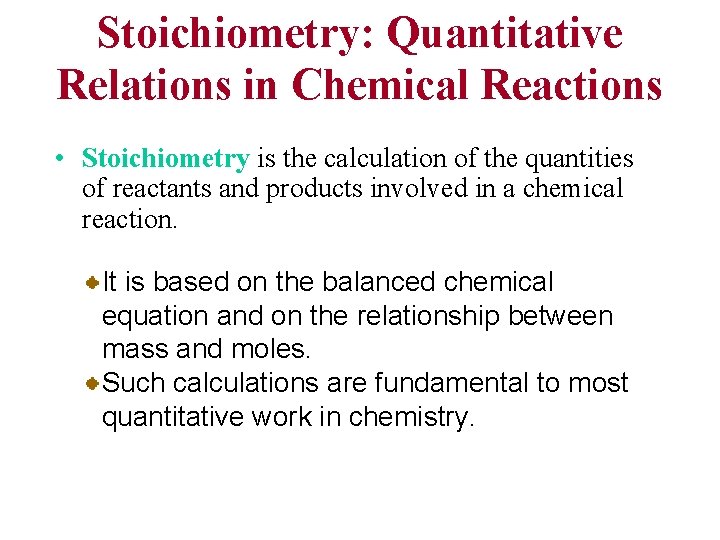
Stoichiometry: Quantitative Relations in Chemical Reactions • Stoichiometry is the calculation of the quantities of reactants and products involved in a chemical reaction. It is based on the balanced chemical equation and on the relationship between mass and moles. Such calculations are fundamental to most quantitative work in chemistry.

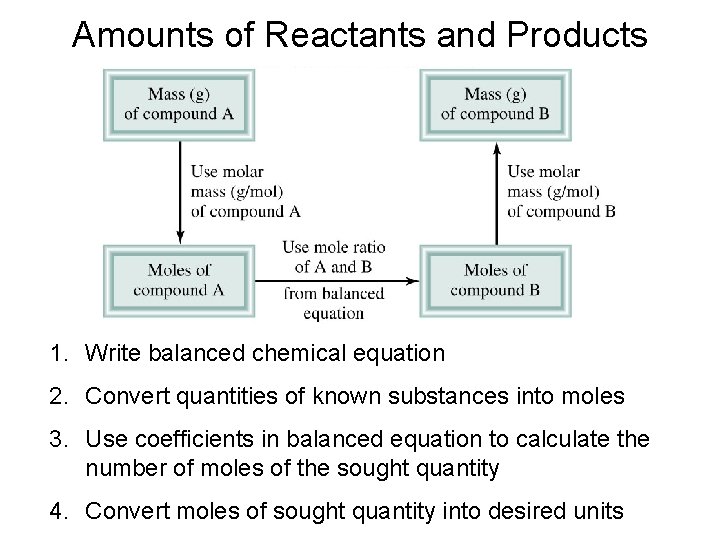
Amounts of Reactants and Products 1. Write balanced chemical equation 2. Convert quantities of known substances into moles 3. Use coefficients in balanced equation to calculate the number of moles of the sought quantity 4. Convert moles of sought quantity into desired units

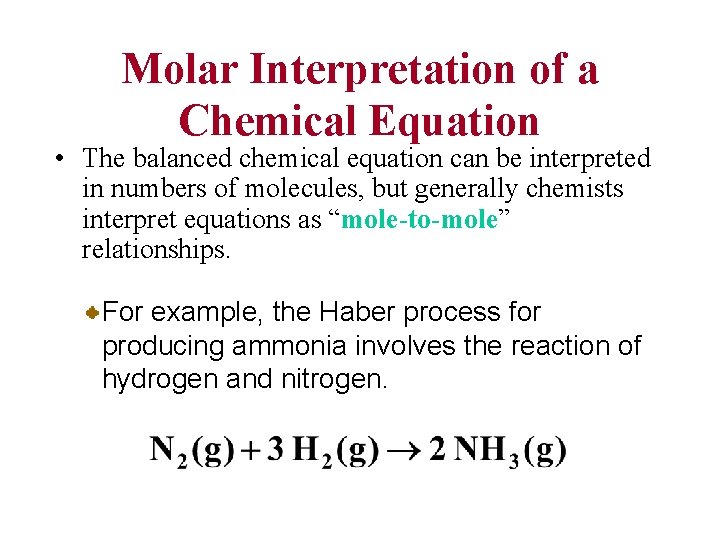
Molar Interpretation of a Chemical Equation • The balanced chemical equation can be interpreted in numbers of molecules, but generally chemists interpret equations as “mole-to-mole” relationships. For example, the Haber process for producing ammonia involves the reaction of hydrogen and nitrogen.

