Law of Conservation of Mass LCM Law of












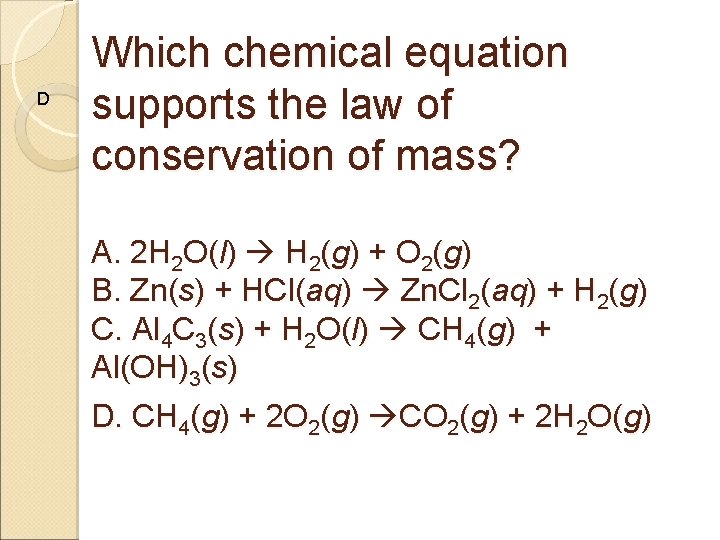


- Slides: 26

Law of Conservation of Mass (LCM)

Law of conservation of mass – states that mass is neither created nor destroyed during chemical reactions or physical changes

Reactant – substance that undergoes a reaction

Product – a new substance formed when reactants undergo chemical change

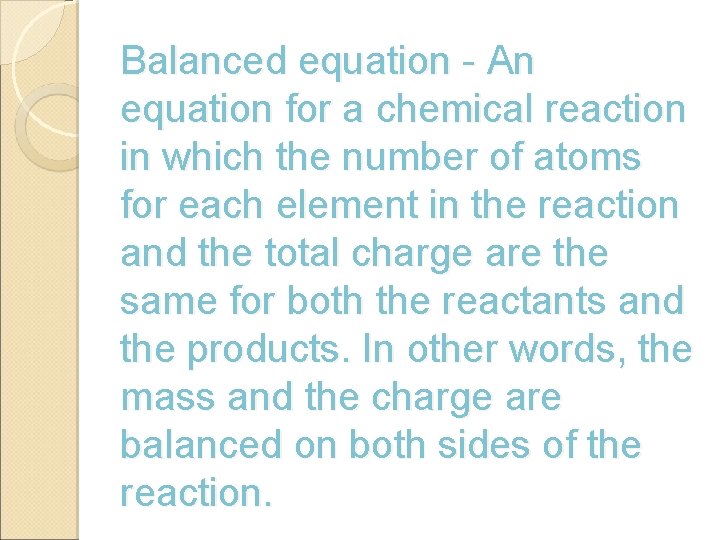
Balanced equation - An equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the products. In other words, the mass and the charge are balanced on both sides of the reaction.

Coefficients – a number placed in front of the parts of a chemical equation to indicate how many are involved ; always a positive whole number.

Subscript –

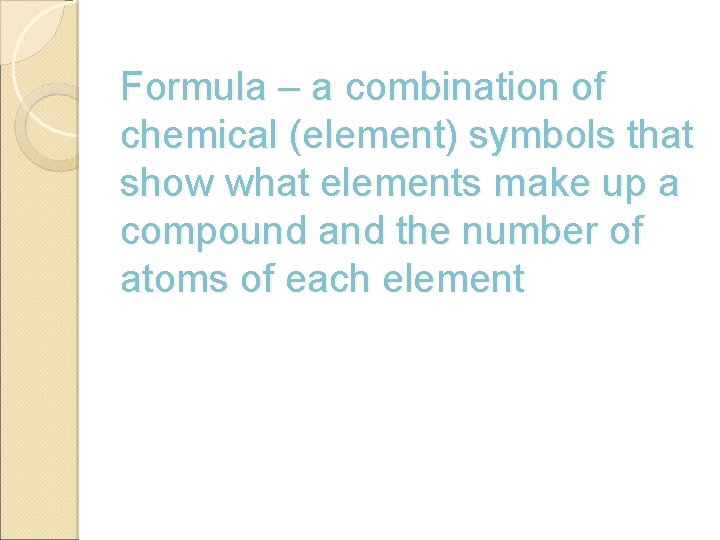
Formula – a combination of chemical (element) symbols that show what elements make up a compound and the number of atoms of each element

