1 Which part of an atom is most

































- Slides: 129

1 Which part of an atom is most directly involved in chemical bonding? A nucleus B electron C proton D neutron

1 Which part of an atom is most directly involved in chemical bonding? A nucleus B electron C proton D neutron

2 Which element has the highest electronegativity? A nitrogen B iodine C fluorine D selenium

2 Which element has the highest electronegativity? A nitrogen B iodine C fluorine D selenium

3 Which set of tools will provide the most precise measurements for calculating the density of an irregularly shaped rock? A flask and beaker B flask and balance C balance and graduated cylinder D beaker and graduated cylinder

3 Which set of tools will provide the most precise measurements for calculating the density of an irregularly shaped rock? A flask and beaker B flask and balance C balance and graduated cylinder D beaker and graduated cylinder

4 Which of these is an example of a chemical change? A Methane is burned in air. B Solid gold is melted to make jewelry. C A bar of copper is stretched into a long copper wire. D Iron is coated with bronze to prevent rusting.

4 Which of these is an example of a chemical change? A Methane is burned in air. B Solid gold is melted to make jewelry. C A bar of copper is stretched into a long copper wire. D Iron is coated with bronze to prevent rusting.

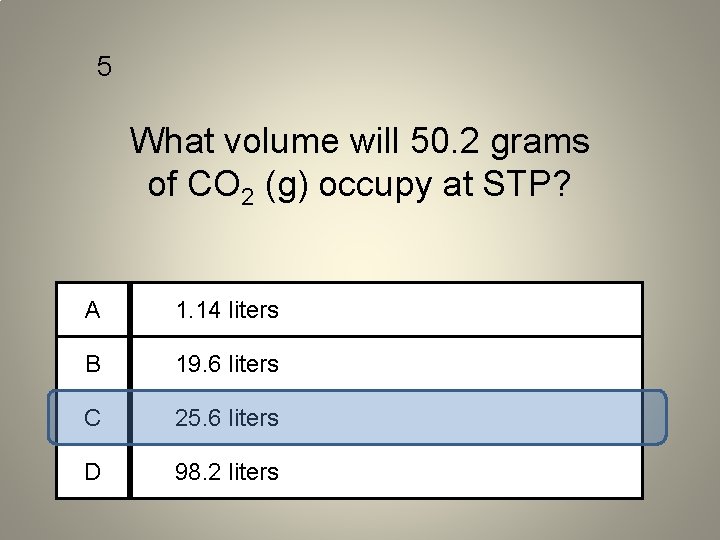
5 What volume will 50. 2 grams of CO 2 (g) occupy at STP? A 1. 14 liters B 19. 6 liters C 25. 6 liters D 98. 2 liters

5 What volume will 50. 2 grams of CO 2 (g) occupy at STP? A 1. 14 liters B 19. 6 liters C 25. 6 liters D 98. 2 liters

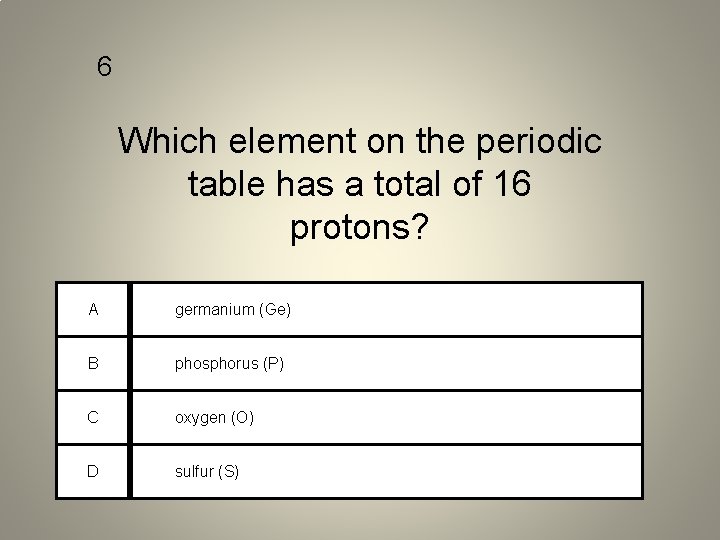
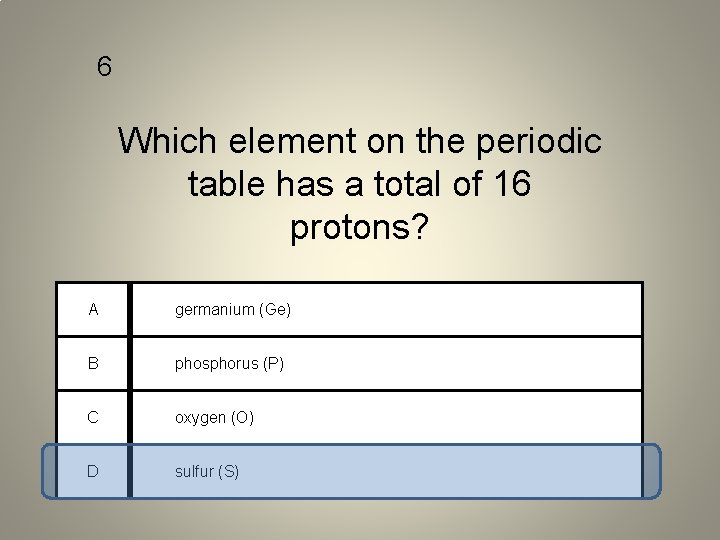
6 Which element on the periodic table has a total of 16 protons? A germanium (Ge) B phosphorus (P) C oxygen (O) D sulfur (S)

6 Which element on the periodic table has a total of 16 protons? A germanium (Ge) B phosphorus (P) C oxygen (O) D sulfur (S)

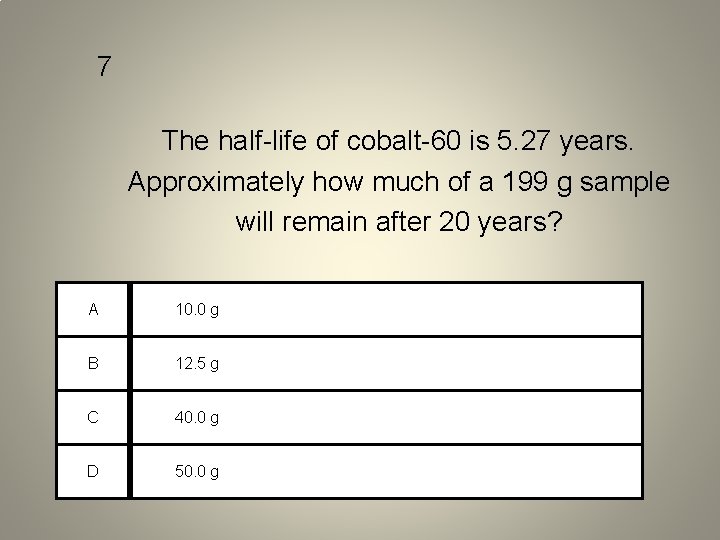
7 The half-life of cobalt-60 is 5. 27 years. Approximately how much of a 199 g sample will remain after 20 years? A 10. 0 g B 12. 5 g C 40. 0 g D 50. 0 g

7 The half-life of cobalt-60 is 5. 27 years. Approximately how much of a 199 g sample will remain after 20 years? A 10. 0 g B 12. 5 g C 40. 0 g D 50. 0 g

8 Scientist Henri Becquerel observed that some minerals, such as potassium uranyl sulfate, could release energy when placed on a photographic plate wrapped in black paper. Becquerel concluded that the potassium uranyl sulfate absorbed energy from the sun and then released the energy to expose the photographic plate. Later Becquerel proposed an alternate explanation for the same experiment: the uranium in potassium uranyl sulfate released energy without energy being absorbed from external sources. A Becquerel was considered the best scientific thinker of the time. B Becquerel’s peers reasoned that his explanation was scientifically sound C Experiments showed that a sample of the uranium could expose a photographic plate even if it was kept in a dark place. D Scientists were unable to determine the mechanism by which uranium could absorb and release solar energy.

8 Scientist Henri Becquerel observed that some minerals, such as potassium uranyl sulfate, could release energy when placed on a photographic plate wrapped in black paper. Becquerel concluded that the potassium uranyl sulfate absorbed energy from the sun and then released the energy to expose the photographic plate. Later Becquerel proposed an alternate explanation for the same experiment: the uranium in potassium uranyl sulfate released energy without energy being absorbed from external sources. A Becquerel was considered the best scientific thinker of the time. B Becquerel’s peers reasoned that his explanation was scientifically sound C Experiments showed that a sample of the uranium could expose a photographic plate even if it was kept in a dark place. D Scientists were unable to determine the mechanism by which uranium could absorb and release solar energy.

