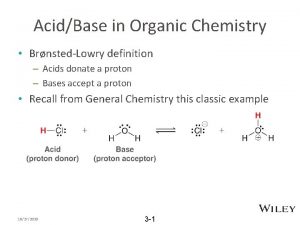
Chemistry 142 Chapter 21 Organic Chemistry Outline I







![Polymers of Alkenes n CH 2=CH 2 ---> -[CH 2]-n Polymers of Alkenes n CH 2=CH 2 ---> -[CH 2]-n](https://slidetodoc.com/presentation_image_h/03a1ab696715791030ef27660fc9be04/image-20.jpg)

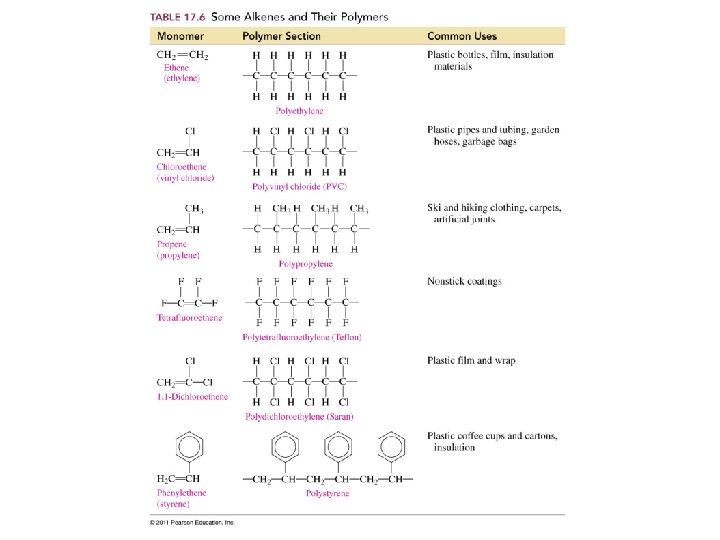













- Slides: 63

Chemistry 142 Chapter 21: Organic Chemistry Outline I. Hydrocarbons A. Alkanes B. Alkenes C. Alkynes D. Aromatics E. Reactions II. Functions Groups A. Alcohols B. Aldehydes C. Ketones D. Carboxylic Acids E. Esters F. Ethers G. Amines H. Amides III. Polymers

Friedrich Wöhler

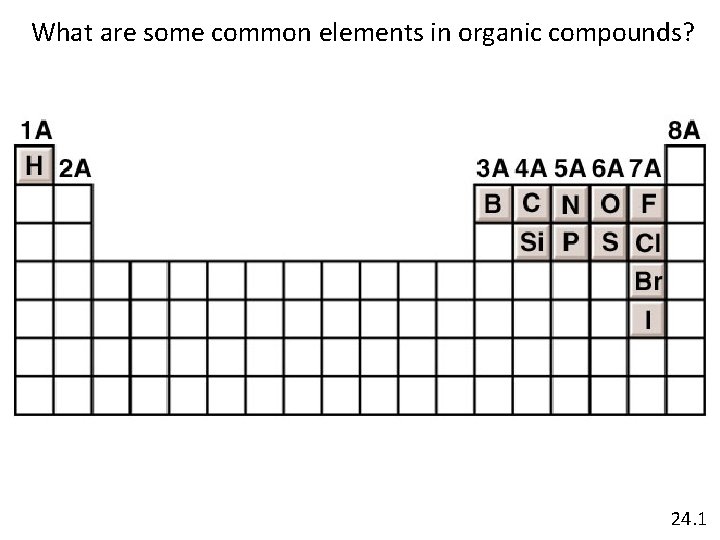
What are some common elements in organic compounds? 24. 1

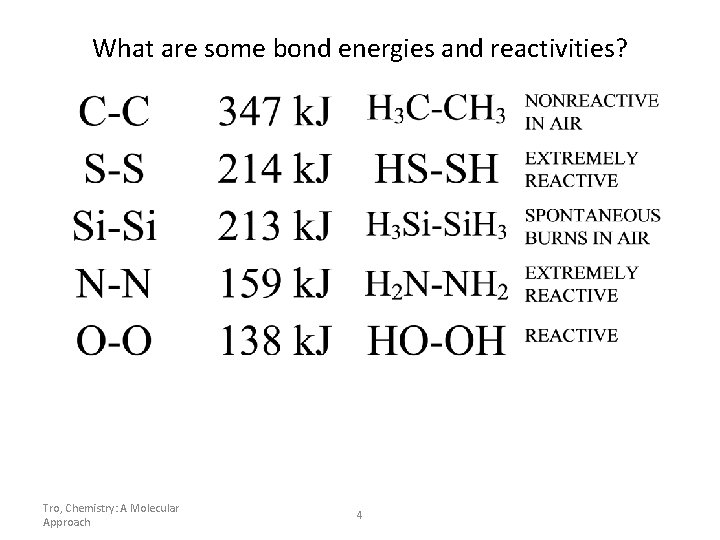
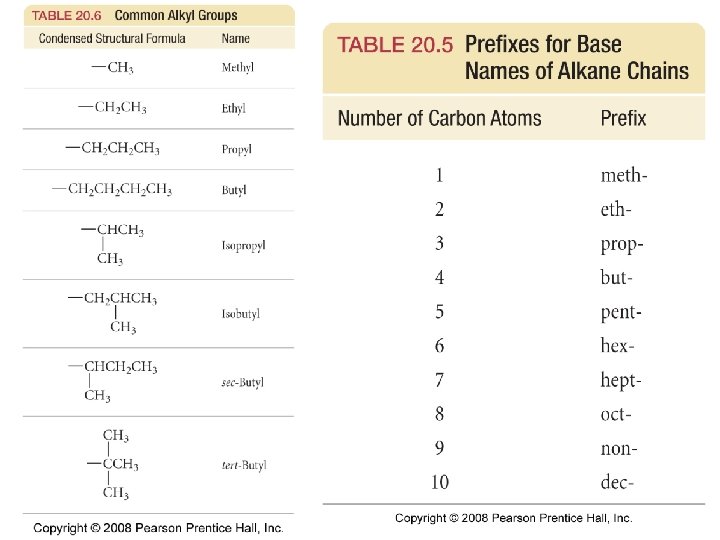
What are some bond energies and reactivities? Tro, Chemistry: A Molecular Approach 4



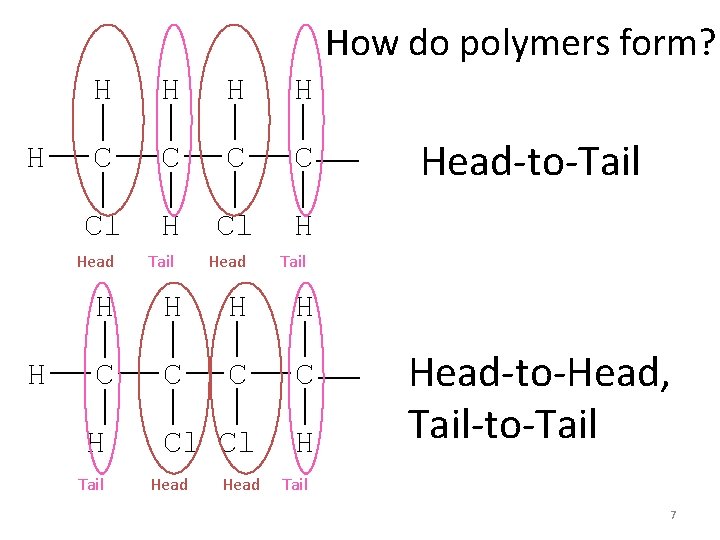
How do polymers form? H H H C C Cl H Head H Tail Head Tail H H C C H Cl Cl Tail Head-to-Tail H Head-to-Head, Tail-to-Tail 7

