Notes SPI 0807 9 11 Law of Conservation


- Slides: 13

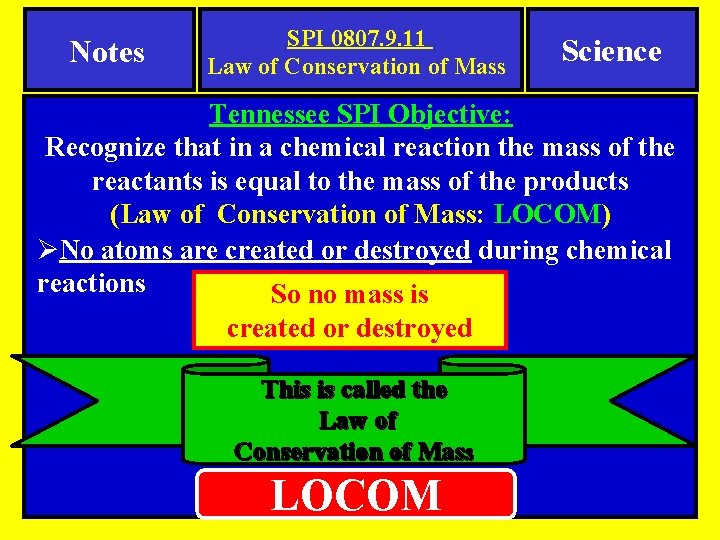
Notes SPI 0807. 9. 11 Law of Conservation of Mass Science Tennessee SPI Objective: Recognize that in a chemical reaction the mass of the reactants is equal to the mass of the products. (Law of Conservation of Mass: LOCOM) Essential Question(s) How do I determine the number and type of atoms on each side of a chemical equation? I Can Statement I can count the numbers of atoms on each side of chemical reactions. Success Criteria I can prove that I understand LOCOM by counting the number of atoms on each side of chemical reactions.

Notes SPI 0807. 9. 11 Law of Conservation of Mass Science Tennessee SPI Objective: Recognize that in a chemical reaction the mass of the reactants is equal to the mass of the products (Law of Conservation of Mass: LOCOM) ØNo atoms are created or destroyed during chemical reactions So no mass is created or destroyed This is called the Law of Conservation of Mass LOCOM

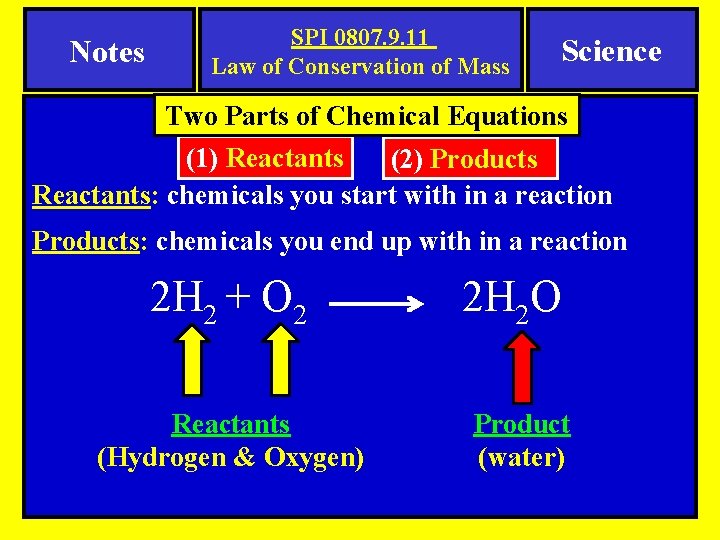
Notes SPI 0807. 9. 11 Law of Conservation of Mass Science Two Parts of Chemical Equations (1) Reactants (2) Products Reactants: chemicals you start with in a reaction Products: chemicals you end up with in a reaction 2 H 2 + O 2 Reactants (Hydrogen & Oxygen) 2 H 2 O Product (water)