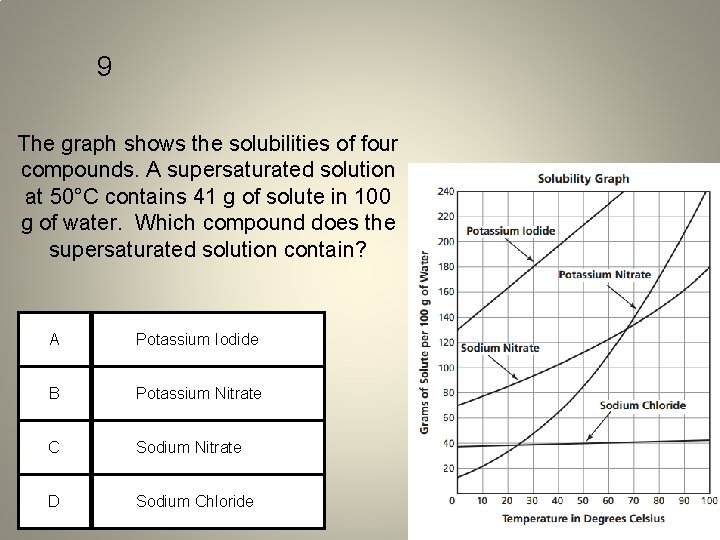
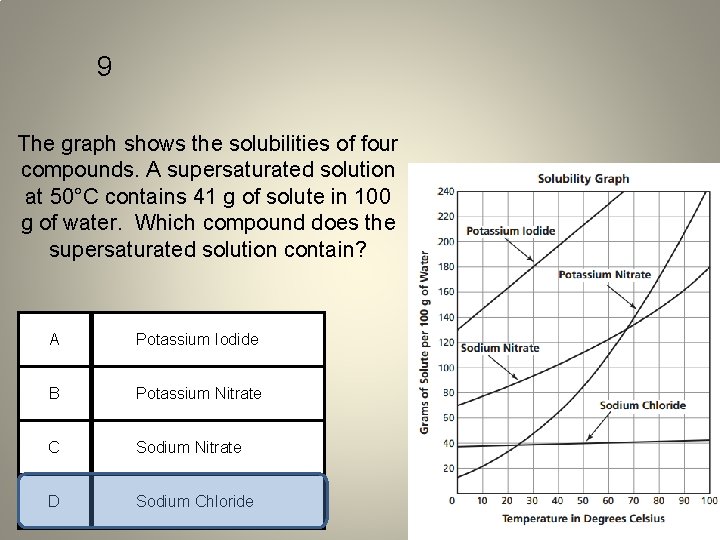
9 The graph shows the solubilities of four compounds. A supersaturated solution at 50°C contains 41 g of solute in 100 g of water. Which compound does the supersaturated solution contain? A Potassium Iodide B Potassium Nitrate C Sodium Nitrate D Sodium Chloride

9 The graph shows the solubilities of four compounds. A supersaturated solution at 50°C contains 41 g of solute in 100 g of water. Which compound does the supersaturated solution contain? A Potassium Iodide B Potassium Nitrate C Sodium Nitrate D Sodium Chloride

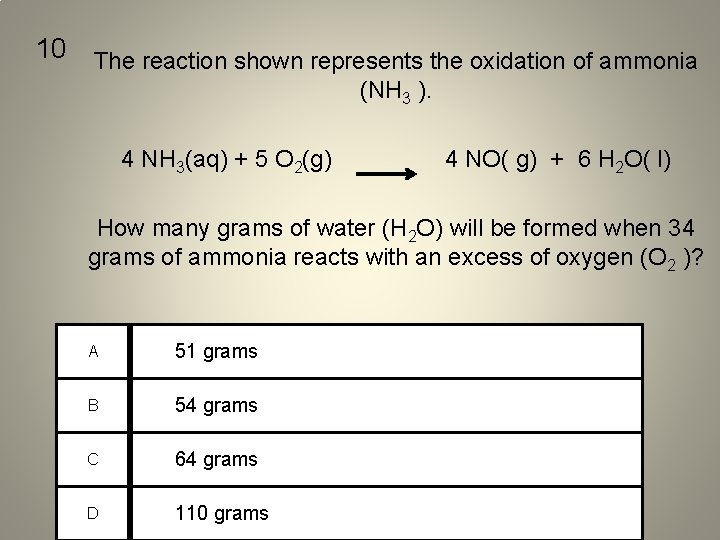
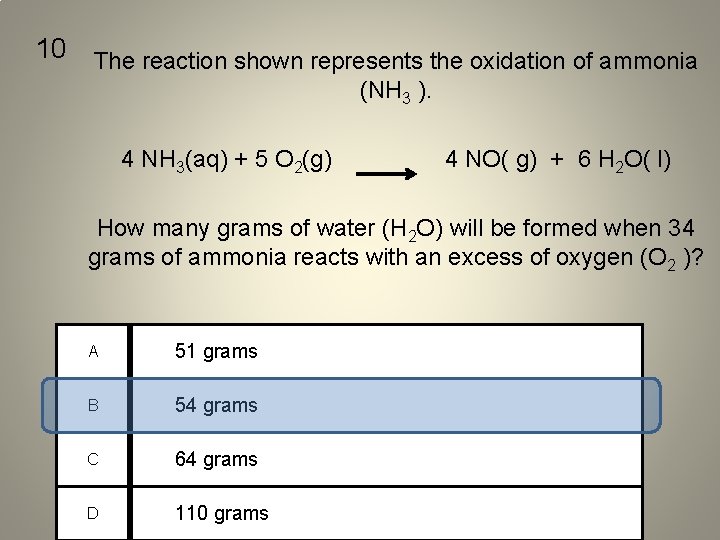
10 The reaction shown represents the oxidation of ammonia (NH 3 ). 4 NH 3(aq) + 5 O 2(g) 4 NO( g) + 6 H 2 O( l) How many grams of water (H 2 O) will be formed when 34 grams of ammonia reacts with an excess of oxygen (O 2 )? A 51 grams B 54 grams C 64 grams D 110 grams

10 The reaction shown represents the oxidation of ammonia (NH 3 ). 4 NH 3(aq) + 5 O 2(g) 4 NO( g) + 6 H 2 O( l) How many grams of water (H 2 O) will be formed when 34 grams of ammonia reacts with an excess of oxygen (O 2 )? A 51 grams B 54 grams C 64 grams D 110 grams

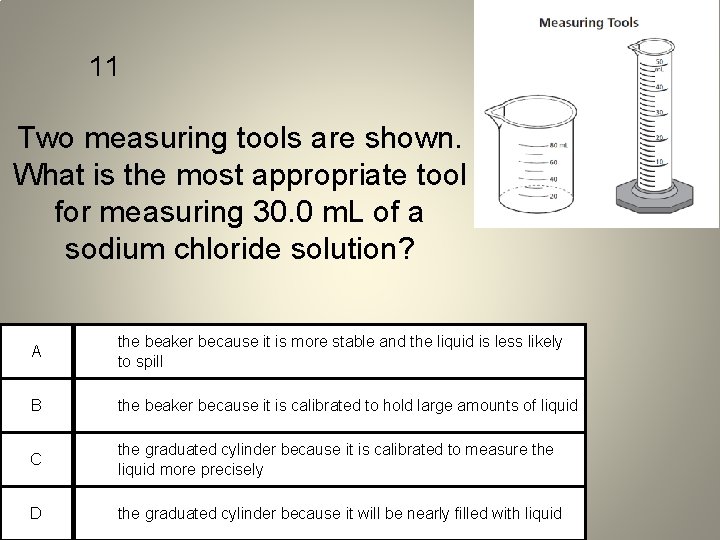
11 Two measuring tools are shown. What is the most appropriate tool for measuring 30. 0 m. L of a sodium chloride solution? A the beaker because it is more stable and the liquid is less likely to spill B the beaker because it is calibrated to hold large amounts of liquid C the graduated cylinder because it is calibrated to measure the liquid more precisely D the graduated cylinder because it will be nearly filled with liquid

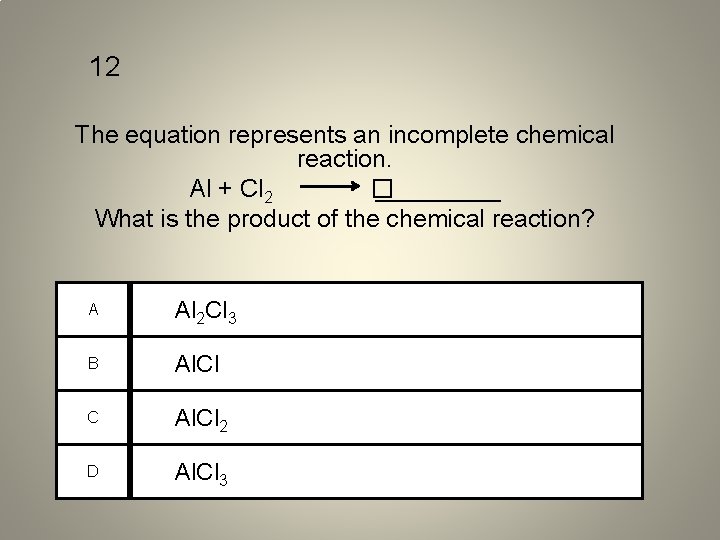
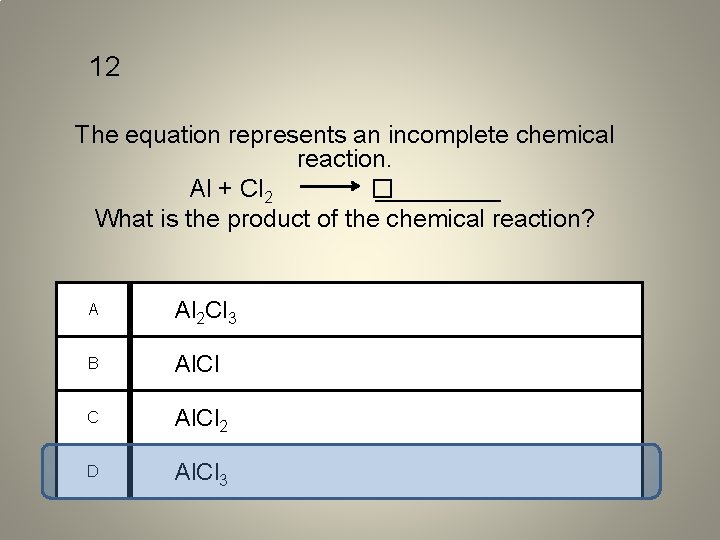
12 The equation represents an incomplete chemical reaction. Al + Cl 2 � _____ What is the product of the chemical reaction? A Al 2 Cl 3 B Al. Cl C Al. Cl 2 D Al. Cl 3