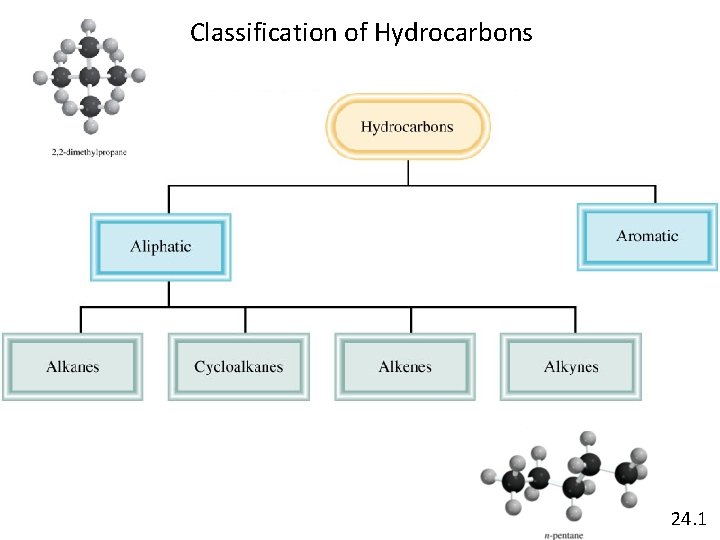
Classification of Hydrocarbons 24. 1

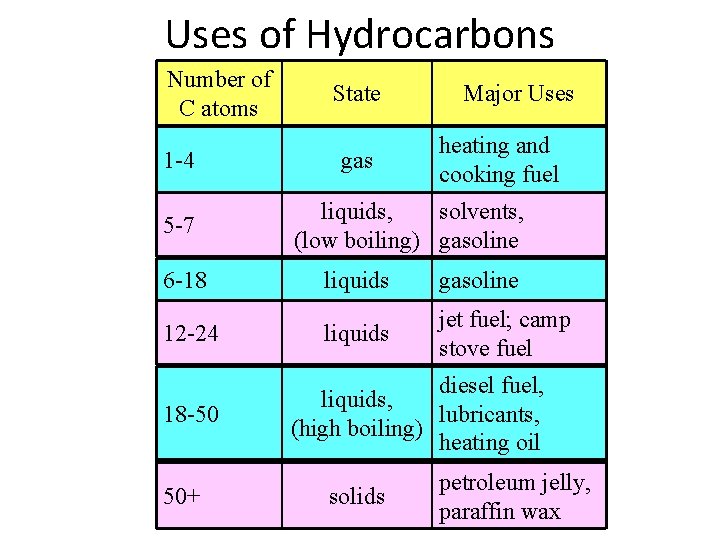
Uses of Hydrocarbons Number of C atoms 1 -4 5 -7 6 -18 12 -24 18 -50 50+50+ State gas Major Uses heating andand cooking fuel solvents, liquids, solvents, gasoline (low boiling) gasoline liquids gasoline fuel; camp jetjetfuel; stove fuel diesel fuel, liquids, lubricants, (high boiling) heating oil petroleum jelly, petroleum solids paraffin wax 9 liquids

Acetylene torch HCCH Parafin wax 20+ carbons Mineral Oil 17 -50 carbon

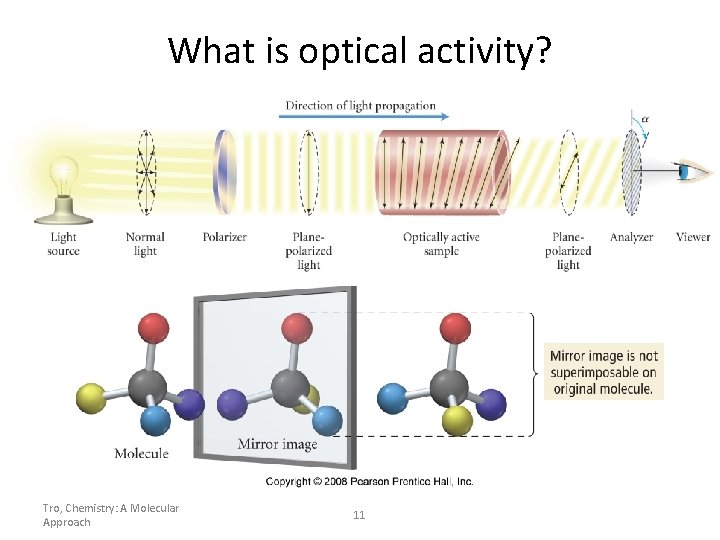
What is optical activity? Tro, Chemistry: A Molecular Approach 11

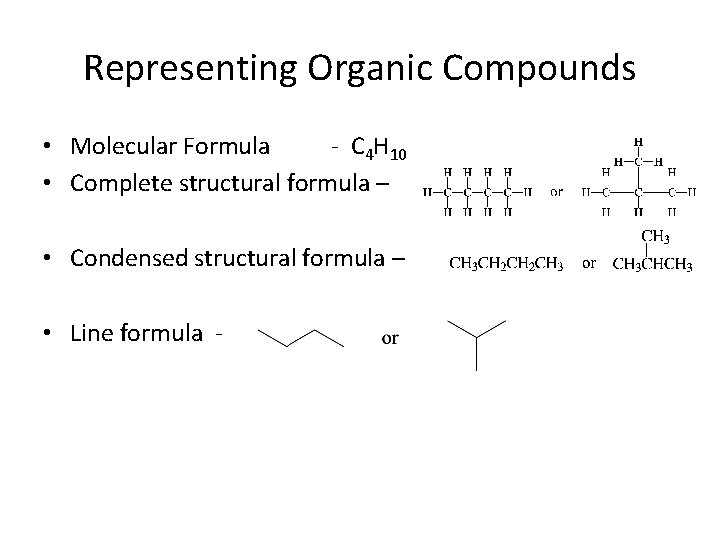
Representing Organic Compounds • Molecular Formula - C 4 H 10 • Complete structural formula – • Condensed structural formula – • Line formula -


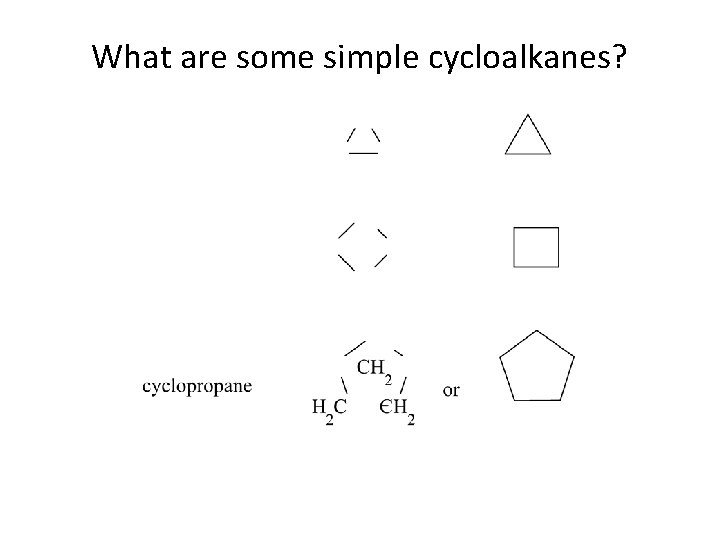
What are some simple cycloalkanes?