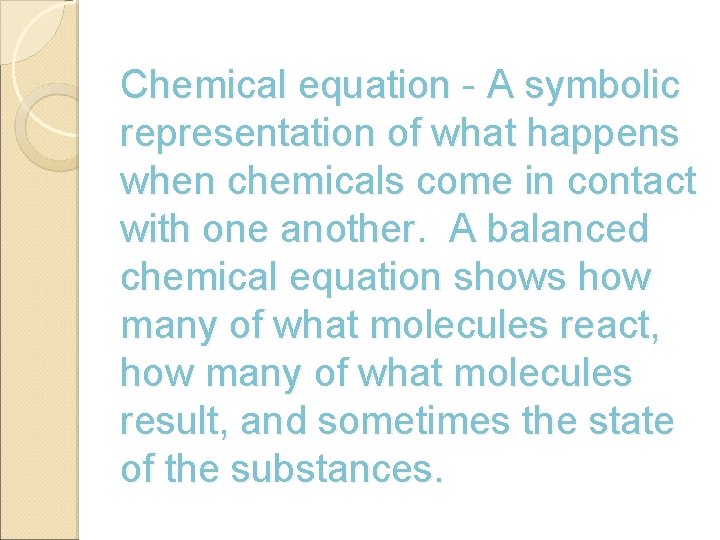
Chemical equation - A symbolic representation of what happens when chemicals come in contact with one another. A balanced chemical equation shows how many of what molecules react, how many of what molecules result, and sometimes the state of the substances.

Demo: LCM – in chemical reactions, you always end up with the same amount of stuff you start with. “Stuff” – very scientific could be atoms or mass.

Demo: What is the formula for aluminum chloride? What is the formula for copper chloride?

Demo: What will happen if I drop aluminum into a copper chloride solution?

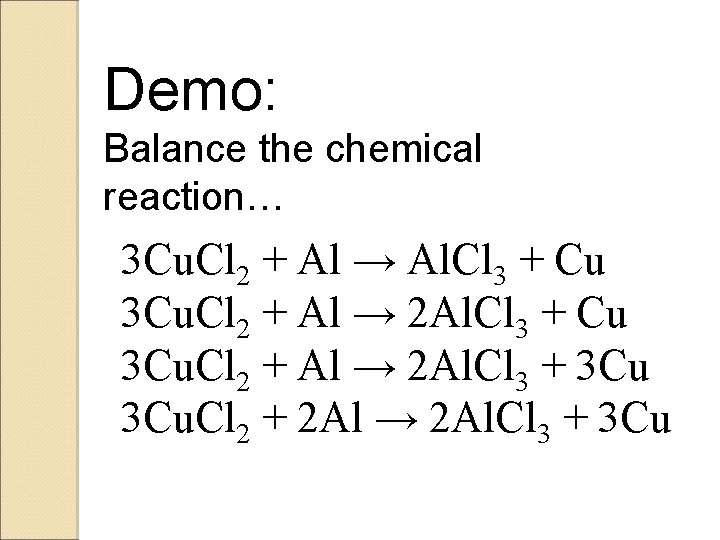
Demo: Write the chemical reaction… Cu. Cl 2 + Al → Al. Cl 3 + Cu Do you notice anything? !

Demo: Balance the chemical reaction… 3 Cu. Cl 2 + Al → Al. Cl 3 + Cu 3 Cu. Cl 2 + Al → 2 Al. Cl 3 + 3 Cu. Cl 2 + 2 Al → 2 Al. Cl 3 + 3 Cu


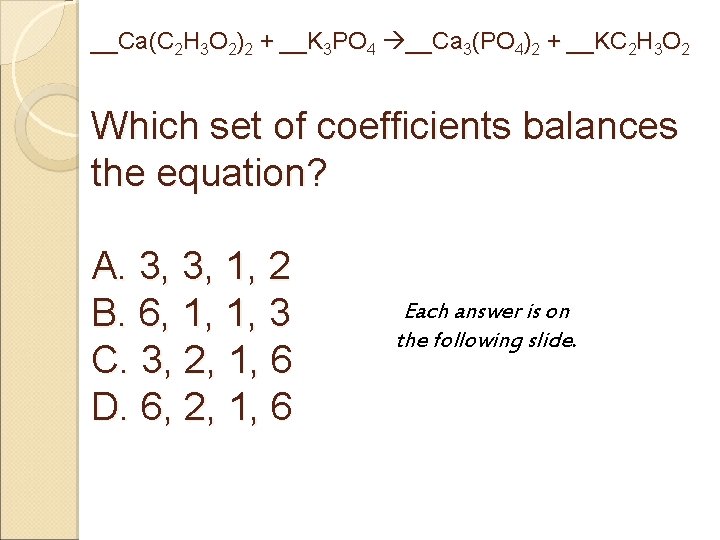
__Ca(C 2 H 3 O 2)2 + __K 3 PO 4 __Ca 3(PO 4)2 + __KC 2 H 3 O 2 Which set of coefficients balances the equation? A. 3, 3, 1, 2 Each answer is on B. 6, 1, 1, 3 the following slide. C. 3, 2, 1, 6 D. 6, 2, 1, 6