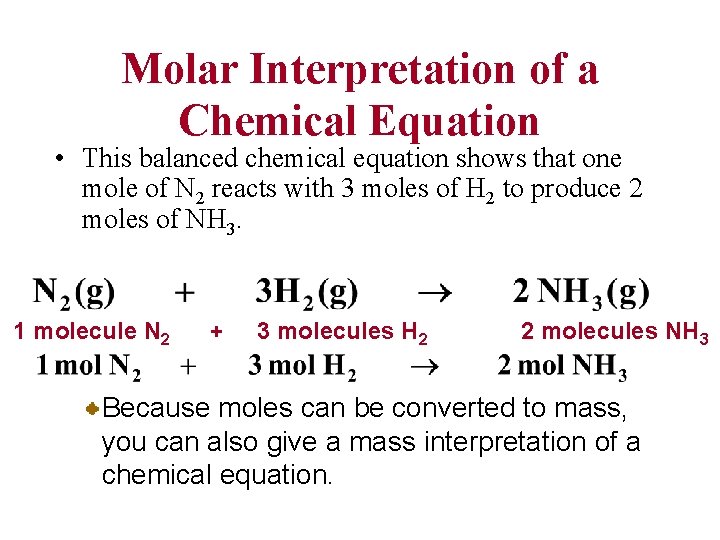
Molar Interpretation of a Chemical Equation • This balanced chemical equation shows that one mole of N 2 reacts with 3 moles of H 2 to produce 2 moles of NH 3. 1 molecule N 2 + 3 molecules H 2 2 molecules NH 3 Because moles can be converted to mass, you can also give a mass interpretation of a chemical equation.

Calculations Using the Chemical 7 Equation • We will learn in this section to calculate quantities of reactants and products in a chemical reaction. • Need a balanced chemical equation for the reaction of interest. • Keep in mind that the coefficients represent the number of moles of each substance in the equation.

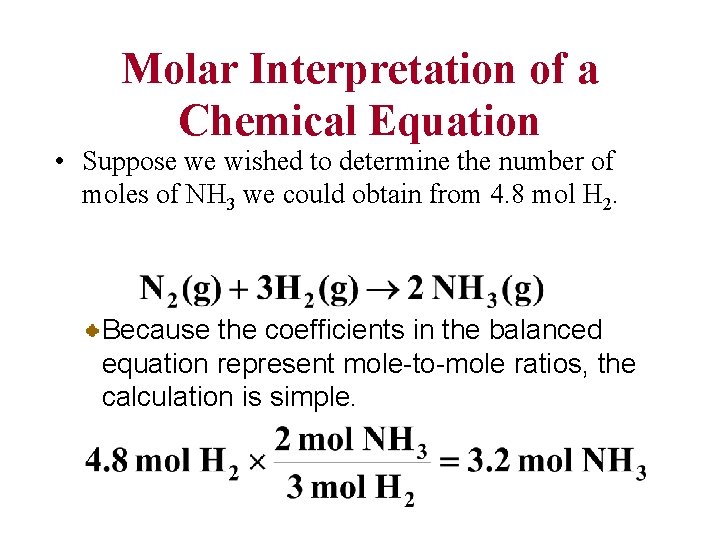
Molar Interpretation of a Chemical Equation • Suppose we wished to determine the number of moles of NH 3 we could obtain from 4. 8 mol H 2. Because the coefficients in the balanced equation represent mole-to-mole ratios, the calculation is simple.
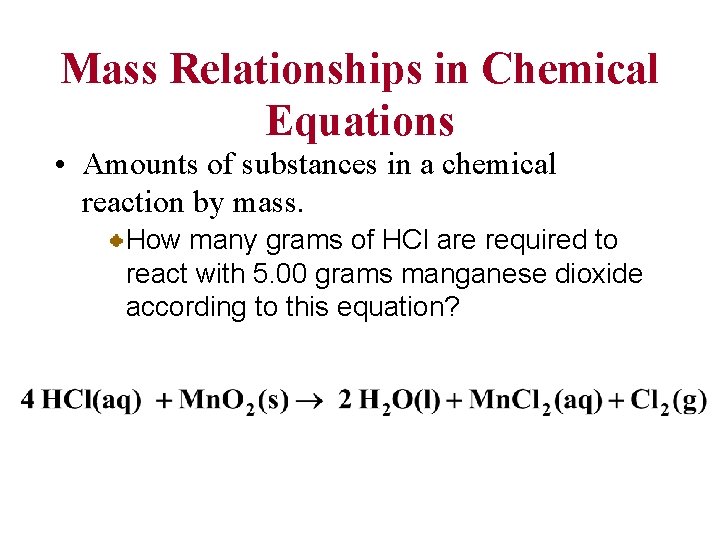
Mass Relationships in Chemical Equations • Amounts of substances in a chemical reaction by mass. How many grams of HCl are required to react with 5. 00 grams manganese dioxide according to this equation?