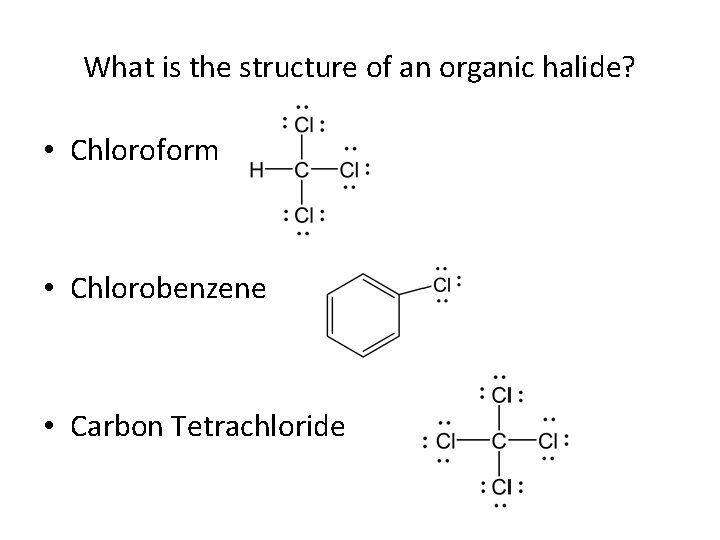
What is the structure of an organic halide? • Chloroform • Chlorobenzene • Carbon Tetrachloride

What makes a hydrocarbon unsaturated? Ethene used to ripen fruit.

Cis-Trans Isomerization in the Vision Process 24. 2

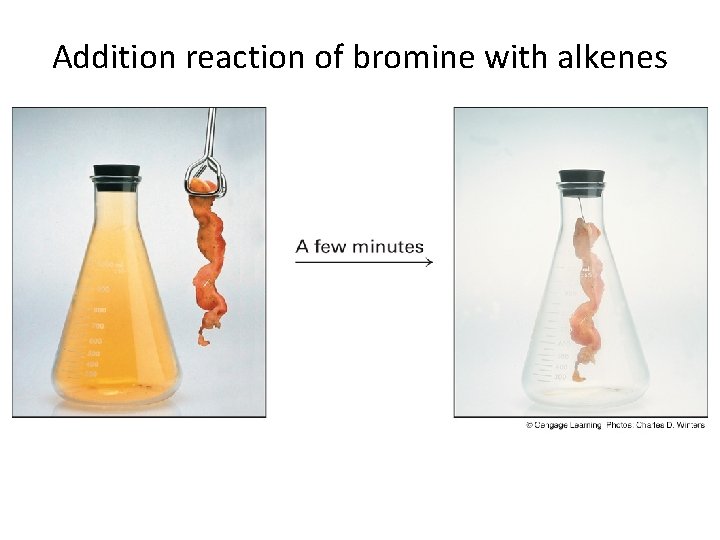
Addition reaction of bromine with alkenes

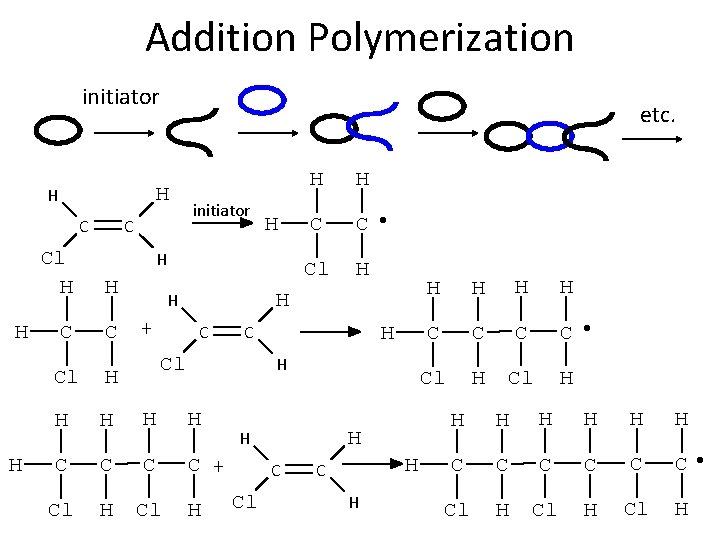
Addition Polymerization initiator H H C H H etc. C initiator Cl H H C C Cl H H H C C + Cl H H H C C • Cl H H H + H C C Cl H H H C Cl H C H H H C C • Cl H H H H C C C • Cl H
![Polymers of Alkenes n CH 2CH 2 CH 2n Polymers of Alkenes n CH 2=CH 2 ---> -[CH 2]-n](https://slidetodoc.com/presentation_image_h/03a1ab696715791030ef27660fc9be04/image-20.jpg)
Polymers of Alkenes n CH 2=CH 2 ---> -[CH 2]-n

Where do we find polymers?



What do the symbols on plastics tell us about their chemical formulas?

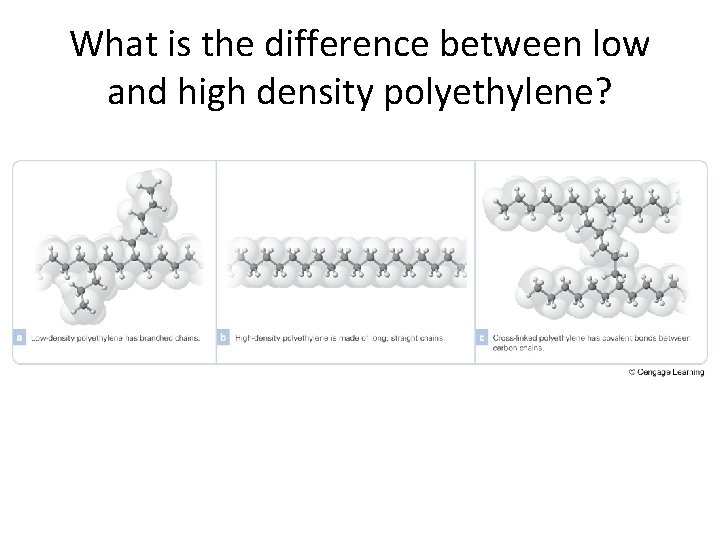
What is the difference between low and high density polyethylene?

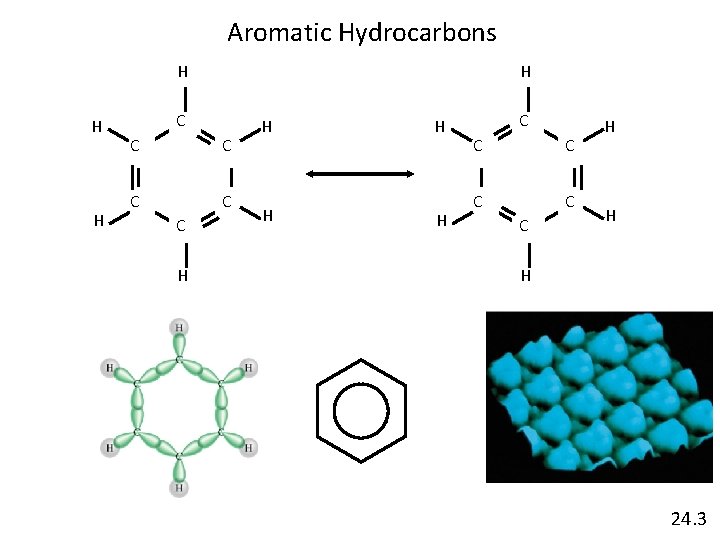
Aromatic Hydrocarbons H H H H C C C H H H 24. 3

What consumer products contain a benzene ring?