C Which of these would support the idea that mass is conserved in a reaction that produces a gas as a product? A. Heating the reactants to ensure the reaction occurs in a gaseous state B. Subtracting the mass of the gas from the mass of the solid and liquid products C. Mixing the reactants and measuring their total mass D. Trapping the gas and measuring its mass

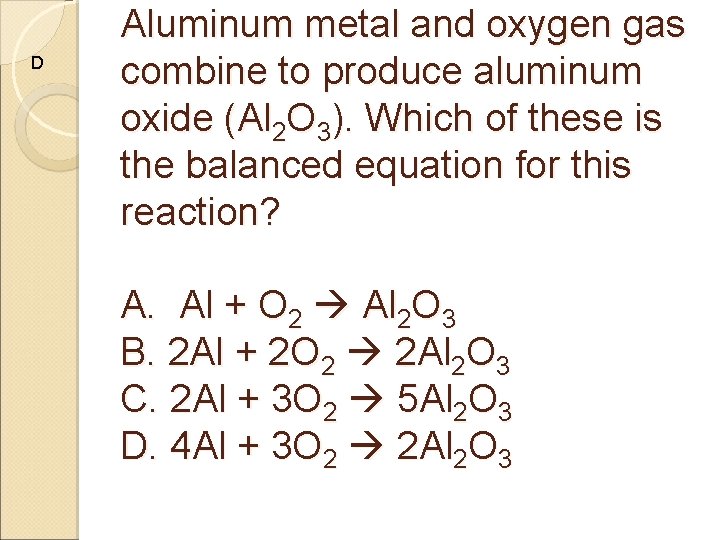
D Aluminum metal and oxygen gas combine to produce aluminum oxide (Al 2 O 3). Which of these is the balanced equation for this reaction? A. Al + O 2 Al 2 O 3 B. 2 Al + 2 O 2 2 Al 2 O 3 C. 2 Al + 3 O 2 5 Al 2 O 3 D. 4 Al + 3 O 2 2 Al 2 O 3

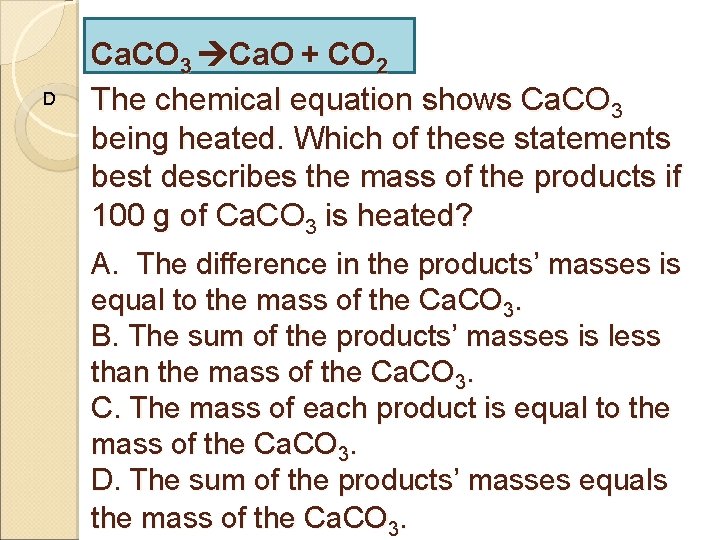
D Ca. CO 3 Ca. O + CO 2 The chemical equation shows Ca. CO 3 being heated. Which of these statements best describes the mass of the products if 100 g of Ca. CO 3 is heated? A. The difference in the products’ masses is equal to the mass of the Ca. CO 3. B. The sum of the products’ masses is less than the mass of the Ca. CO 3. C. The mass of each product is equal to the mass of the Ca. CO 3. D. The sum of the products’ masses equals the mass of the Ca. CO 3.
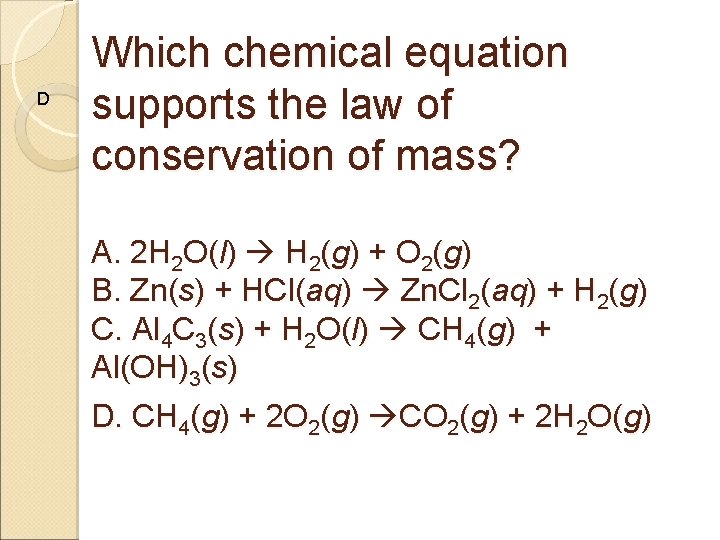
D Which chemical equation supports the law of conservation of mass? A. 2 H 2 O(l) H 2(g) + O 2(g) B. Zn(s) + HCl(aq) Zn. Cl 2(aq) + H 2(g) C. Al 4 C 3(s) + H 2 O(l) CH 4(g) + Al(OH)3(s) D. CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g)