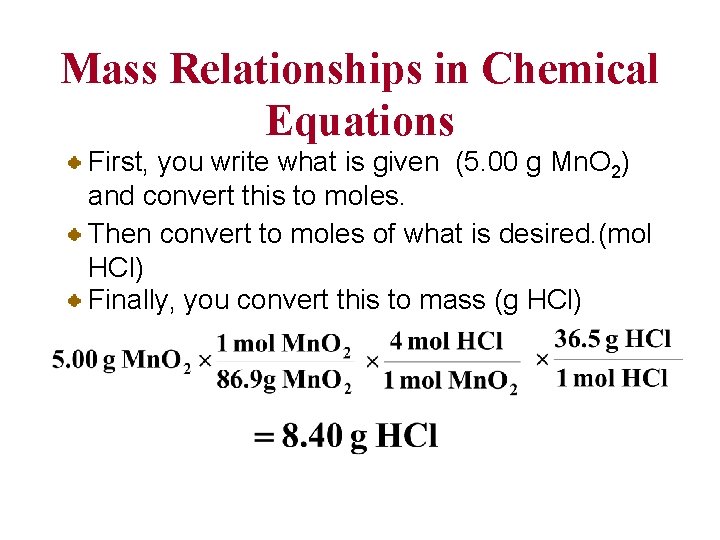
Mass Relationships in Chemical Equations First, you write what is given (5. 00 g Mn. O 2) and convert this to moles. Then convert to moles of what is desired. (mol HCl) Finally, you convert this to mass (g HCl)

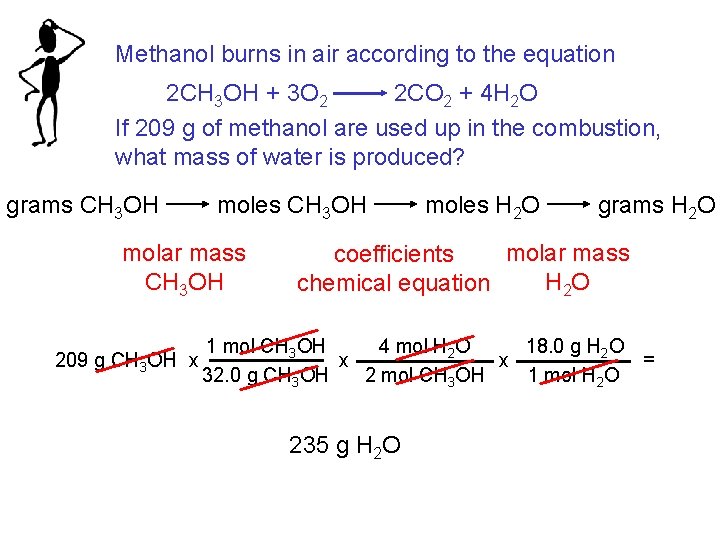
Methanol burns in air according to the equation 2 CH 3 OH + 3 O 2 2 CO 2 + 4 H 2 O If 209 g of methanol are used up in the combustion, what mass of water is produced? grams CH 3 OH moles CH 3 OH molar mass CH 3 OH 209 g CH 3 OH x moles H 2 O grams H 2 O molar mass coefficients H 2 O chemical equation 4 mol H 2 O 18. 0 g H 2 O 1 mol CH 3 OH = x x 32. 0 g CH 3 OH 1 mol H 2 O 2 mol CH 3 OH 235 g H 2 O