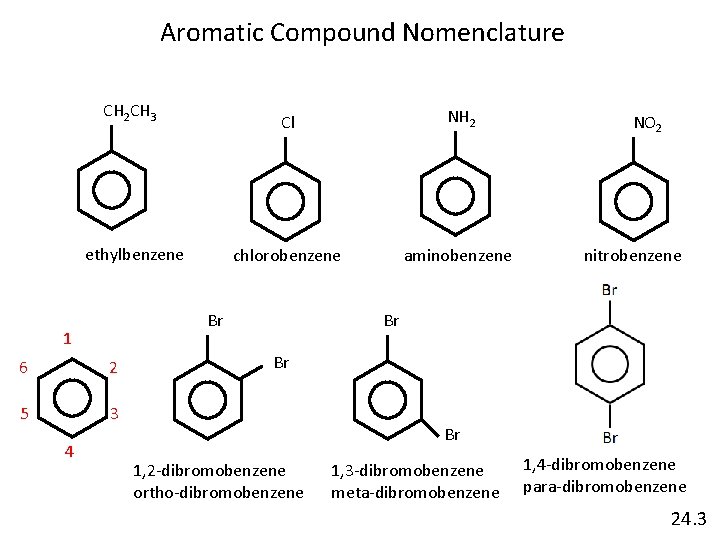
Aromatic Compound Nomenclature CH 2 CH 3 Cl NH 2 ethylbenzene chlorobenzene aminobenzene Br 1 6 2 5 3 4 NO 2 nitrobenzene Br Br Br 1, 2 -dibromobenzene ortho-dibromobenzene 1, 3 -dibromobenzene meta-dibromobenzene 1, 4 -dibromobenzene para-dibromobenzene 24. 3

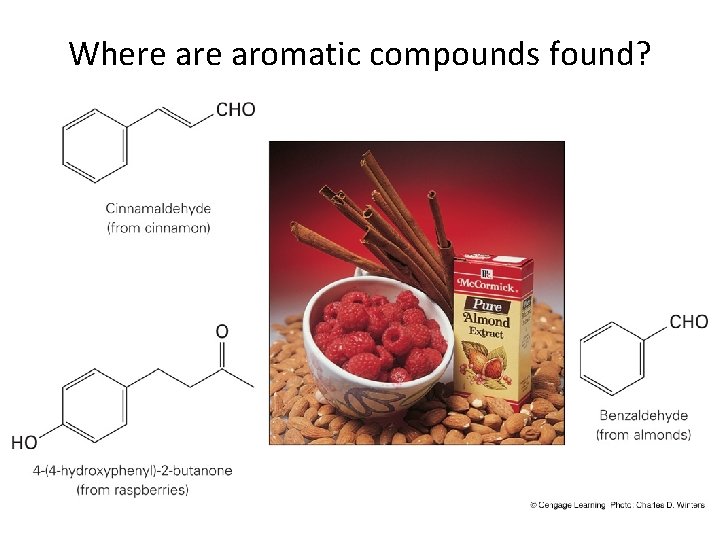
Where aromatic compounds found?

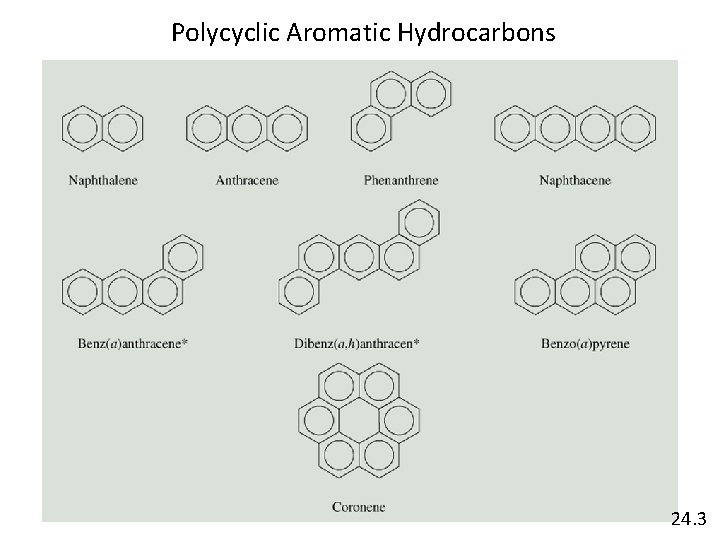
Polycyclic Aromatic Hydrocarbons 24. 3

Polymers Containing Aromatic Rings


What is the structure of an alcohol? • Cyclohexanol • 1 -propanol

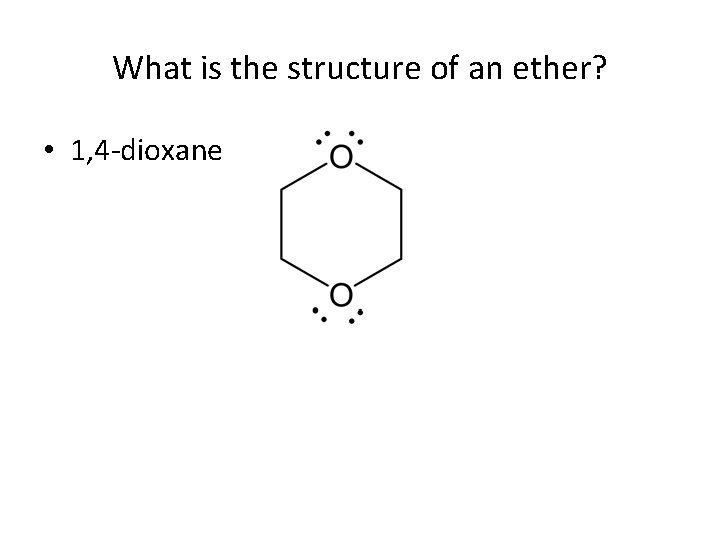
What is the structure of an ether? • 1, 4 -dioxane

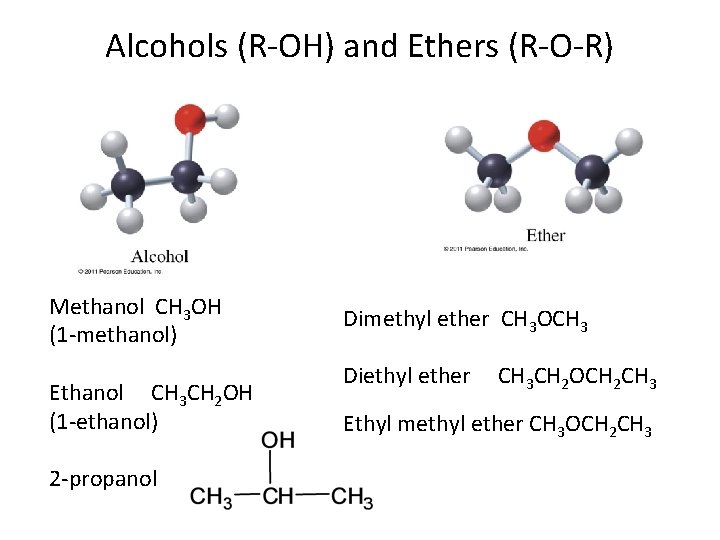
Alcohols (R-OH) and Ethers (R-O-R) Methanol CH 3 OH (1 -methanol) Ethanol CH 3 CH 2 OH (1 -ethanol) 2 -propanol Dimethyl ether CH 3 OCH 3 Diethyl ether CH 3 CH 2 OCH 2 CH 3 Ethyl methyl ether CH 3 OCH 2 CH 3