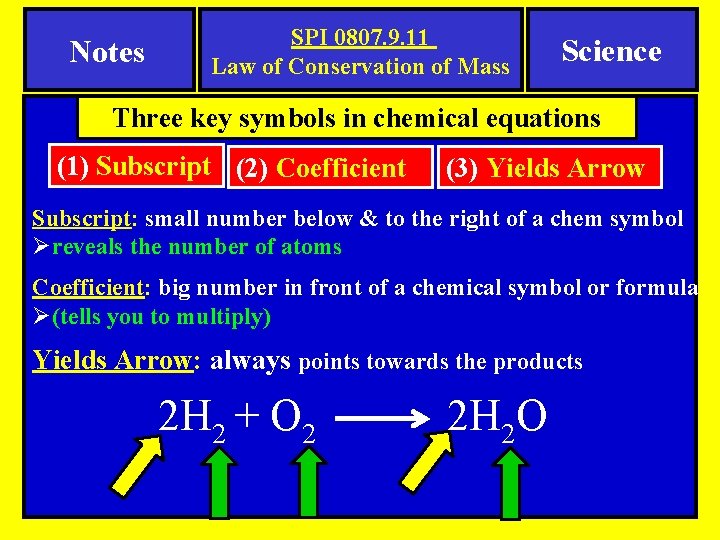
Notes SPI 0807. 9. 11 Law of Conservation of Mass Science Three key symbols in chemical equations (1) Subscript (2) Coefficient (3) Yields Arrow Subscript: small number below & to the right of a chem symbol Øreveals the number of atoms Coefficient: big number in front of a chemical symbol or formula Ø(tells you to multiply) Yields Arrow: always points towards the products 2 H 2 + O 2 2 H 2 O

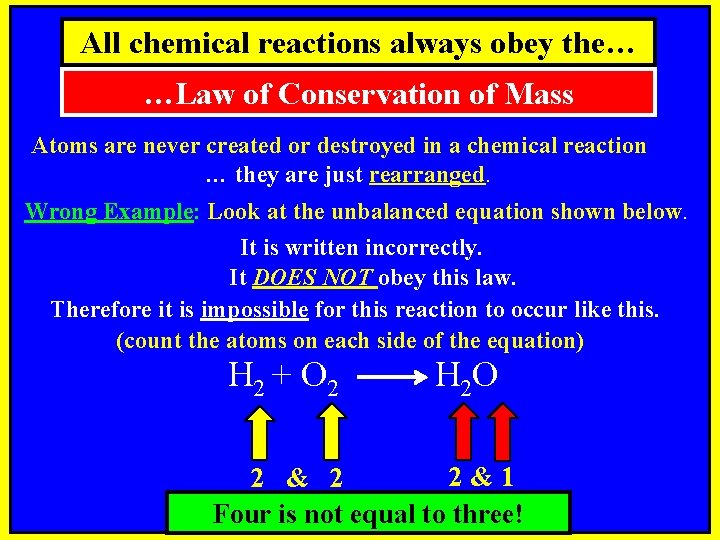
All chemical reactions always obey the… …Law of Conservation of Mass Atoms are never created or destroyed in a chemical reaction … they are just rearranged. Wrong Example: Look at the unbalanced equation shown below. It is written incorrectly. It DOES NOT obey this law. Therefore it is impossible for this reaction to occur like this. (count the atoms on each side of the equation) H 2 + O 2 H 2 O 2&1 2 & 2 Four is not equal to three!