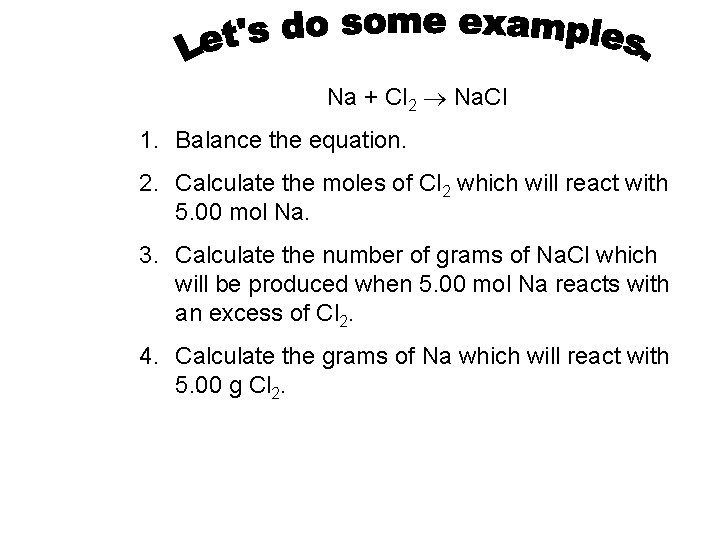
Na + Cl 2 Na. Cl 1. Balance the equation. 2. Calculate the moles of Cl 2 which will react with 5. 00 mol Na. 3. Calculate the number of grams of Na. Cl which will be produced when 5. 00 mol Na reacts with an excess of Cl 2. 4. Calculate the grams of Na which will react with 5. 00 g Cl 2.

Limiting Reagent • The limiting reactant (or limiting reagent) is the reactant that is entirely consumed when the reaction goes to completion. The limiting reagent ultimately determines how much product can be obtained. For example, bicycles require one frame and two wheels. If you have 20 wheels but only 5 frames, it is clear that the number of frames will determine how many bicycles can be made.

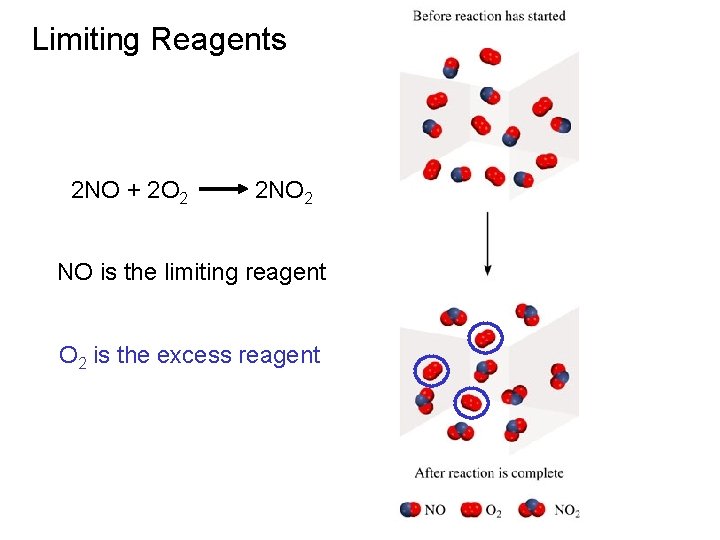
Limiting Reagents 2 NO + 2 O 2 2 NO 2 NO is the limiting reagent O 2 is the excess reagent

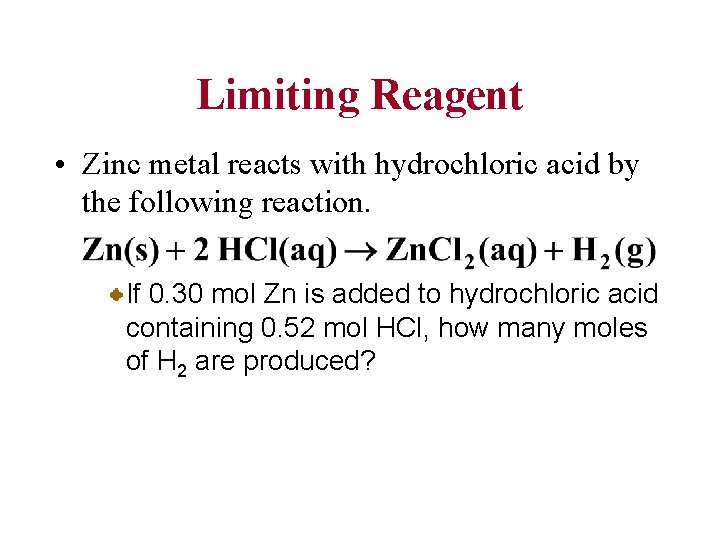
Limiting Reagent • Zinc metal reacts with hydrochloric acid by the following reaction. If 0. 30 mol Zn is added to hydrochloric acid containing 0. 52 mol HCl, how many moles of H 2 are produced?