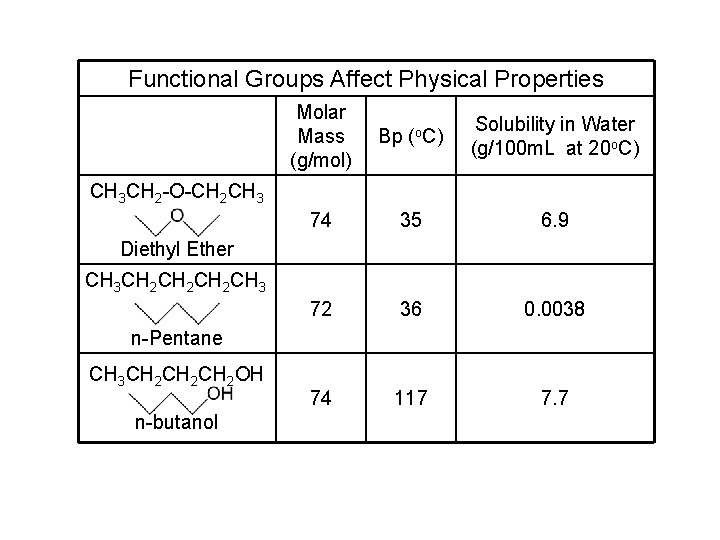
Functional Groups Affect Physical Properties Molar Mass (g/mol) Bp (o. C) Solubility in Water (g/100 m. L at 20 o. C) 74 35 6. 9 72 36 0. 0038 74 117 7. 7 CH 3 CH 2 -O-CH 2 CH 3 Diethyl Ether CH 3 CH 2 CH 2 CH 3 n-Pentane CH 3 CH 2 CH 2 OH n-butanol

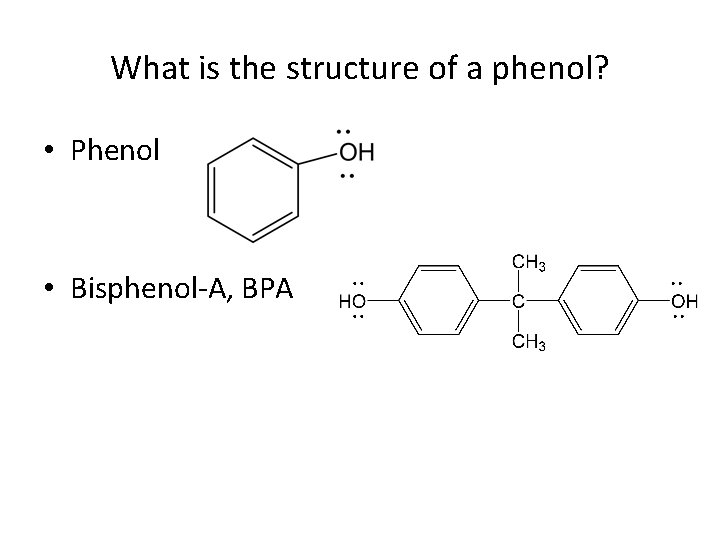
What is the structure of a phenol? • Phenol • Bisphenol-A, BPA

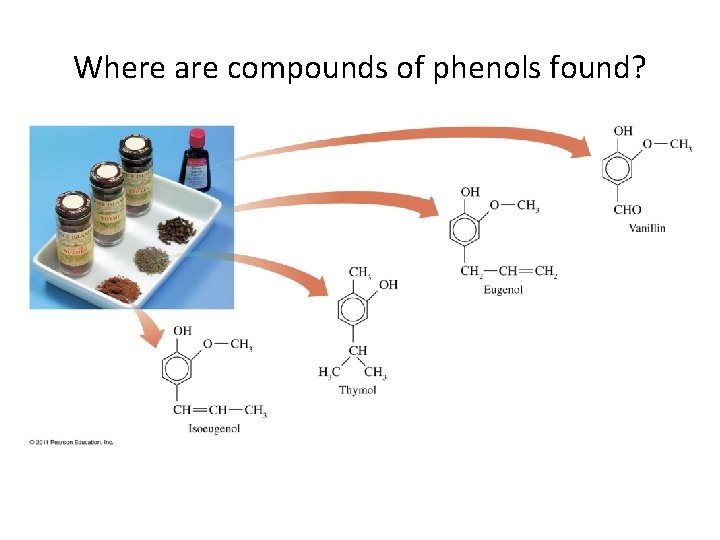
Where are compounds of phenols found?

