Law of Conservation of Mass Is the mass





- Slides: 10

Law of Conservation of Mass Is the mass of the following equations conserved (balanced) or not? Explain.

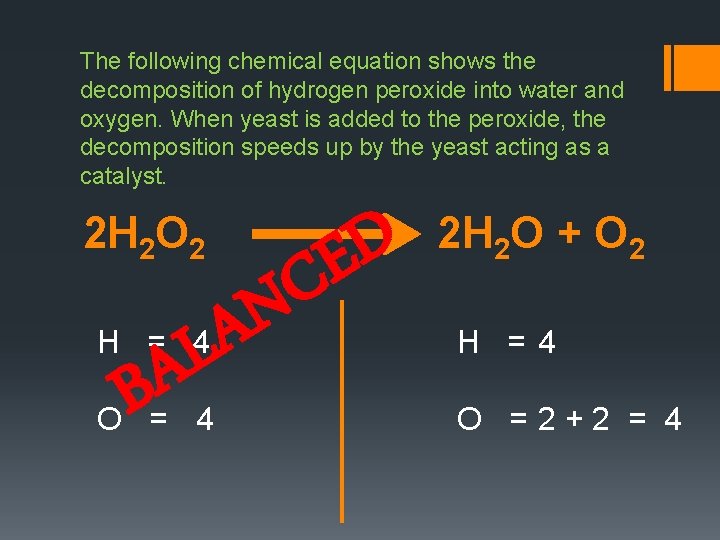
The following chemical equation shows the decomposition of hydrogen peroxide into water and oxygen. When yeast is added to the peroxide, the decomposition speeds up by the yeast acting as a catalyst. D E C 2 H 2 O 2 N A L H = 4 A B O = 4 2 H 2 O + O 2 H =4 O =2+2 = 4

Acetic acid (vinegar) reacts with sodium bicarbonate (baking soda) and produces sodium acetate, water, and carbon dioxide. CH 3 COOH + Na. HCO 3 C= 3 H= 5 O= 5 Na = 1 D E C N A L A B CH 3 COONa + H 2 O + CO 2 C= 3 H= 5 O= 5 Na = 1

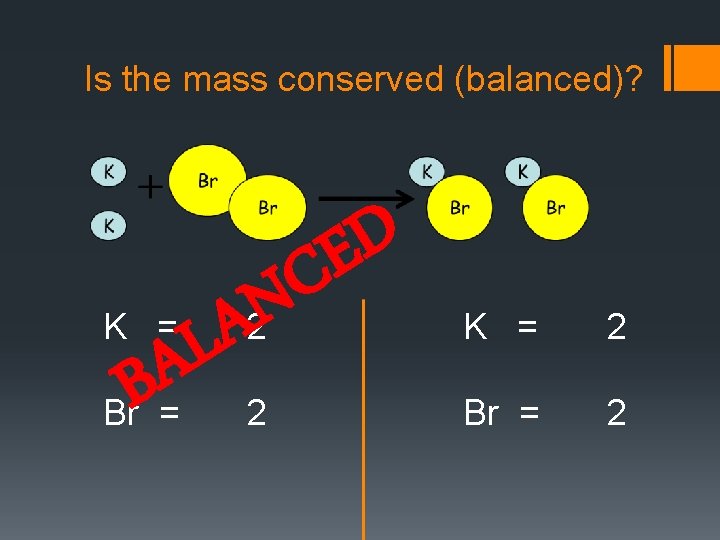
Is the mass conserved (balanced)? K = A B Br = N A L D E C 2 2 K = 2 Br = 2

Is the following chemical equation indicate a chemical reaction? L A C I M E H C N Ag + H a. O Fe + H S O I T T O A N EQU 2 2

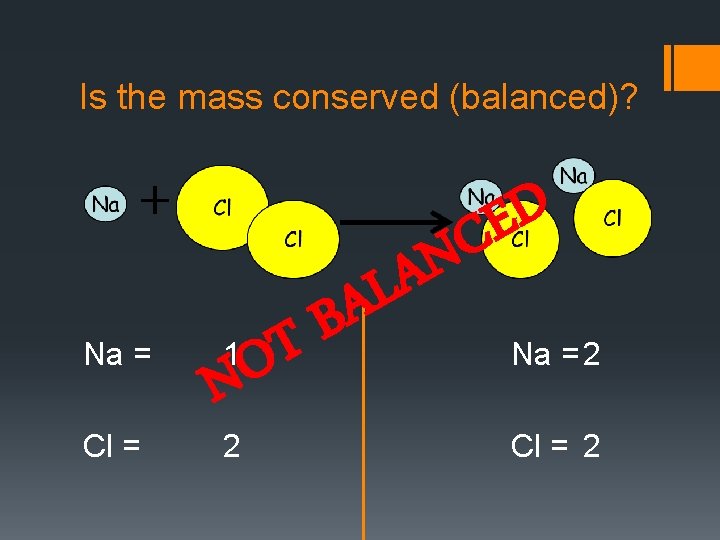
Is the mass conserved (balanced)? Na = Cl = N LA D E C A B 1 T O N 2 Na = 2 Cl = 2