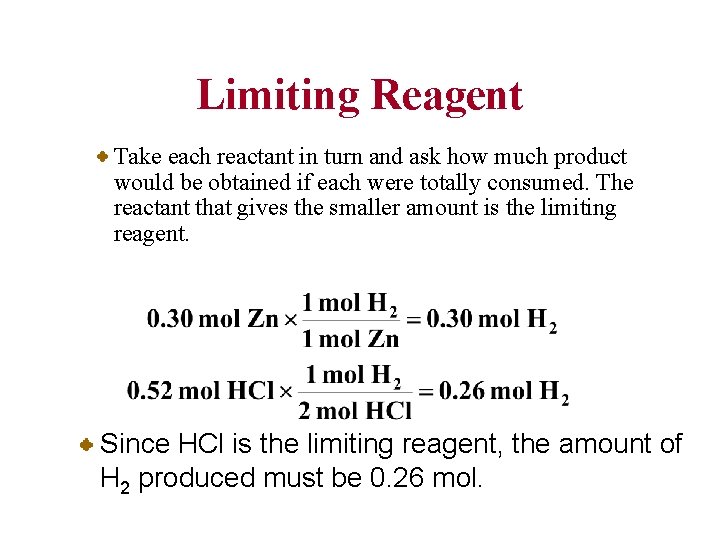
Limiting Reagent Take each reactant in turn and ask how much product would be obtained if each were totally consumed. The reactant that gives the smaller amount is the limiting reagent. Since HCl is the limiting reagent, the amount of H 2 produced must be 0. 26 mol.

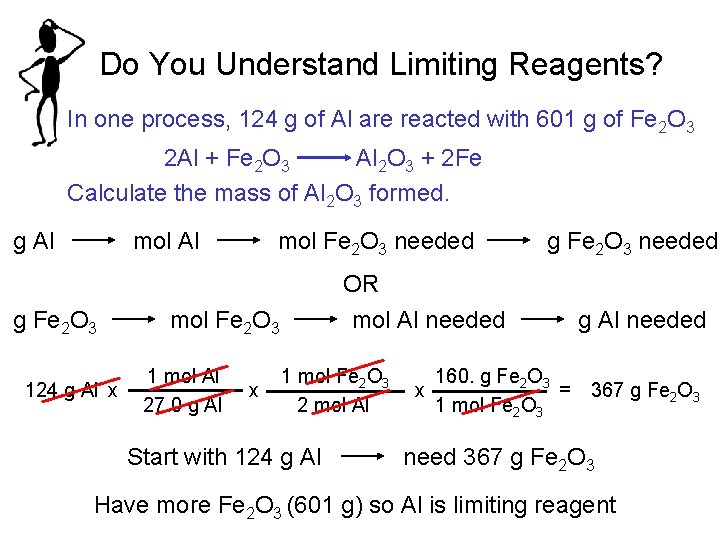
Do You Understand Limiting Reagents? In one process, 124 g of Al are reacted with 601 g of Fe 2 O 3 2 Al + Fe 2 O 3 Al 2 O 3 + 2 Fe Calculate the mass of Al 2 O 3 formed. g Al mol Al g Fe 2 O 3 124 g Al x mol Fe 2 O 3 needed OR mol Al needed mol Fe 2 O 3 1 mol Al 27. 0 g Al x g Fe 2 O 3 needed 1 mol Fe 2 O 3 2 mol Al Start with 124 g Al 160. g Fe 2 O 3 = x 1 mol Fe 2 O 3 g Al needed 367 g Fe 2 O 3 need 367 g Fe 2 O 3 Have more Fe 2 O 3 (601 g) so Al is limiting reagent

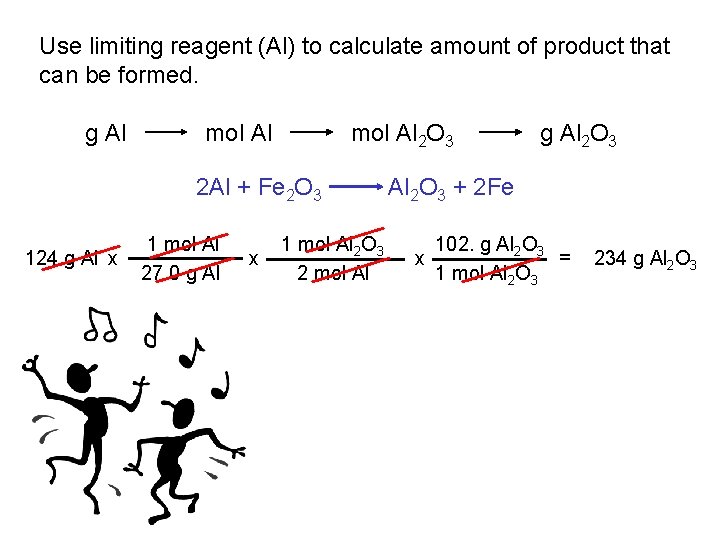
Use limiting reagent (Al) to calculate amount of product that can be formed. g Al mol Al 2 O 3 2 Al + Fe 2 O 3 124 g Al x 1 mol Al 27. 0 g Al x 1 mol Al 2 O 3 2 mol Al g Al 2 O 3 + 2 Fe 102. g Al 2 O 3 = x 1 mol Al 2 O 3 234 g Al 2 O 3