12 The equation represents an incomplete chemical reaction. Al + Cl 2 � _____ What is the product of the chemical reaction? A Al 2 Cl 3 B Al. Cl C Al. Cl 2 D Al. Cl 3

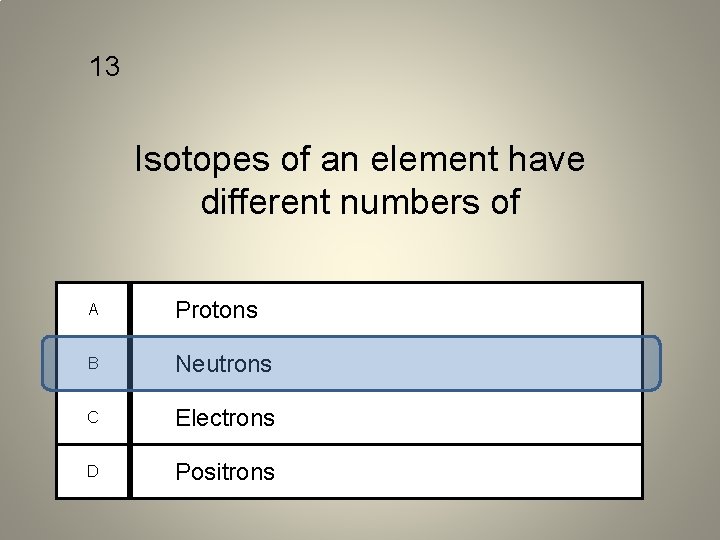
13 Isotopes of an element have different numbers of A Protons B Neutrons C Electrons D Positrons

13 Isotopes of an element have different numbers of A Protons B Neutrons C Electrons D Positrons

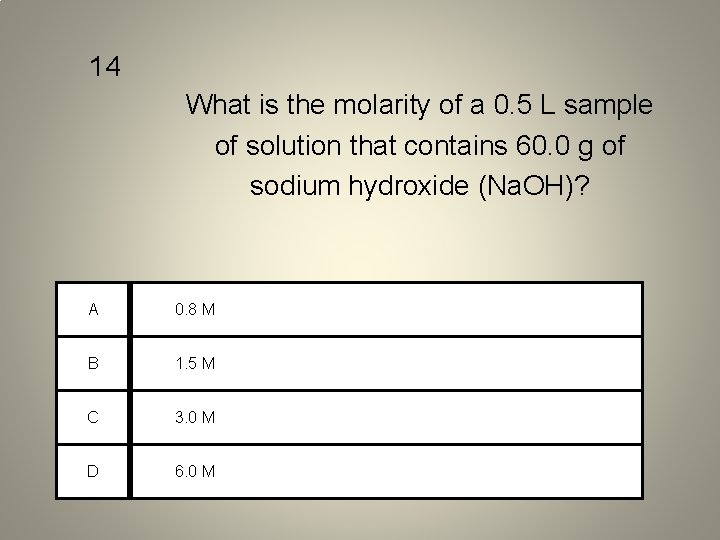
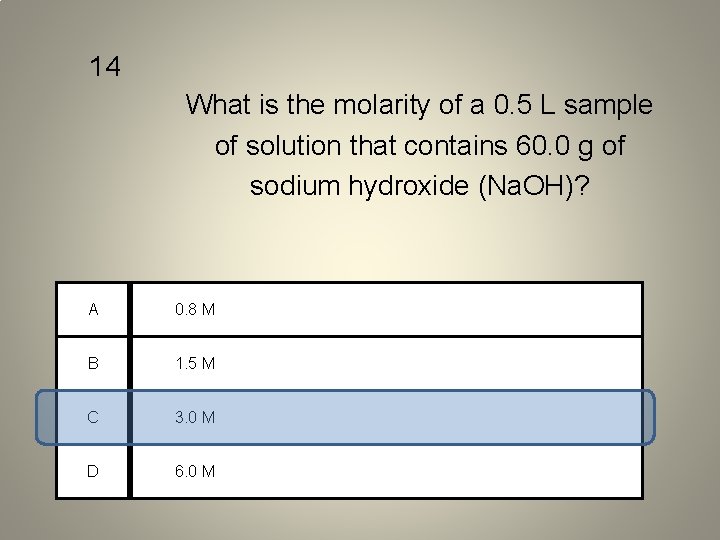
14 What is the molarity of a 0. 5 L sample of solution that contains 60. 0 g of sodium hydroxide (Na. OH)? A 0. 8 M B 1. 5 M C 3. 0 M D 6. 0 M

14 What is the molarity of a 0. 5 L sample of solution that contains 60. 0 g of sodium hydroxide (Na. OH)? A 0. 8 M B 1. 5 M C 3. 0 M D 6. 0 M

15 Which element has the highest electronegativity? A Berrylium (Be) B Fluorine (F) C Silver (Ag) D Silicon (Si)

15 Which element has the highest electronegativity? A Berrylium (Be) B Fluorine (F) C Silver (Ag) D Silicon (Si)

16 Air pollution from automobile exhaust is minimized by using electric cars powered by lead-acid batteries. What will be a negative effect of using lead-acid batteries? A Toxic metal in the batteries will enter the environment. B Sulfuric acid in the batteries will generate electricity. C Oxygen and hydrogen will be produced by the batteries. D Dense and malleable metal will be used in the batteries.

16 Air pollution from automobile exhaust is minimized by using electric cars powered by lead-acid batteries. What will be a negative effect of using lead-acid batteries? A Toxic metal in the batteries will enter the environment. B Sulfuric acid in the batteries will generate electricity. C Oxygen and hydrogen will be produced by the batteries. D Dense and malleable metal will be used in the batteries.

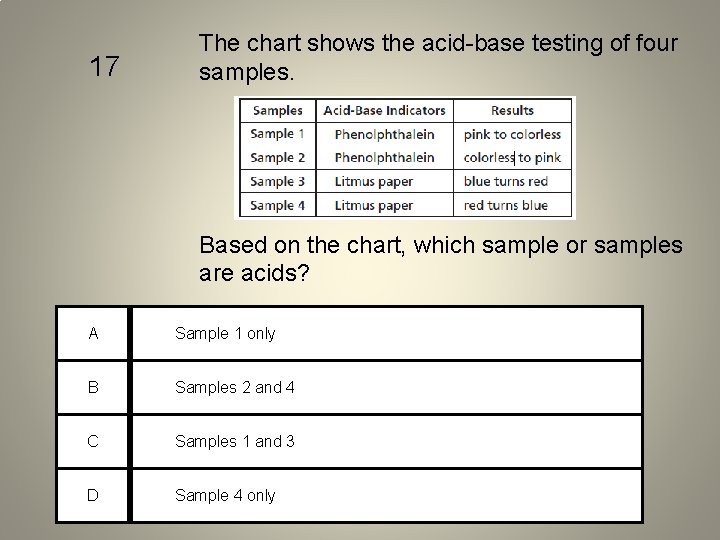
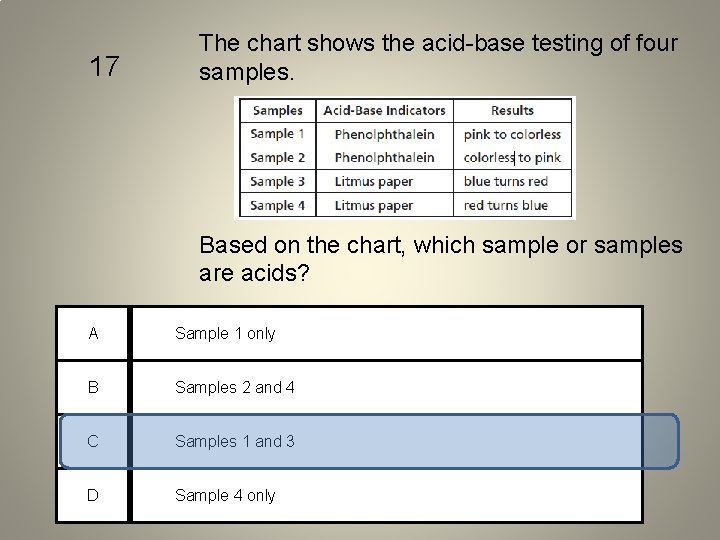
17 The chart shows the acid-base testing of four samples. Based on the chart, which sample or samples are acids? A Sample 1 only B Samples 2 and 4 C Samples 1 and 3 D Sample 4 only