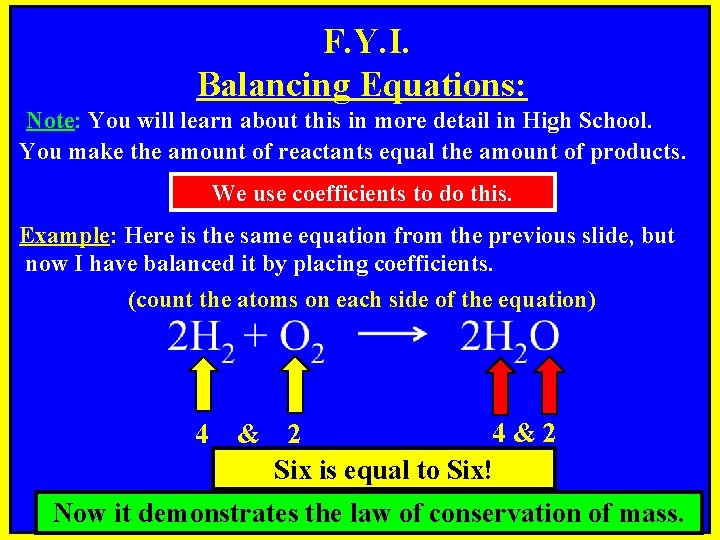
F. Y. I. Balancing Equations: Note: You will learn about this in more detail in High School. You make the amount of reactants equal the amount of products. We use coefficients to do this. Example: Here is the same equation from the previous slide, but now I have balanced it by placing coefficients. (count the atoms on each side of the equation) 4 & 4&2 2 Six is equal to Six! Now it demonstrates the law of conservation of mass.

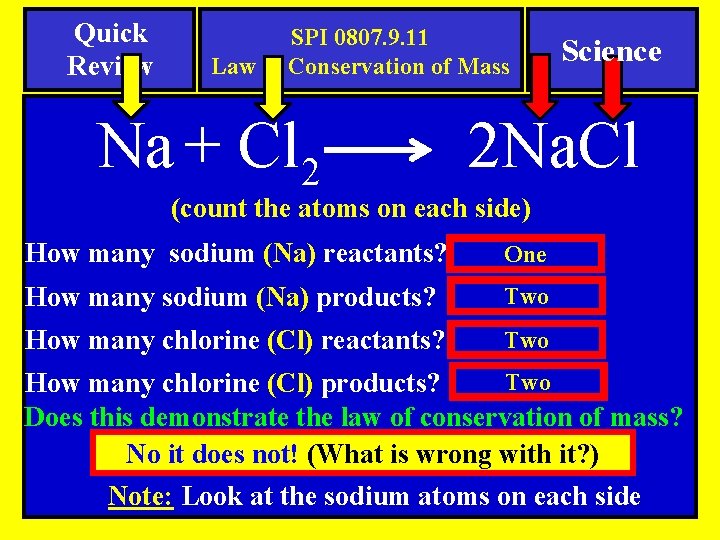
Quick Review SPI 0807. 9. 11 Law of Conservation of Mass Na + Cl 2 Science 2 Na. Cl (count the atoms on each side) How many sodium (Na) reactants? One How many sodium (Na) products? Two How many chlorine (Cl) reactants? Two How many chlorine (Cl) products? Does this demonstrate the law of conservation of mass? No it does not! (What is wrong with it? ) Note: Look at the sodium atoms on each side

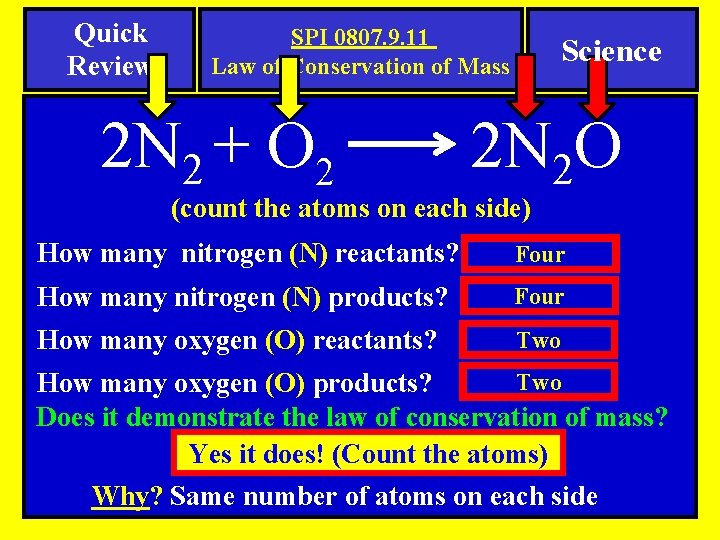
Quick Review SPI 0807. 9. 11 Law of Conservation of Mass 2 N 2 + O 2 Science 2 N 2 O (count the atoms on each side) How many nitrogen (N) reactants? Four How many nitrogen (N) products? Four How many oxygen (O) reactants? Two How many oxygen (O) products? Does it demonstrate the law of conservation of mass? Yes it does! (Count the atoms) Why? Same number of atoms on each side