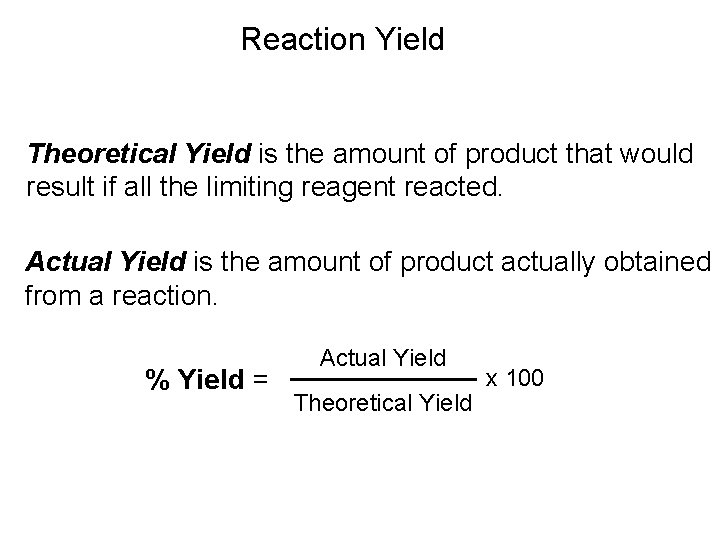
Reaction Yield Theoretical Yield is the amount of product that would result if all the limiting reagent reacted. Actual Yield is the amount of product actually obtained from a reaction. % Yield = Actual Yield Theoretical Yield x 100

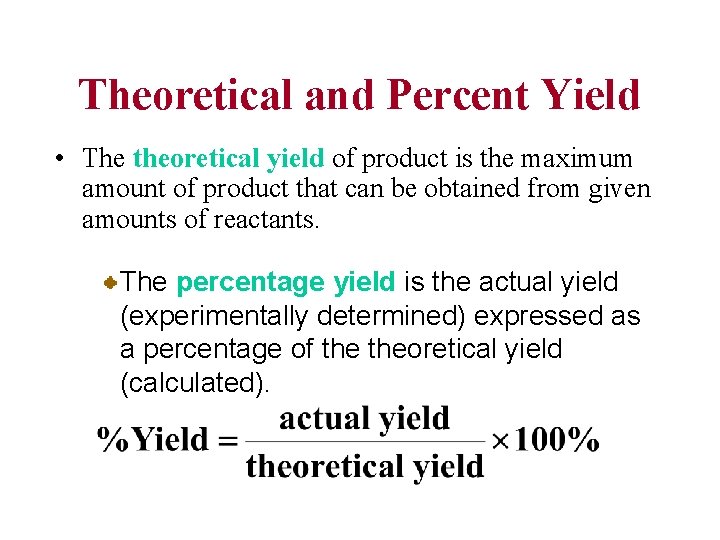
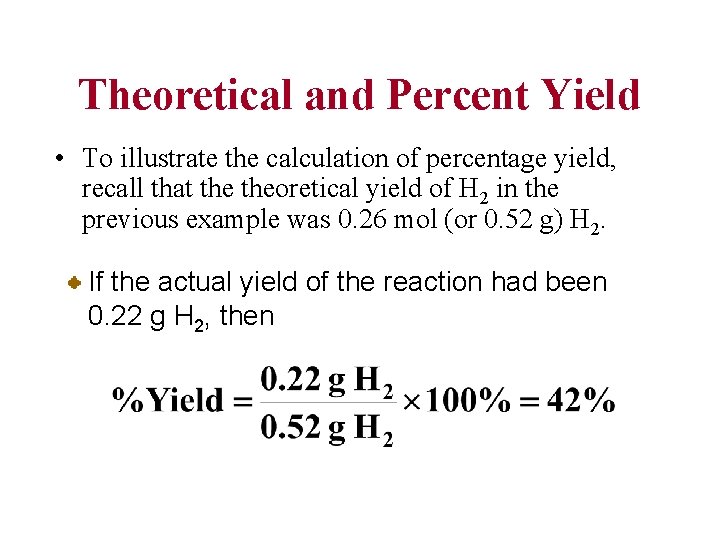
Theoretical and Percent Yield • The theoretical yield of product is the maximum amount of product that can be obtained from given amounts of reactants. The percentage yield is the actual yield (experimentally determined) expressed as a percentage of theoretical yield (calculated).