17 The chart shows the acid-base testing of four samples. Based on the chart, which sample or samples are acids? A Sample 1 only B Samples 2 and 4 C Samples 1 and 3 D Sample 4 only

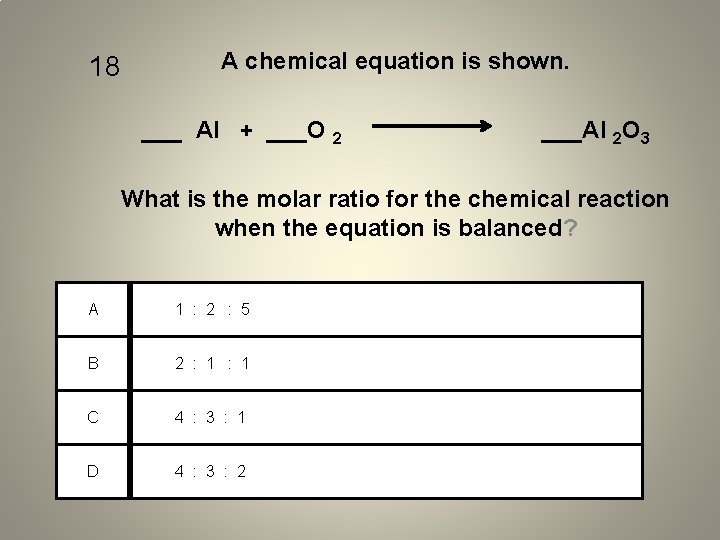
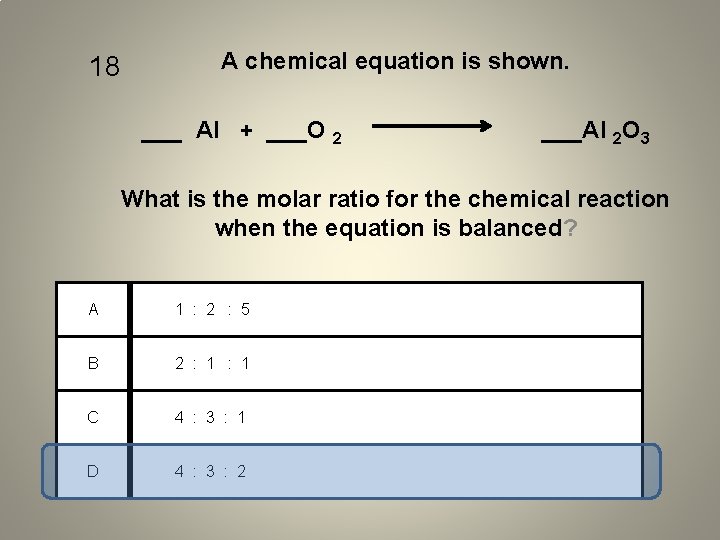
18 A chemical equation is shown. ___ Al + ___O 2 ___Al 2 O 3 What is the molar ratio for the chemical reaction when the equation is balanced? A 1 : 2 : 5 B 2 : 1 C 4 : 3 : 1 D 4 : 3 : 2

18 A chemical equation is shown. ___ Al + ___O 2 ___Al 2 O 3 What is the molar ratio for the chemical reaction when the equation is balanced? A 1 : 2 : 5 B 2 : 1 C 4 : 3 : 1 D 4 : 3 : 2

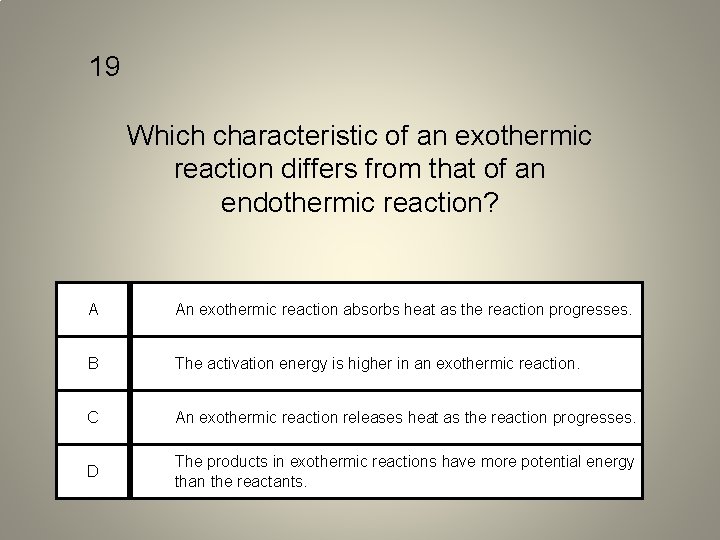
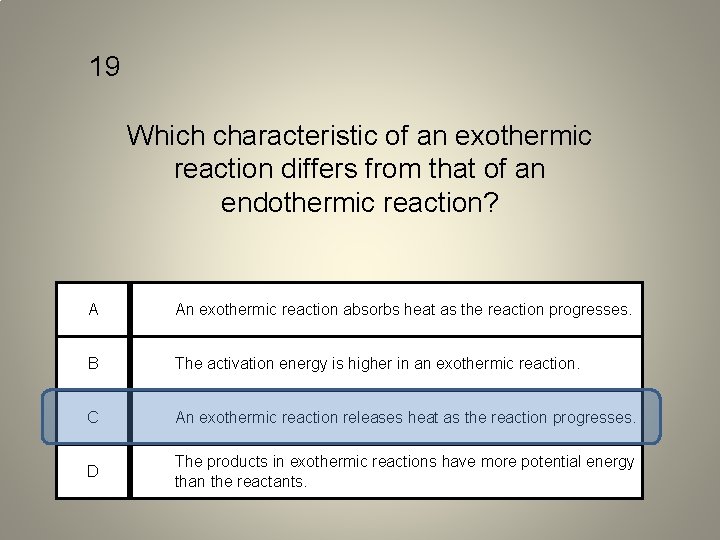
19 Which characteristic of an exothermic reaction differs from that of an endothermic reaction? A An exothermic reaction absorbs heat as the reaction progresses. B The activation energy is higher in an exothermic reaction. C An exothermic reaction releases heat as the reaction progresses. D The products in exothermic reactions have more potential energy than the reactants.

19 Which characteristic of an exothermic reaction differs from that of an endothermic reaction? A An exothermic reaction absorbs heat as the reaction progresses. B The activation energy is higher in an exothermic reaction. C An exothermic reaction releases heat as the reaction progresses. D The products in exothermic reactions have more potential energy than the reactants.

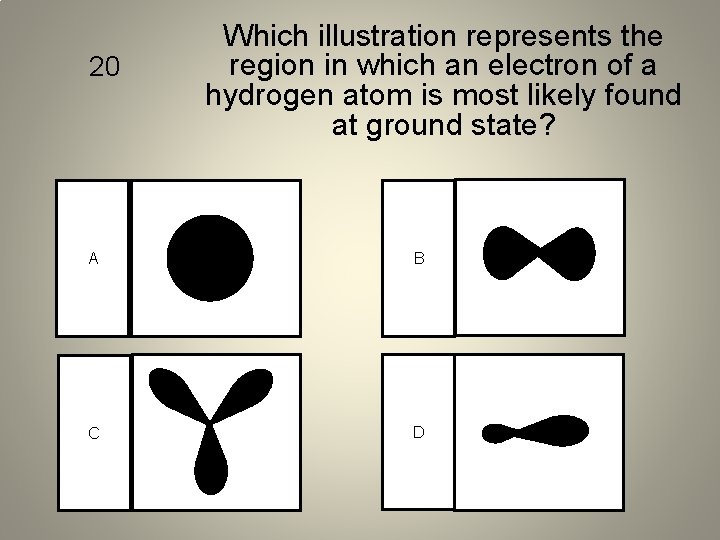
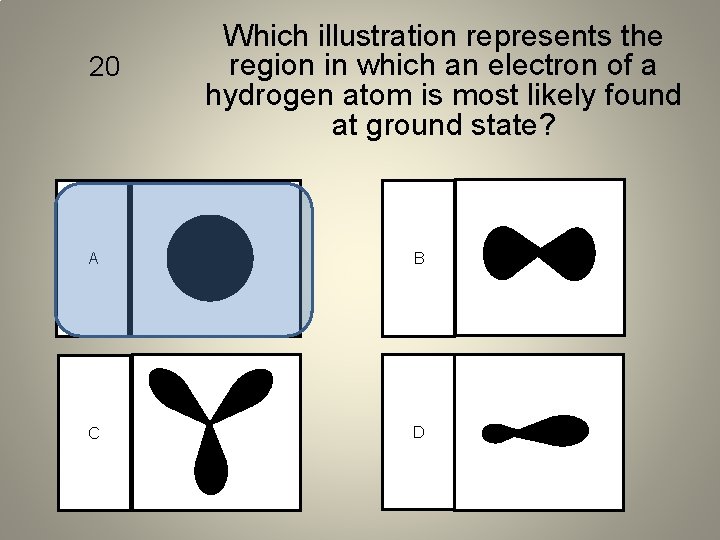
20 Which illustration represents the region in which an electron of a hydrogen atom is most likely found at ground state? A B C D

20 Which illustration represents the region in which an electron of a hydrogen atom is most likely found at ground state? A B C D