D 2 Cu + O 2 2 Cu. O When 127 g of copper reacts with 32 g of oxygen gas to form copper (II) oxide, no copper or oxygen is left over. How much copper (II) oxide is produced? A. 32 g B. 95 g C. 127 g D. 159 g

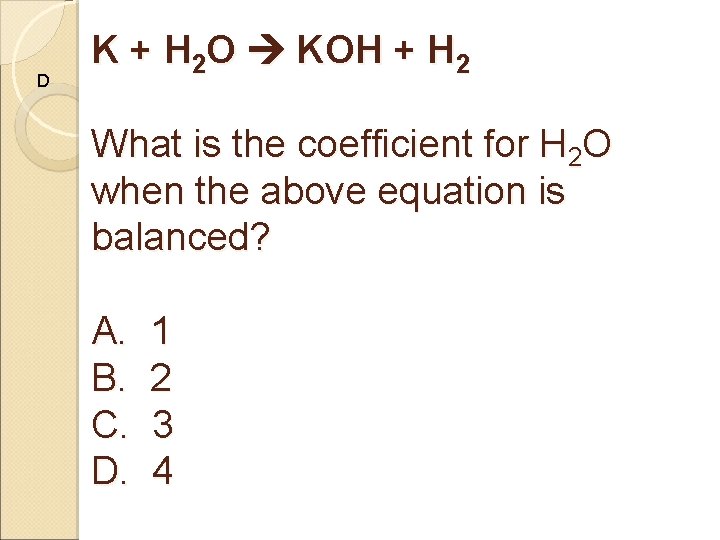
D K + H 2 O KOH + H 2 What is the coefficient for H 2 O when the above equation is balanced? A. 1 B. 2 C. 3 D. 4

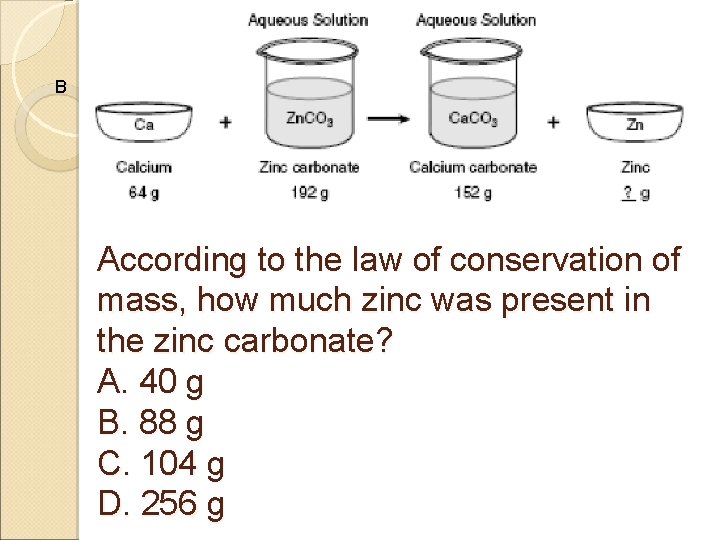
B According to the law of conservation of mass, how much zinc was present in the zinc carbonate? A. 40 g B. 88 g C. 104 g D. 256 g