Theoretical and Percent Yield • To illustrate the calculation of percentage yield, recall that theoretical yield of H 2 in the previous example was 0. 26 mol (or 0. 52 g) H 2. If the actual yield of the reaction had been 0. 22 g H 2, then

Operational Skills • • • Calculating the formula weight from a formula. Calculating the mass of an atom or molecule. Converting moles of substance to grams and vice versa. Calculating the number of molecules in a given mass. Calculating the percentage composition from the formula. Calculating the mass of an element in a given mass of compound. Calculating the percentages C and H by combustion. Determining the empirical formula from percentage composition. Determining the true molecular formula. Relating quantities in a chemical equation. Calculating with a limiting reagent.




Worked Example 3. 4


Worked Example 3. 6

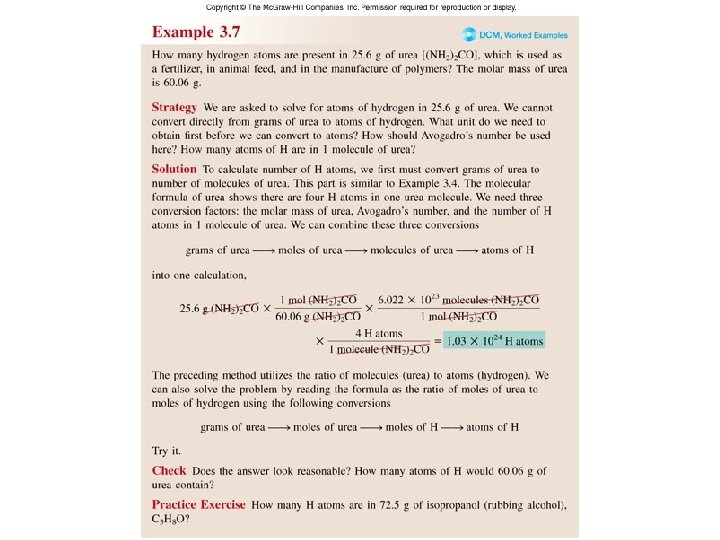
Worked Example 3. 7


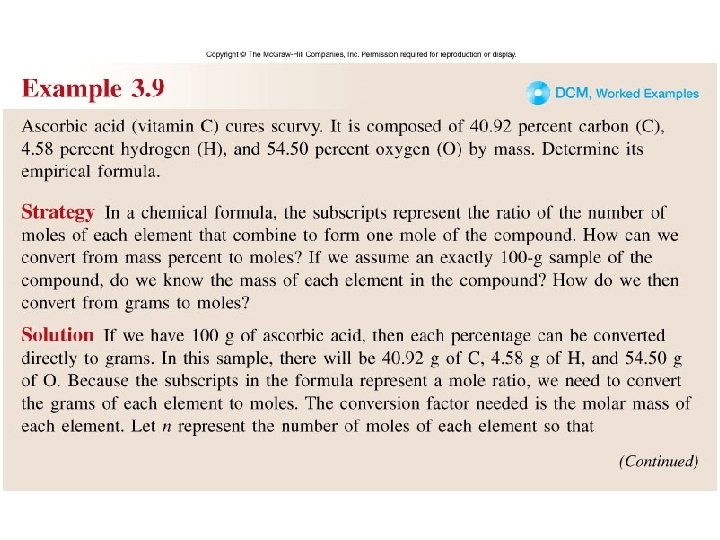
Worked Example 3. 9


Worked Example 3. 11

Worked Example 3. 12

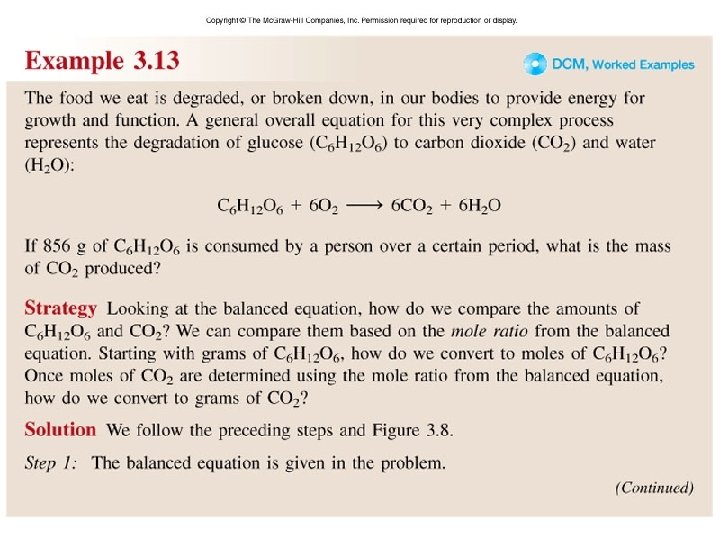
Worked Example 3. 13 a

Worked Example 3. 13 b


Worked Example 3. 15 a



 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Mass relationships in chemical reactions
Mass relationships in chemical reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of reactions
Types of reactions Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions Sodium acetate reaction with water equation
Sodium acetate reaction with water equation Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key 5 types of chemical reactions
5 types of chemical reactions What are active metals
What are active metals Redox reaction examples
Redox reaction examples Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Trinitrogen monosulfide formula
Trinitrogen monosulfide formula Unit 5 chemical reactions
Unit 5 chemical reactions Different types of redox reactions
Different types of redox reactions Identify types of reactions
Identify types of reactions Types of reactions chemistry
Types of reactions chemistry Reaction type
Reaction type Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Principles of immunochemical reaction
Principles of immunochemical reaction Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products synthesis
Predicting products synthesis Activity series of metals
Activity series of metals Unit 11 chemical reactions
Unit 11 chemical reactions Lesson 68 toxic reactions chemical equations
Lesson 68 toxic reactions chemical equations Four types of chemical reactions
Four types of chemical reactions Www.biology-roots.com
Www.biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life 5 general types of chemical reactions
5 general types of chemical reactions Chemical reactions reactants and products
Chemical reactions reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Chemical reactions study guide