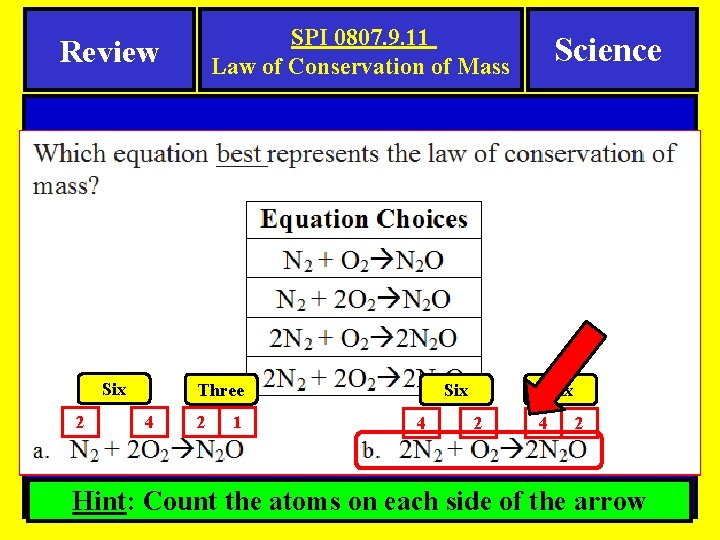
SPI 0807. 9. 11 Law of Conservation of Mass Review Six 2 Three 4 2 1 Science Six 4 Six 2 4 2 Hint: Count the atoms on each side of the arrow

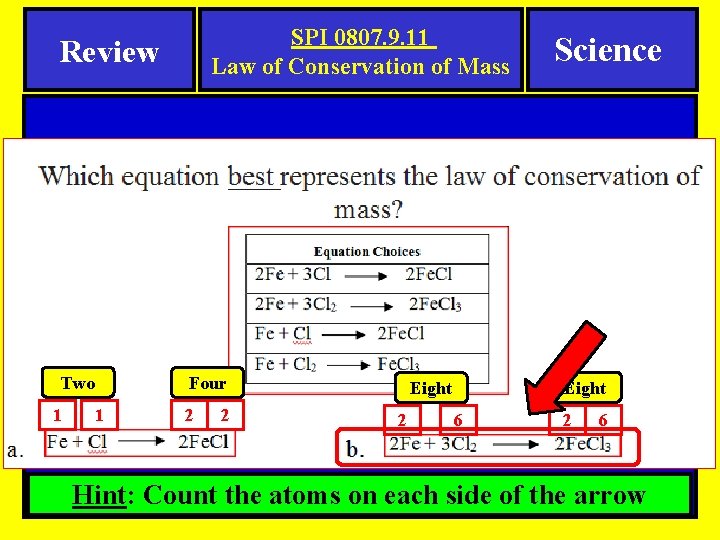
SPI 0807. 9. 11 Law of Conservation of Mass Review Two 1 1 Four 2 2 Eight 2 Science 6 2 6 Hint: Count the atoms on each side of the arrow