21 Magnesium (Mg) and oxygen( O 2) react to form magnesium oxide (Mg. O). 2 Mg (s) + O 2 2 Mg. O (s) Which type of chemical reaction does the equation represent? A decomposition B double replacement C single replacement D composition

21 Magnesium (Mg) and oxygen( O 2) react to form magnesium oxide (Mg. O). 2 Mg (s) + O 2 2 Mg. O (s) Which type of chemical reaction does the equation represent? A decomposition B double replacement C single replacement D composition

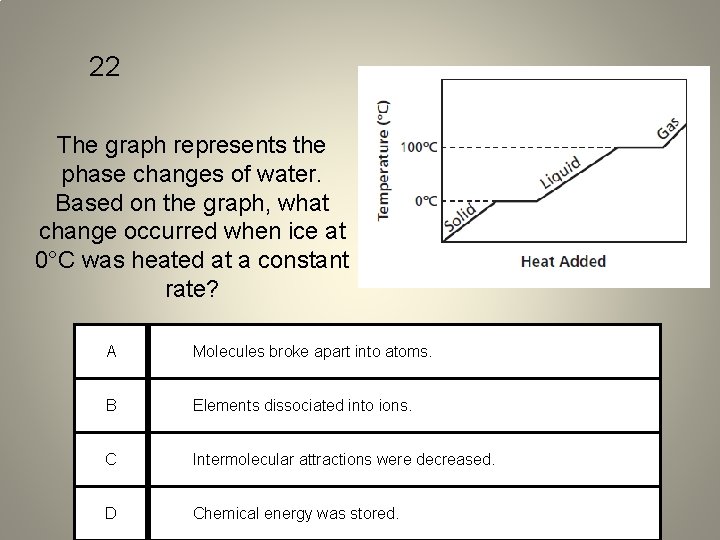
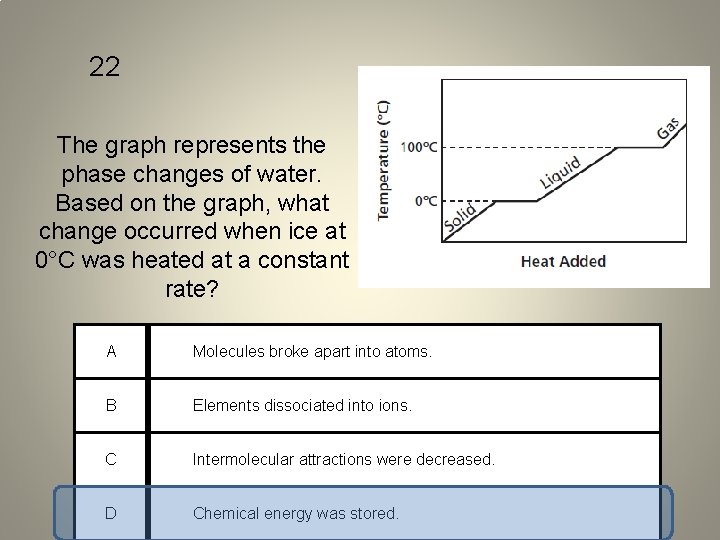
22 The graph represents the phase changes of water. Based on the graph, what change occurred when ice at 0°C was heated at a constant rate? A Molecules broke apart into atoms. B Elements dissociated into ions. C Intermolecular attractions were decreased. D Chemical energy was stored.

22 The graph represents the phase changes of water. Based on the graph, what change occurred when ice at 0°C was heated at a constant rate? A Molecules broke apart into atoms. B Elements dissociated into ions. C Intermolecular attractions were decreased. D Chemical energy was stored.

23 A 2135 cm 3 sample of dry air has a pressure of 98. 4 k. Pa at 127°C. What is the volume of the sample if the temperature is increased to 206°C when the pressure is kept constant? A 1320 cm 3 B 1780 cm 3 C 2560 cm 3 D 3460 cm 3

23 A 2135 cm 3 sample of dry air has a pressure of 98. 4 k. Pa at 127°C. What is the volume of the sample if the temperature is increased to 206°C when the pressure is kept constant? A 1320 cm 3 B 1780 cm 3 C 2560 cm 3 D 3460 cm 3

24 Which property do the liquid phase and the gas phase of a substance have in common? A Both phases are highly compressible. B Both phases lack a definite shape. C Both phases have high densities. D Both phases have equal kinetic energy.

24 Which property do the liquid phase and the gas phase of a substance have in common? A Both phases are highly compressible. B Both phases lack a definite shape. C Both phases have high densities. D Both phases have equal kinetic energy.

25 What is the percent composition by mass of sulfur in ammonium sulfate, (NH 4)2 SO 4? A 6. 7 % B 24 % C 28 % D 32 %

25 What is the percent composition by mass of sulfur in ammonium sulfate, (NH 4)2 SO 4? A 6. 7 % B 24 % C 28 % D 32 %

26 Anhydrous copper (II) sulfate is a gray-white powder. When a student heated 250 g of bright blue copper(II) sulfate pentahydrate (Cu. S 04 • 5 H 20 ) in a test tube, the sample crumbled and turned gray white. The sample also lost 90 g. Based on this information, what is the most acceptable conclusion? A The color of the sample changed when copper reacted with sulfate ions. B The mass of the sample decreased after the copper(II) sulfate evaporated. C The sample became dehydrated when it was heated. D The sample went from the solid phase to the gaseous phase.

26 Anhydrous copper (II) sulfate is a gray-white powder. When a student heated 250 g of bright blue copper(II) sulfate pentahydrate (Cu. S 04 • 5 H 20 ) in a test tube, the sample crumbled and turned gray white. The sample also lost 90 g. Based on this information, what is the most acceptable conclusion? A The color of the sample changed when copper reacted with sulfate ions. B The mass of the sample decreased after the copper(II) sulfate evaporated. C The sample became dehydrated when it was heated. D The sample went from the solid phase to the gaseous phase.

27 Which of these is a base? A Li. OH B Ba. Cl 2 C KI D KNO 3

27 Which of these is a base? A Li. OH B Ba. Cl 2 C KI D KNO 3

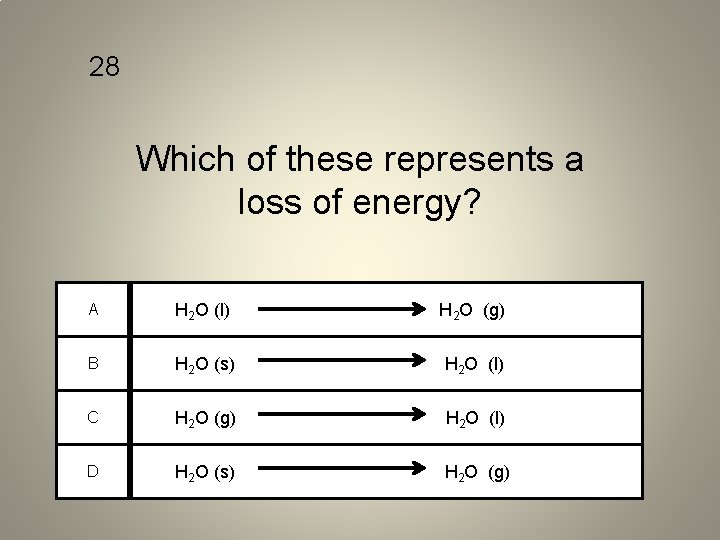
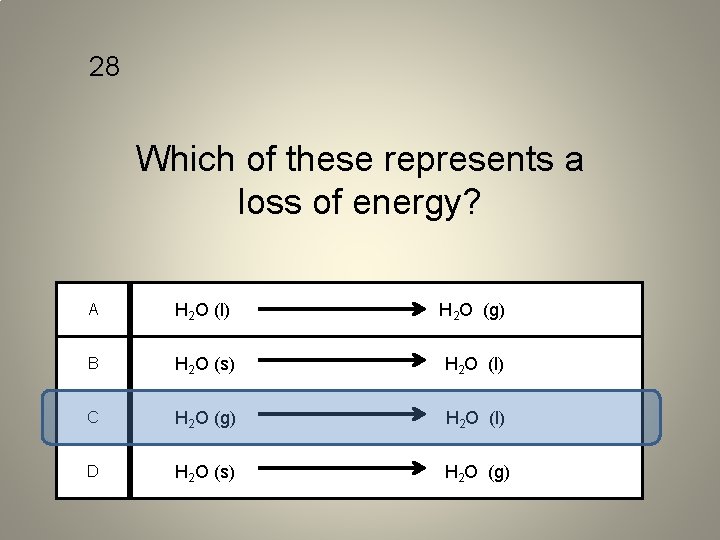
28 Which of these represents a loss of energy? A H 2 O (l) H 2 O (g) B H 2 O (s) H 2 O (l) C H 2 O (g) H 2 O (l) D H 2 O (s) H 2 O (g)

28 Which of these represents a loss of energy? A H 2 O (l) H 2 O (g) B H 2 O (s) H 2 O (l) C H 2 O (g) H 2 O (l) D H 2 O (s) H 2 O (g)