Polymer of Alcohols and Ethers

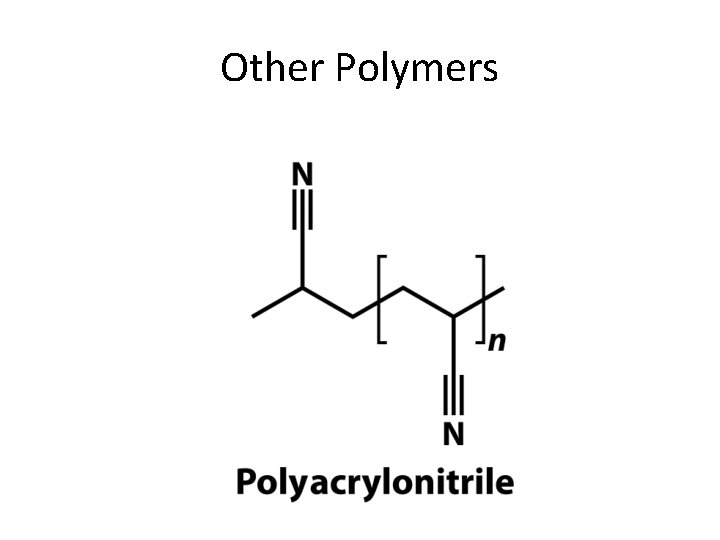
Other Polymers

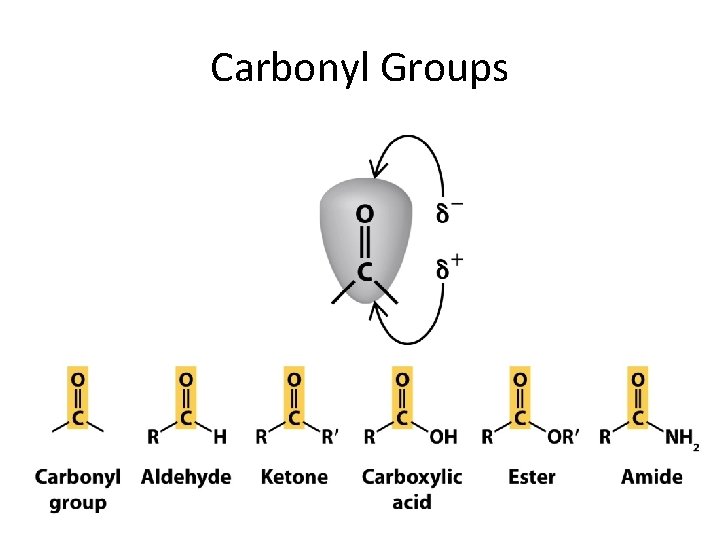
Carbonyl Groups

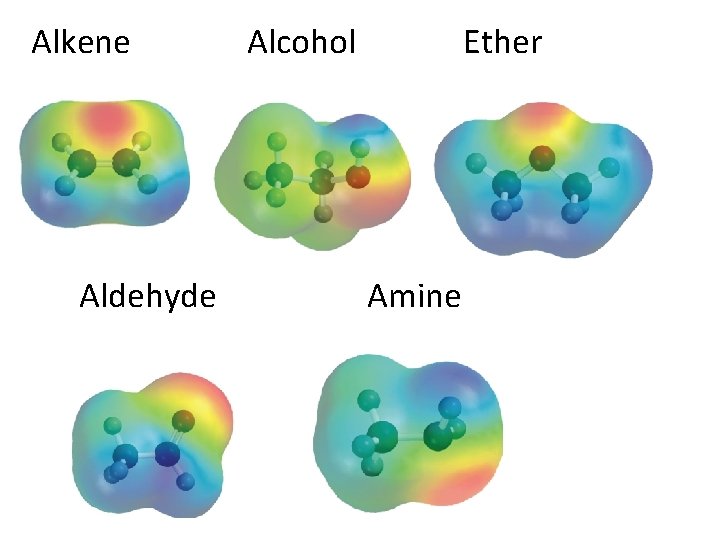
Alkene Aldehyde Alcohol Ether Amine

Carbonyl Group Tro, Chemistry: A Molecular Approach 50

What is the structure of an aldehyde? • ethanal

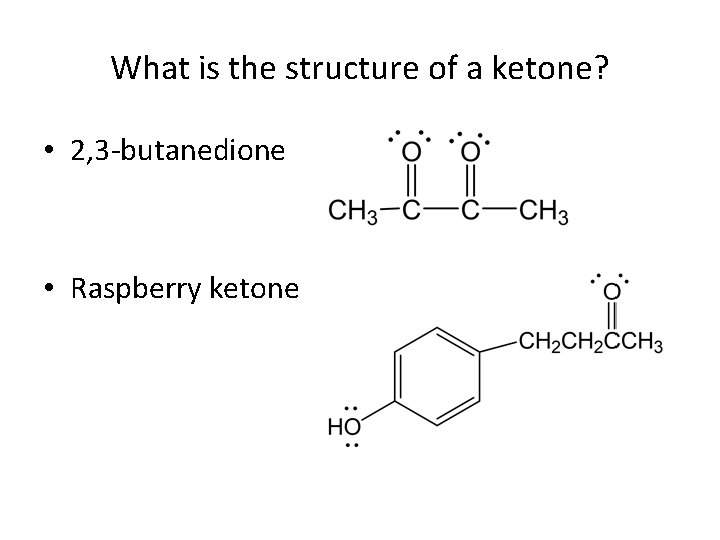
What is the structure of a ketone? • 2, 3 -butanedione • Raspberry ketone

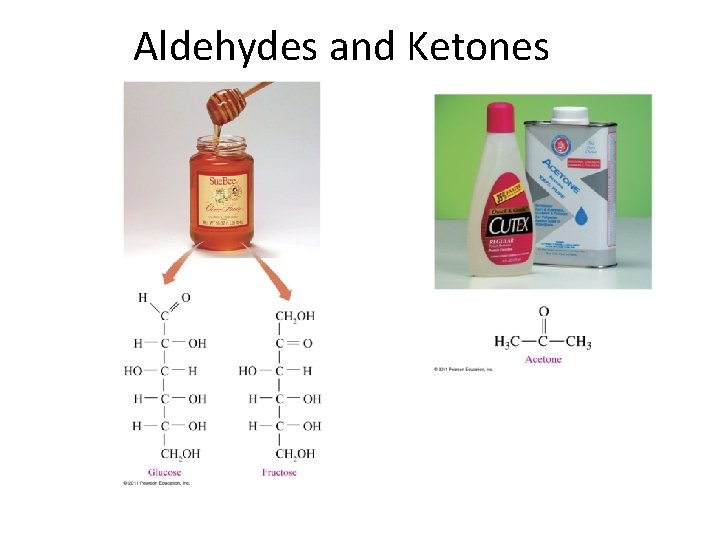
Aldehydes and Ketones

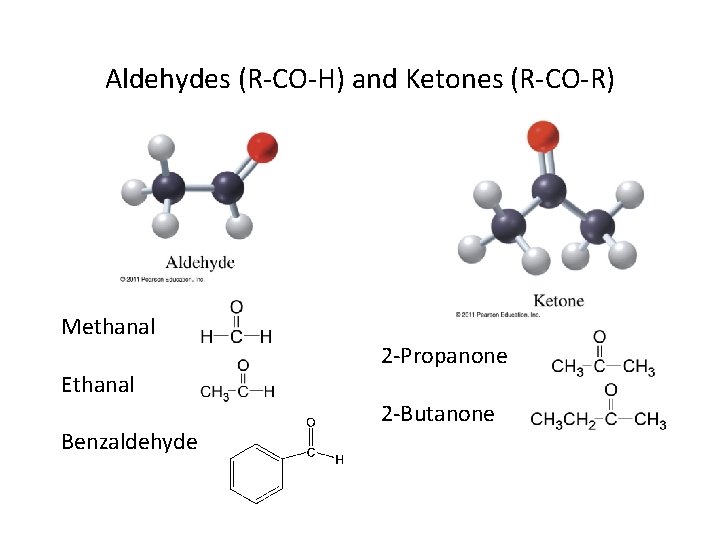
Aldehydes (R-CO-H) and Ketones (R-CO-R) Methanal Ethanal Benzaldehyde 2 -Propanone 2 -Butanone

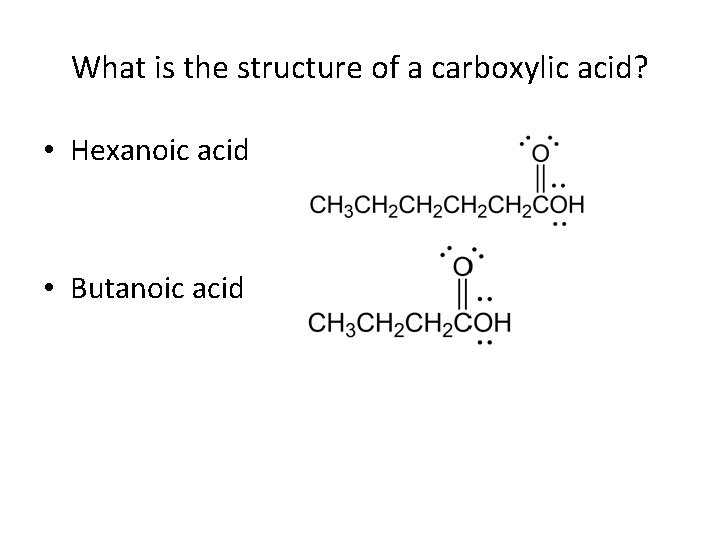
What is the structure of a carboxylic acid? • Hexanoic acid • Butanoic acid

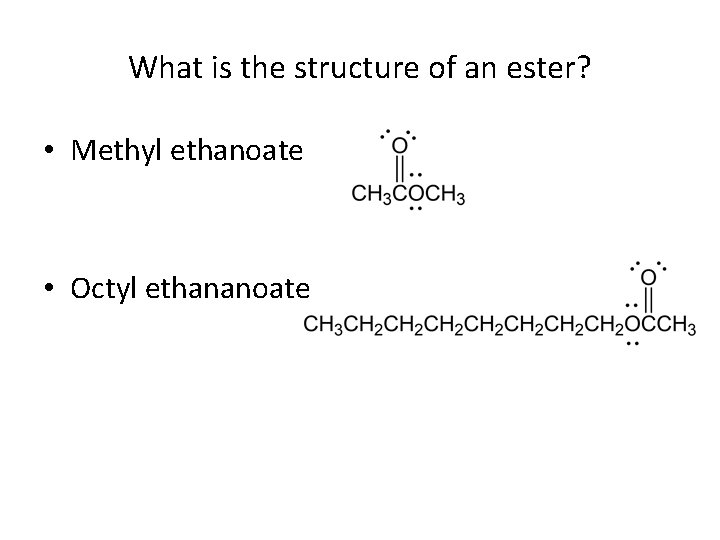
What is the structure of an ester? • Methyl ethanoate • Octyl ethananoate