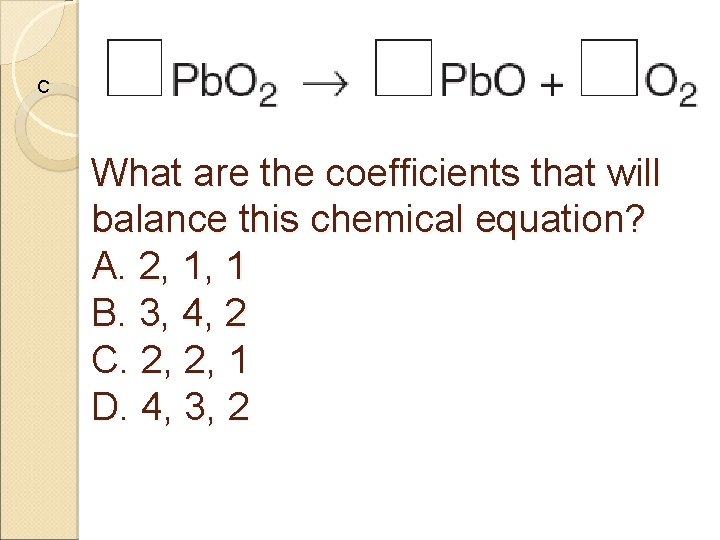
C What are the coefficients that will balance this chemical equation? A. 2, 1, 1 B. 3, 4, 2 C. 2, 2, 1 D. 4, 3, 2

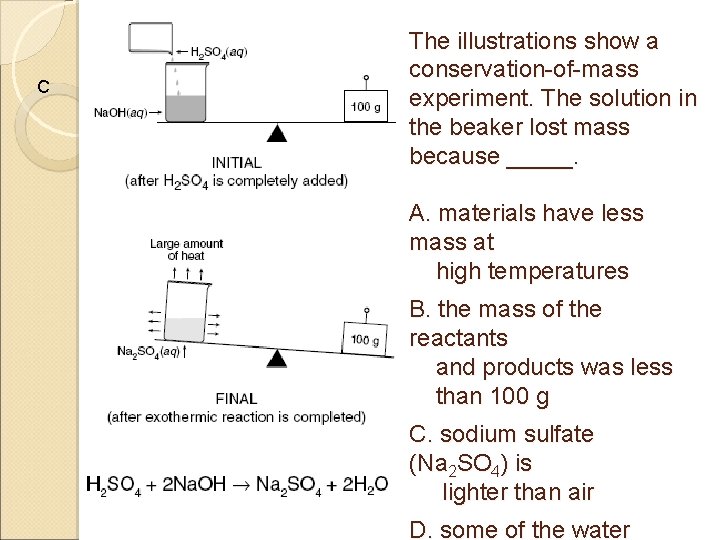
C The illustrations show a conservation-of-mass experiment. The solution in the beaker lost mass because _____. A. materials have less mass at high temperatures B. the mass of the reactants and products was less than 100 g C. sodium sulfate (Na 2 SO 4) is lighter than air D. some of the water

D
 Antoine lavoisier law of conservation of mass
Antoine lavoisier law of conservation of mass Law of conservation od mass
Law of conservation od mass Law of conservation of mass virtual lab answer key
Law of conservation of mass virtual lab answer key Law of conservation of mass
Law of conservation of mass The law of conservation of mass
The law of conservation of mass Law of conservation of mass
Law of conservation of mass Law of conservation of mass
Law of conservation of mass Law of conservation of mass
Law of conservation of mass Br3cl9
Br3cl9 Law of conservation of mass example
Law of conservation of mass example State law of conservation of mass
State law of conservation of mass Law of conservation of mass
Law of conservation of mass Conservation of mass
Conservation of mass Example of law of conservation of mass
Example of law of conservation of mass Derive the equation of continuity
Derive the equation of continuity Mass conservation equation
Mass conservation equation Ideal fluid
Ideal fluid Conservation of mass momentum and energy equations
Conservation of mass momentum and energy equations Conservation of mass problems
Conservation of mass problems Rsc conservation of mass
Rsc conservation of mass Mass conservation
Mass conservation Imapp radboud
Imapp radboud Energy formula
Energy formula Momentum means
Momentum means How momentum conservation is applied in vehicular accidents
How momentum conservation is applied in vehicular accidents Law of conservation of momentum animation
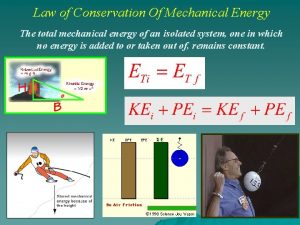
Law of conservation of momentum animation Conservation of mechanical energy formula
Conservation of mechanical energy formula