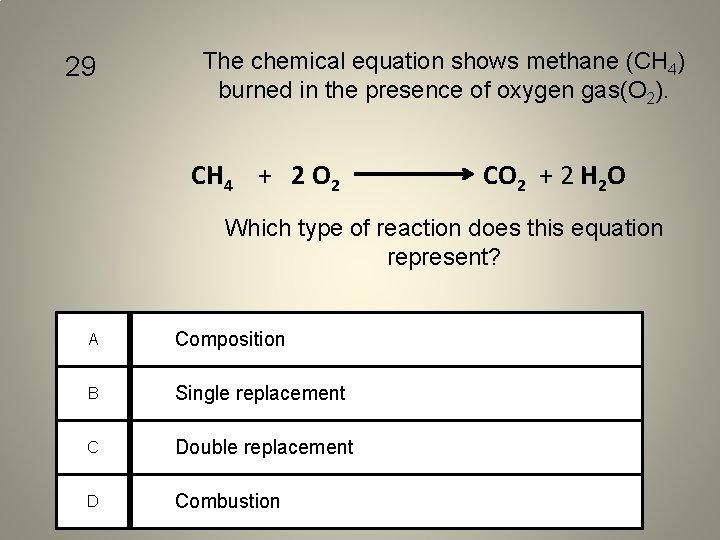
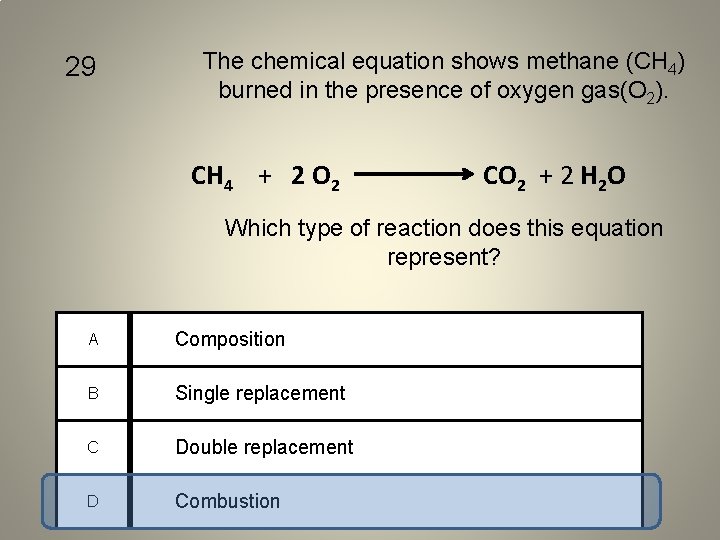
29 The chemical equation shows methane (CH 4) burned in the presence of oxygen gas(O 2). CH 4 + 2 O 2 CO 2 + 2 H 2 O Which type of reaction does this equation represent? A Composition B Single replacement C Double replacement D Combustion

29 The chemical equation shows methane (CH 4) burned in the presence of oxygen gas(O 2). CH 4 + 2 O 2 CO 2 + 2 H 2 O Which type of reaction does this equation represent? A Composition B Single replacement C Double replacement D Combustion

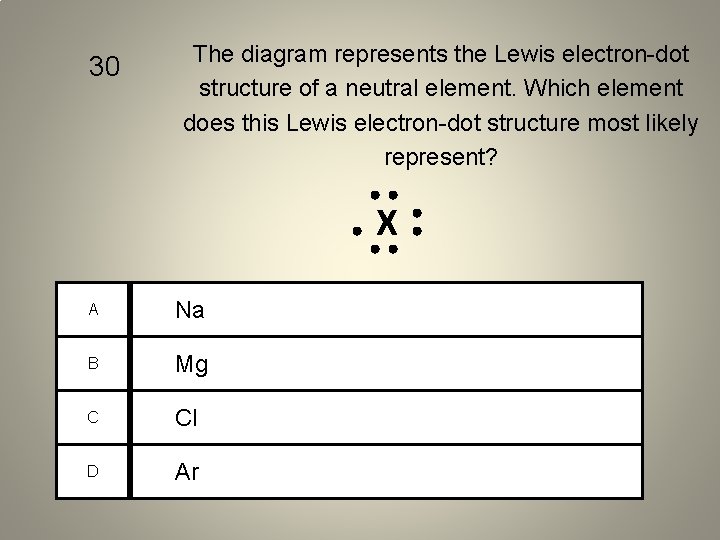
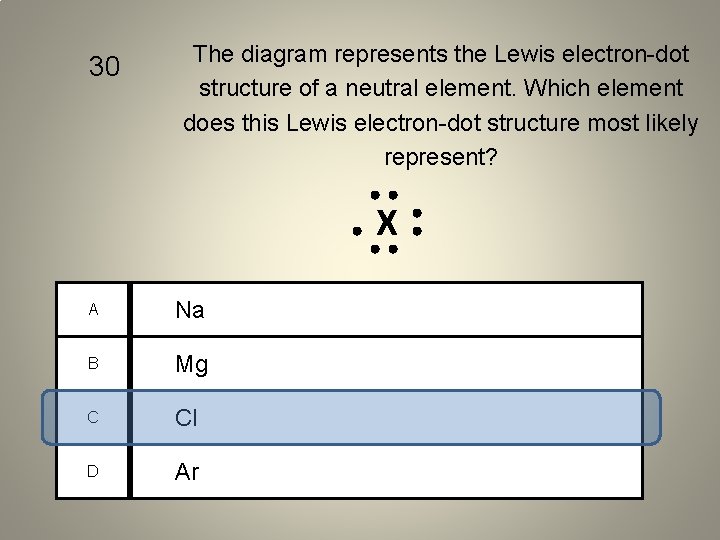
30 The diagram represents the Lewis electron-dot structure of a neutral element. Which element does this Lewis electron-dot structure most likely represent? X A Na B Mg C Cl D Ar

30 The diagram represents the Lewis electron-dot structure of a neutral element. Which element does this Lewis electron-dot structure most likely represent? X A Na B Mg C Cl D Ar

31 A student fills a flask with 5. 0 moles of nitrogen gas and then seals the flask. Which change will happen when the student warms the flask? A The temperature of the nitrogen gas will decrease. B The pressure inside the flask will increase. C The volume inside the flask will decrease. D The molar mass of the nitrogen gas will increase.

31 A student fills a flask with 5. 0 moles of nitrogen gas and then seals the flask. Which change will happen when the student warms the flask? A The temperature of the nitrogen gas will decrease. B The pressure inside the flask will increase. C The volume inside the flask will decrease. D The molar mass of the nitrogen gas will increase.

32 Which characteristic is more similar in liquids and solids as compared to gases? A the masses of particles B the distance between particles C the degree to which particles are organized D the strength of chemical bonds within particles

32 Which characteristic is more similar in liquids and solids as compared to gases? A the masses of particles B the distance between particles C the degree to which particles are organized D the strength of chemical bonds within particles

33 Which statement best describes the Bohr model of the atom? A Electrons are arranged in energy clouds. B Electrons have properties similar to waves. C Electrons orbit around the nucleus in set paths. D Electrons make up most of the mass of an atom.

33 Which statement best describes the Bohr model of the atom? A Electrons are arranged in energy clouds. B Electrons have properties similar to waves. C Electrons orbit around the nucleus in set paths. D Electrons make up most of the mass of an atom.

34 What is the approximate percent composition of phosphorus in phosphoric acid (H 3 PO 4)? A 29. 5% B 31. 6% C 65. 3% D 90. 0%

34 What is the approximate percent composition of phosphorus in phosphoric acid (H 3 PO 4)? A 29. 5% B 31. 6% C 65. 3% D 90. 0%

35 Which of these is an example of a homogeneous mixture? A a bowl of noodle soup B a container of water and sand C a glass of salt water D a bottle of oil and vinegar

35 Which of these is an example of a homogeneous mixture? A a bowl of noodle soup B a container of water and sand C a glass of salt water D a bottle of oil and vinegar

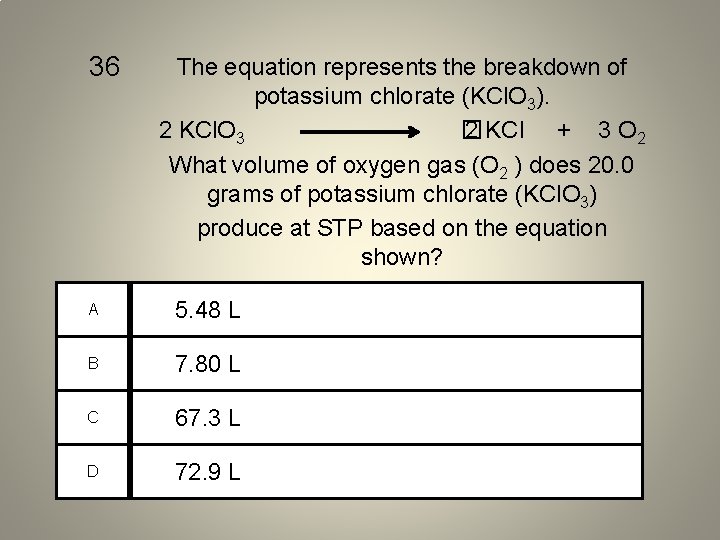
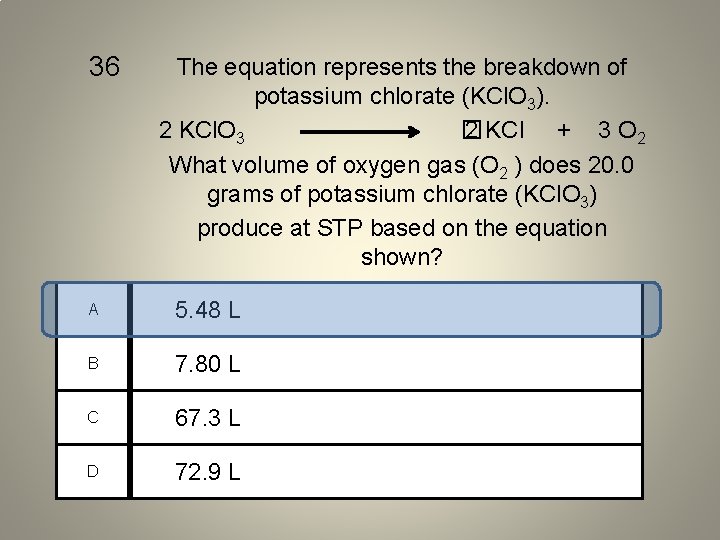
36 The equation represents the breakdown of potassium chlorate (KCl. O 3). 2 KCl. O 3 � 2 KCl + 3 O 2 What volume of oxygen gas (O 2 ) does 20. 0 grams of potassium chlorate (KCl. O 3) produce at STP based on the equation shown? A 5. 48 L B 7. 80 L C 67. 3 L D 72. 9 L