Carboxylic Acids (R-COOH) and Esters (R-COO-R) Methanoic acid Ethylbutanoate Ethanoic acid Ethylmethanoate


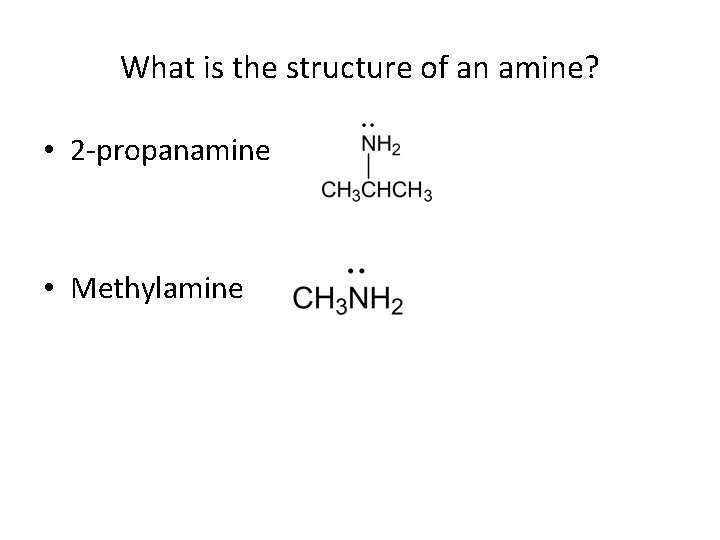
What is the structure of an amine? • 2 -propanamine • Methylamine

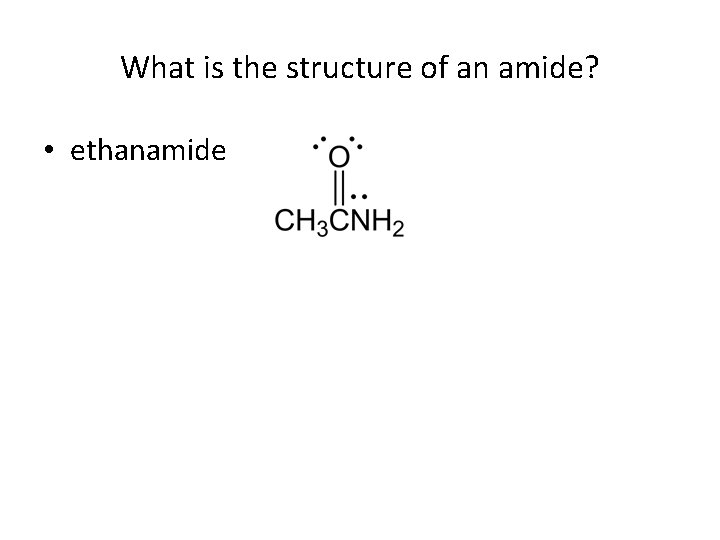
What is the structure of an amide? • ethanamide

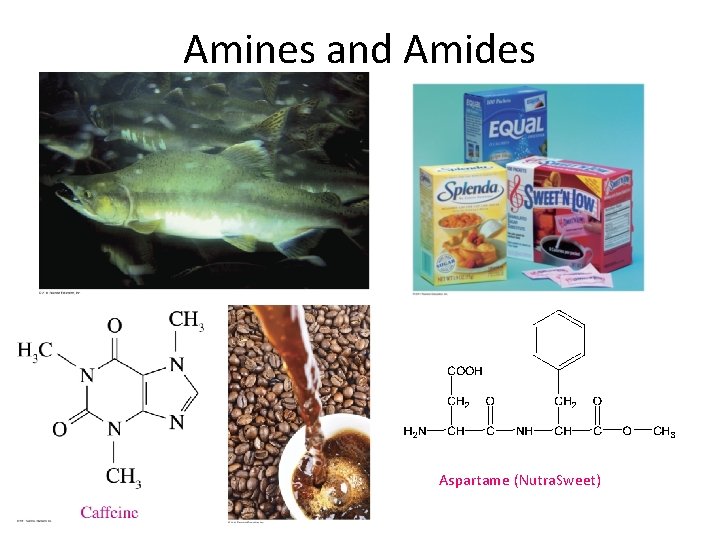
Amines and Amides Aspartame (Nutra. Sweet)

Amines (R 2 -NH) and Amides (R-CO-NH 2) Triethylamine Methanamide Nicotine Ethanamide Propanamide

Amino Acids

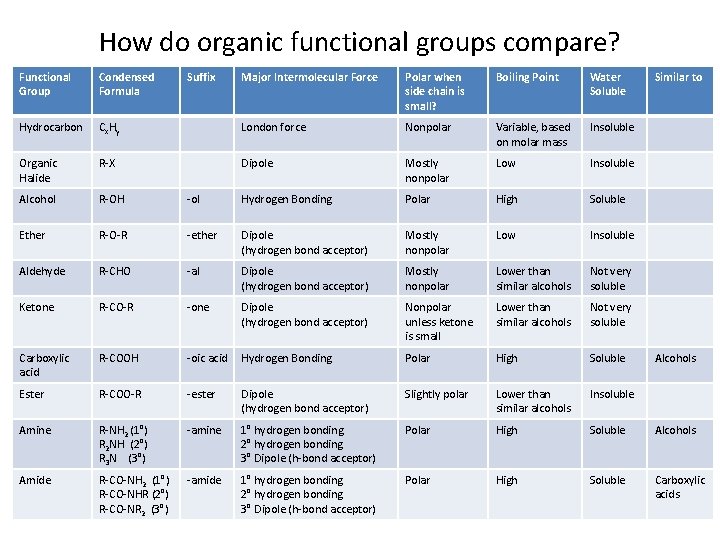
How do organic functional groups compare? Functional Group Condensed Formula Hydrocarbon Suffix Major Intermolecular Force Polar when side chain is small? Boiling Point Water Soluble Similar to Cx. Hy London force Nonpolar Variable, based on molar mass Insoluble Organic Halide R-X Dipole Mostly nonpolar Low Insoluble Alcohol R-OH -ol Hydrogen Bonding Polar High Soluble Ether R-O-R -ether Dipole (hydrogen bond acceptor) Mostly nonpolar Low Insoluble Aldehyde R-CHO -al Dipole (hydrogen bond acceptor) Mostly nonpolar Lower than similar alcohols Not very soluble Ketone R-CO-R -one Dipole (hydrogen bond acceptor) Nonpolar unless ketone is small Lower than similar alcohols Not very soluble Carboxylic acid R-COOH -oic acid Hydrogen Bonding Polar High Soluble Ester R-COO-R -ester Dipole (hydrogen bond acceptor) Slightly polar Lower than similar alcohols Insoluble Amine R-NH 2 (1°) R 2 NH (2°) R 3 N (3°) -amine 1° hydrogen bonding 2° hydrogen bonding 3° Dipole (h-bond acceptor) Polar High Soluble Alcohols Amide R-CO-NH 2 (1°) R-CO-NHR (2°) R-CO-NR 2 (3°) -amide 1° hydrogen bonding 2° hydrogen bonding 3° Dipole (h-bond acceptor) Polar High Soluble Carboxylic acids Alcohols