Is the following chemical equation indicate a chemical reaction? 4 Fe + 3 O 2 C A E H I M L A C N O I T A U 2 Fe O Q E 2 3

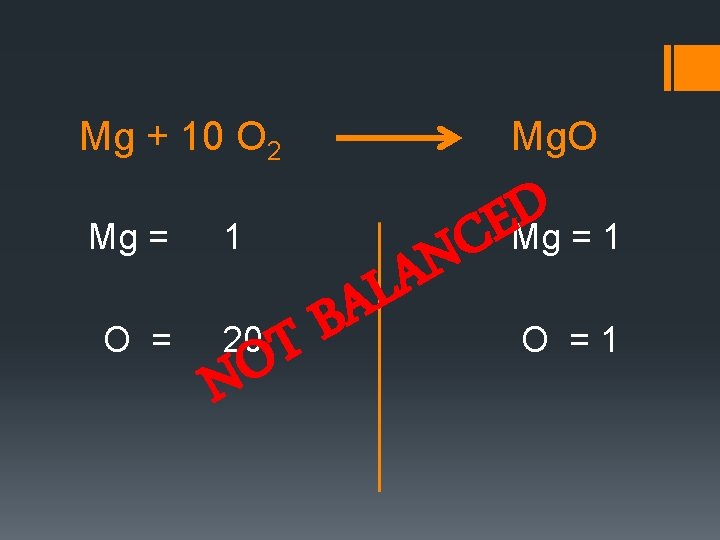
Mg + 10 O 2 Mg = O = 1 Mg. O A B 20 T O N N LA D E C Mg = 1 O =1

Does the following chemical equation follows the law of conservation of mass? L A C I M E Zn + C SH Ca. O N a O I T T Zn = 1 Ca =1 O A N S E= Q 1 U O = 1

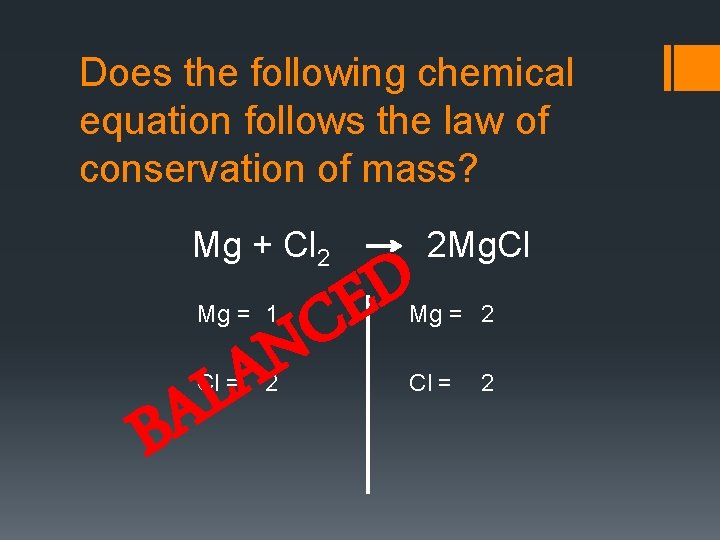
Does the following chemical equation follows the law of conservation of mass? Mg + Cl 2 Mg = 1 N A L Cl = A B D E C 2 2 Mg. Cl Mg = 2 Cl = 2
 Antoine lavoisier law
Antoine lavoisier law Law of conservation od mass
Law of conservation od mass Lab conservation of mass worksheet answers
Lab conservation of mass worksheet answers Law of conservation of mass
Law of conservation of mass The law of conservation of mass
The law of conservation of mass Law of conservation of mass
Law of conservation of mass Control volume approach
Control volume approach Law of conservation of mass
Law of conservation of mass Br3cl9 compound name
Br3cl9 compound name Example of law of conservation of mass
Example of law of conservation of mass State the law of conservation of mass
State the law of conservation of mass

