36 The equation represents the breakdown of potassium chlorate (KCl. O 3). 2 KCl. O 3 � 2 KCl + 3 O 2 What volume of oxygen gas (O 2 ) does 20. 0 grams of potassium chlorate (KCl. O 3) produce at STP based on the equation shown? A 5. 48 L B 7. 80 L C 67. 3 L D 72. 9 L

37 A student sets up an experiment to investigate the effect of temperature on the volume of 50 grams of gas inside a balloon. Which statement correctly describes the design of the experiment? A The temperature is an experimental control, and the volume is the independent variable. B The volume is an experimental control, and the temperature is the dependent variable. C The mass of the gas is an experimental control, and the temperature is the independent variable. D The temperature is an experimental control, and the mass of the gas is the dependent variable.

37 A student sets up an experiment to investigate the effect of temperature on the volume of 50 grams of gas inside a balloon. Which statement correctly describes the design of the experiment? A The temperature is an experimental control, and the volume is the independent variable. B The volume is an experimental control, and the temperature is the dependent variable. C The mass of the gas is an experimental control, and the temperature is the independent variable. D The temperature is an experimental control, and the mass of the gas is the dependent variable.

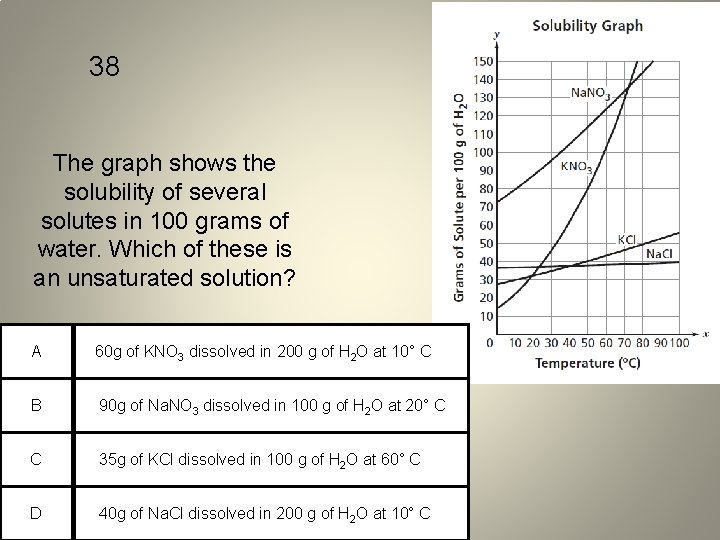
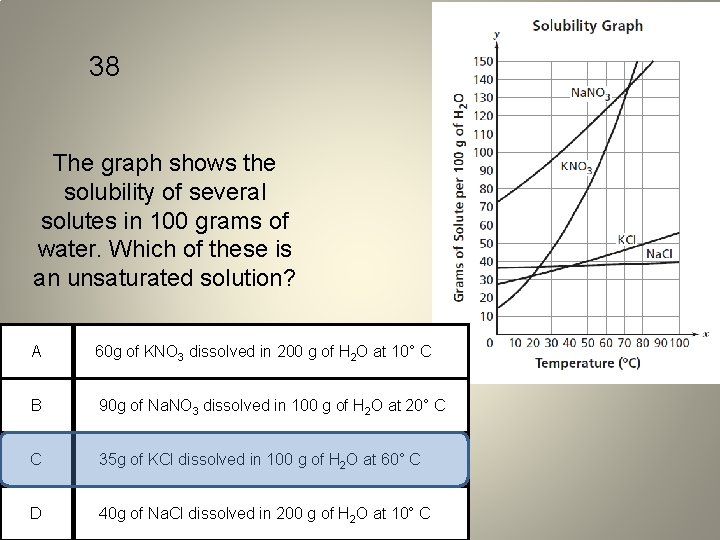
38 The graph shows the solubility of several solutes in 100 grams of water. Which of these is an unsaturated solution? A 60 g of KNO 3 dissolved in 200 g of H 2 O at 10° C B 90 g of Na. NO 3 dissolved in 100 g of H 2 O at 20° C C 35 g of KCl dissolved in 100 g of H 2 O at 60° C D 40 g of Na. Cl dissolved in 200 g of H 2 O at 10° C

38 The graph shows the solubility of several solutes in 100 grams of water. Which of these is an unsaturated solution? A 60 g of KNO 3 dissolved in 200 g of H 2 O at 10° C B 90 g of Na. NO 3 dissolved in 100 g of H 2 O at 20° C C 35 g of KCl dissolved in 100 g of H 2 O at 60° C D 40 g of Na. Cl dissolved in 200 g of H 2 O at 10° C

39 An engine cylinder contains 250 m. L of gas at a pressure of 1. 0 atm. As the engine runs, it compresses the cylinder, reducing the volume of the gas to 25 m. L. What is the new pressure of the gas at this volume? A 0. 10 atm B 10. 0 atm C 25 atm D 250 atm

39 An engine cylinder contains 250 m. L of gas at a pressure of 1. 0 atm. As the engine runs, it compresses the cylinder, reducing the volume of the gas to 25 m. L. What is the new pressure of the gas at this volume? A 0. 10 atm B 10. 0 atm C 25 atm D 250 atm

40 What is the approximate mass of 1. 50 moles of ammonia (NH 3)? A 10. 0 grams B 15. 0 grams C 17. 0 grams D 25. 5 grams

40 What is the approximate mass of 1. 50 moles of ammonia (NH 3)? A 10. 0 grams B 15. 0 grams C 17. 0 grams D 25. 5 grams

41 What is the total number of electrons in all s orbitals of a neutral atom of phosphorus? A 2 B 4 C 6 D 8

41 What is the total number of electrons in all s orbitals of a neutral atom of phosphorus? A 2 B 4 C 6 D 8

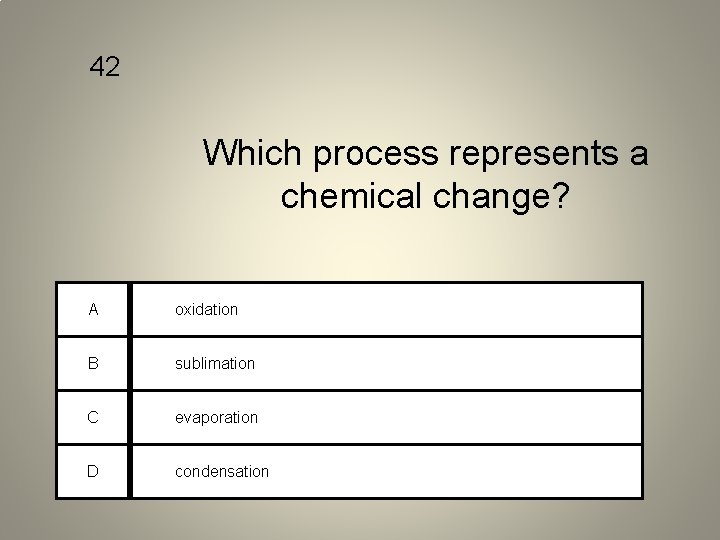
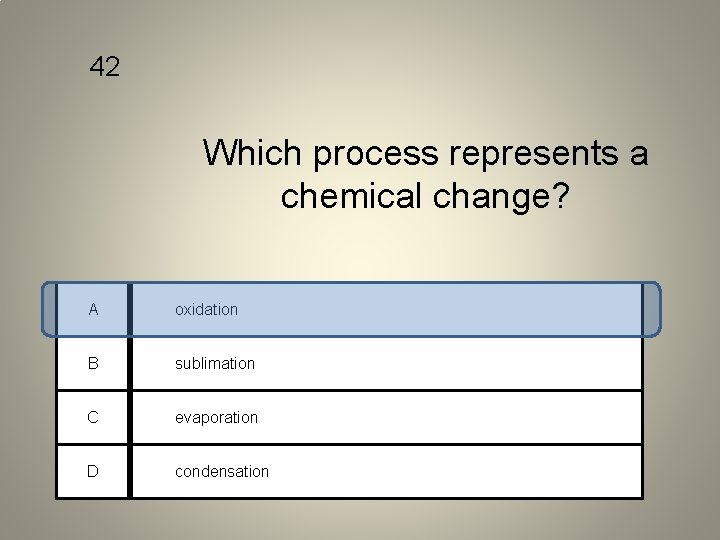
42 Which process represents a chemical change? A oxidation B sublimation C evaporation D condensation