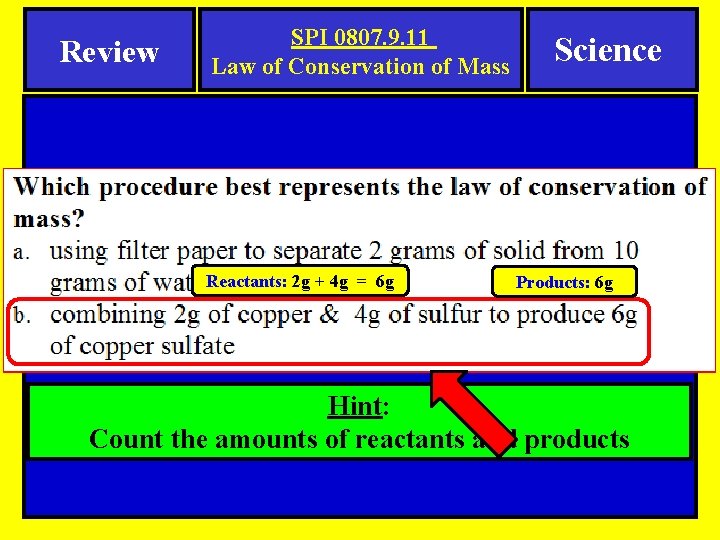
Review SPI 0807. 9. 11 Law of Conservation of Mass Reactants: 2 g + 4 g = 6 g Science Products: 6 g Hint: Count the amounts of reactants and products

Review SPI 0807. 9. 11 Law of Conservation of Mass Science Hint: Use substitution: K= 39 grams, O= 32 grams …so 39 + 32 ? ? ?

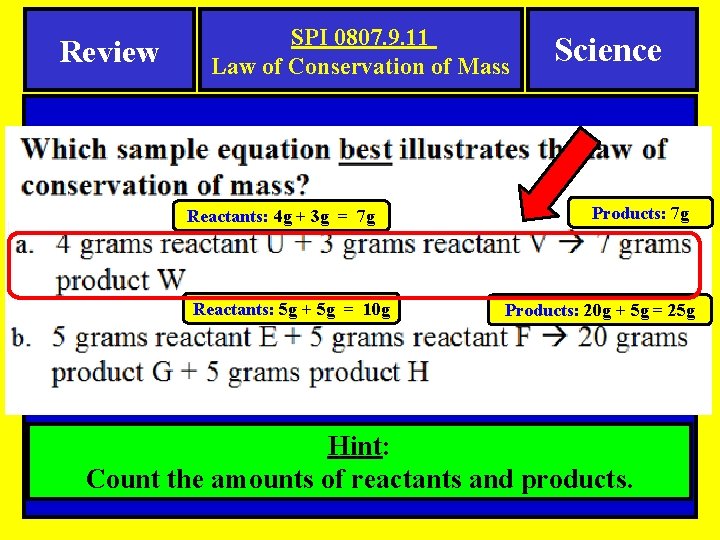
Review SPI 0807. 9. 11 Law of Conservation of Mass Reactants: 4 g + 3 g = 7 g Reactants: 5 g + 5 g = 10 g Science Products: 7 g Products: 20 g + 5 g = 25 g Hint: Count the amounts of reactants and products.
 Energy conservation
Energy conservation Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law V=k/p
V=k/p Avogadro's law constant
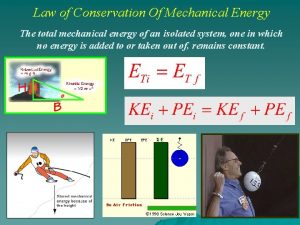
Avogadro's law constant Law of conservation of mechanical energy
Law of conservation of mechanical energy Momentum means
Momentum means Momentum conservation law
Momentum conservation law Law of conservation of linear momentum
Law of conservation of linear momentum Principle of conservation of mechanical energy
Principle of conservation of mechanical energy Antoine lavoisier law
Antoine lavoisier law Law of conservation of energy worksheets
Law of conservation of energy worksheets Energy conservation law
Energy conservation law The law of conservation of energy states that
The law of conservation of energy states that