Chemical reactions study guide Rate constant and equilibrium constant
Rate constant and equilibrium constant What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions 5 chemical reactions
5 chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes How to name carboxylic acid
How to name carboxylic acid Chemical reactions summary
Chemical reactions summary Bread chemical reaction
Bread chemical reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Solvent in chemical reactions
Solvent in chemical reactions Fatoumata dembele chef
Fatoumata dembele chef Indications of chemical reaction
Indications of chemical reaction Describing chemical reactions
Describing chemical reactions Rules for chemical reactions
Rules for chemical reactions Chemical reaction rearrangement of atoms
Chemical reaction rearrangement of atoms Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Classification of chemical reactions worksheet
Classification of chemical reactions worksheet What are the five general types of chemical reactions
What are the five general types of chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical reactions of copper and percent yield report sheet
Chemical reactions of copper and percent yield report sheet Laser beam welding (lbw)
Laser beam welding (lbw) Chemistry unit 4 grade 11
Chemistry unit 4 grade 11 Describing chemical reactions
Describing chemical reactions Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 2 chemical reactions answer key
Section 2 chemical reactions answer key Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions What is released or absorbed whenever chemical
What is released or absorbed whenever chemical What is this
What is this Protonic and non protonic solvents
Protonic and non protonic solvents Are all chemical reactions reversible
Are all chemical reactions reversible Chemical reactions in water
Chemical reactions in water Solvent in chemical reactions
Solvent in chemical reactions Chemical reactions in soil
Chemical reactions in soil Homogenous solution
Homogenous solution Solvent in chemical reactions
Solvent in chemical reactions Alkyne to alkene
Alkyne to alkene Jared investigated chemical reactions
Jared investigated chemical reactions Toxic reactions chemical equations
Toxic reactions chemical equations Describing chemical reactions worksheet answers
Describing chemical reactions worksheet answers Solvent in chemical reactions
Solvent in chemical reactions Chemical reactions that release energy are called
Chemical reactions that release energy are called Type of chemical reactions
Type of chemical reactions In chemical reactions atoms are rearranged
In chemical reactions atoms are rearranged Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Enzyme catalyzed reaction
Enzyme catalyzed reaction Indications of chemical reactions
Indications of chemical reactions