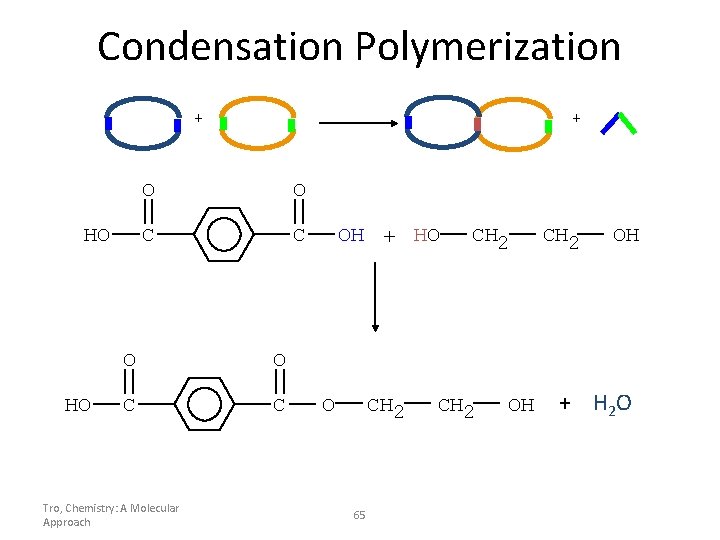
Condensation Polymerization + HO HO + O O C C Tro, Chemistry: A Molecular Approach OH + HO O CH 2 65 CH 2 OH + H 2 O

Synthesis of Polyesters

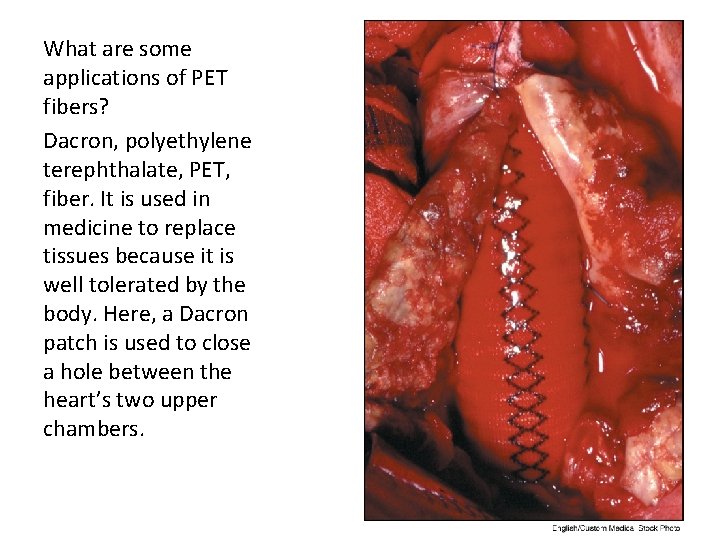
What are some applications of PET fibers? Dacron, polyethylene terephthalate, PET, fiber. It is used in medicine to replace tissues because it is well tolerated by the body. Here, a Dacron patch is used to close a hole between the heart’s two upper chambers.

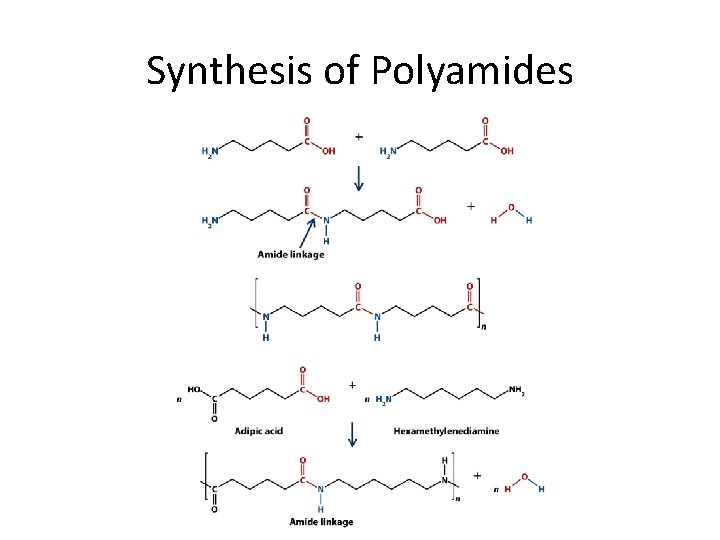
Synthesis of Polyamides

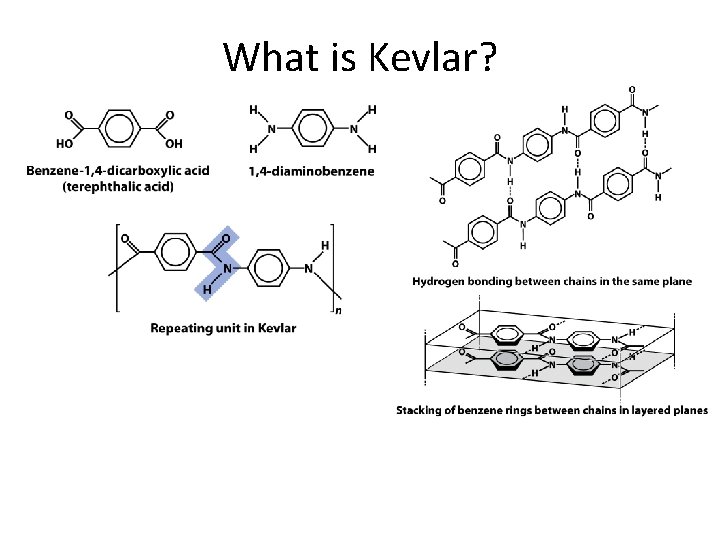
What is Kevlar?

Tro, Chemistry: A Molecular Approach 70
 Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Introduction to organic chemistry
Introduction to organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Acid chloride + grignard reagent
Acid chloride + grignard reagent Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 organic chemistry
Chapter 7 organic chemistry Entane
Entane Analytical chemistry chapter 1
Analytical chemistry chapter 1 Halohydrin
Halohydrin Numbering carbon chains
Numbering carbon chains Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry Cooh
Cooh Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Nomenclature of ethers
Nomenclature of ethers Is alkane an organic compound
Is alkane an organic compound Ario practice problems
Ario practice problems Priority of functional groups
Priority of functional groups Organic chemistry lab report example
Organic chemistry lab report example Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Organic chemistry grade 10
Organic chemistry grade 10 Cyclo organic chemistry
Cyclo organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis Meth eth prop but order
Meth eth prop but order Chemistry cracking
Chemistry cracking Organic chemistry vs biochemistry
Organic chemistry vs biochemistry Organic chemistry myanmar
Organic chemistry myanmar Electrophilic addition hbr
Electrophilic addition hbr Gc organic chemistry
Gc organic chemistry Hono organic chemistry
Hono organic chemistry Geminal and vicinal
Geminal and vicinal Topic 11 organic chemistry
Topic 11 organic chemistry Organic chemistry reaction pathways
Organic chemistry reaction pathways Organic chemistry nomenclature
Organic chemistry nomenclature What is organic chemistry like
What is organic chemistry like Organic vs inorganic molecules
Organic vs inorganic molecules Organic chemistry vocabulary
Organic chemistry vocabulary Organic chemistry laboratory ch 2540 manual
Organic chemistry laboratory ch 2540 manual A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Biological importance of fats
Biological importance of fats Leveling effect organic chemistry
Leveling effect organic chemistry Calculating percentage yield
Calculating percentage yield Polarimetry organic chemistry
Polarimetry organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry
Organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Organic chemistry
Organic chemistry Chemistry ethics case studies
Chemistry ethics case studies Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Octane lewis structure
Octane lewis structure Carbohydrates organic chemistry
Carbohydrates organic chemistry Resonance in benzyl carbocation
Resonance in benzyl carbocation Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Macromolecules cheat sheet
Macromolecules cheat sheet Mindup mind map
Mindup mind map Ethos
Ethos Ir spectroscopy
Ir spectroscopy