42 Which process represents a chemical change? A oxidation B sublimation C evaporation D condensation

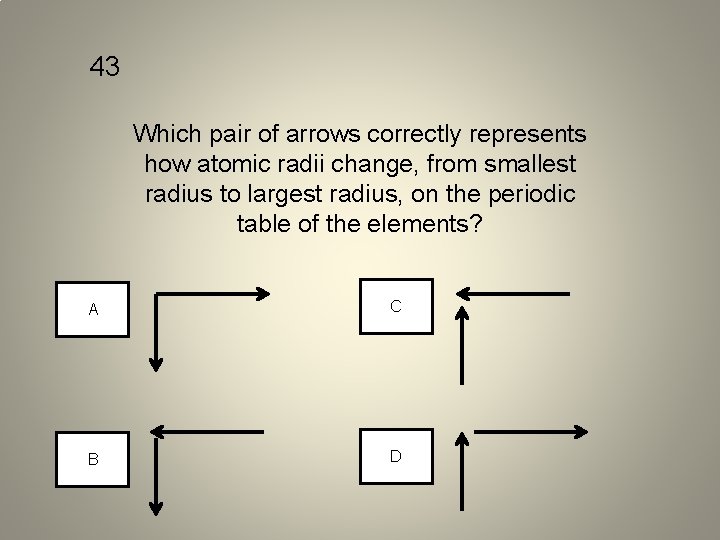
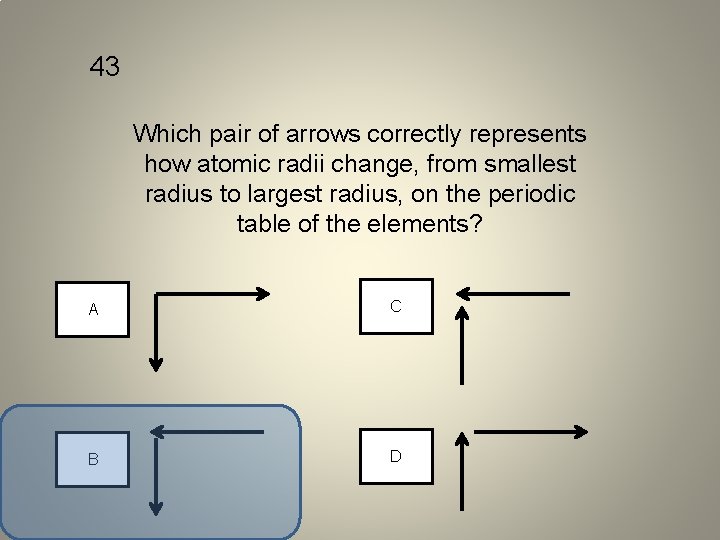
43 Which pair of arrows correctly represents how atomic radii change, from smallest radius to largest radius, on the periodic table of the elements? A C B D

43 Which pair of arrows correctly represents how atomic radii change, from smallest radius to largest radius, on the periodic table of the elements? A C B D

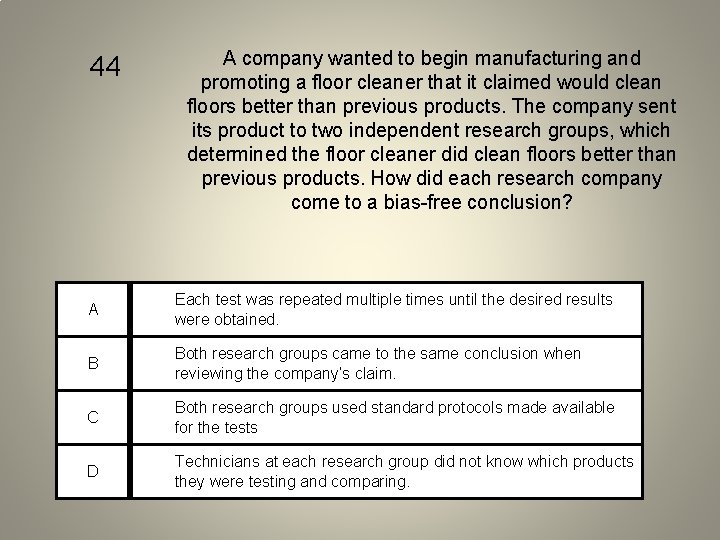
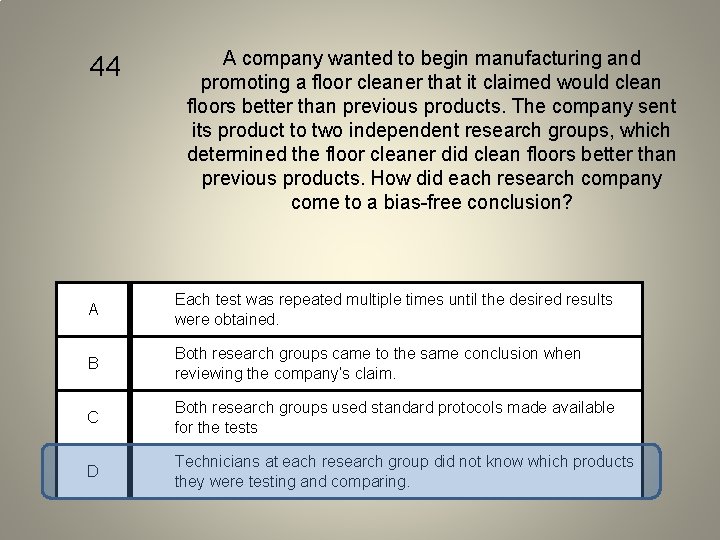
44 A company wanted to begin manufacturing and promoting a floor cleaner that it claimed would clean floors better than previous products. The company sent its product to two independent research groups, which determined the floor cleaner did clean floors better than previous products. How did each research company come to a bias-free conclusion? A Each test was repeated multiple times until the desired results were obtained. B Both research groups came to the same conclusion when reviewing the company’s claim. C Both research groups used standard protocols made available for the tests D Technicians at each research group did not know which products they were testing and comparing.

44 A company wanted to begin manufacturing and promoting a floor cleaner that it claimed would clean floors better than previous products. The company sent its product to two independent research groups, which determined the floor cleaner did clean floors better than previous products. How did each research company come to a bias-free conclusion? A Each test was repeated multiple times until the desired results were obtained. B Both research groups came to the same conclusion when reviewing the company’s claim. C Both research groups used standard protocols made available for the tests D Technicians at each research group did not know which products they were testing and comparing.

45 What are the products of the decomposition of mercury(II) oxide (Hg. O)? A Hg 2 and O 2 B Hg 2 and O C Hg and O 2 D Hg. O and O 2

45 What are the products of the decomposition of mercury(II) oxide (Hg. O)? A Hg 2 and O 2 B Hg 2 and O C Hg and O 2 D Hg. O and O 2

46 How did the quantum mechanical model of the atom improve on Bohr’s atomic model? A by showing that most of the atom is empty space B by establishing the probability clouds of the electrons C by predicting the particle nature of the electrons D by measuring the absorption spectra of discrete orbits

46 How did the quantum mechanical model of the atom improve on Bohr’s atomic model? A by showing that most of the atom is empty space B by establishing the probability clouds of the electrons C by predicting the particle nature of the electrons D by measuring the absorption spectra of discrete orbits

47 Early in the history of the periodic table, a scientist observed the relationships between element characteristics and atomic mass. The scientist used this information to predict the existence of unknown elements with specific atomic masses and characteristics. How was this information applied by modern scientists? A Scientists were able to predict the properties of new elements. B Scientists were able to change the atomic makeup of elements. C Scientists were able to make computer parts because of the unpredictable properties of silicon D Scientists were able to create new elements with unpredictable physical and chemical properties.

47 Early in the history of the periodic table, a scientist observed the relationships between element characteristics and atomic mass. The scientist used this information to predict the existence of unknown elements with specific atomic masses and characteristics. How was this information applied by modern scientists? A Scientists were able to predict the properties of new elements. B Scientists were able to change the atomic makeup of elements. C Scientists were able to make computer parts because of the unpredictable properties of silicon D Scientists were able to create new elements with unpredictable physical and chemical properties.

48 What is the molarity of a 2. 0 -liter solution containing 58 grams of Na. Cl? A 0. 50 M B 1. 0 M C 2. 0 M D 3. 2 M

48 What is the molarity of a 2. 0 -liter solution containing 58 grams of Na. Cl? A 0. 50 M B 1. 0 M C 2. 0 M D 3. 2 M

49 The electron configuration of a neutral atom of calcium is shown. 1 s 22 p 63 s 23 p 64 s 2 How many valence electrons are in the atom? A 2 B 4 C 8 D 20

49 The electron configuration of a neutral atom of calcium is shown. 1 s 22 p 63 s 23 p 64 s 2 How many valence electrons are in the atom? A 2 B 4 C 8 D 20

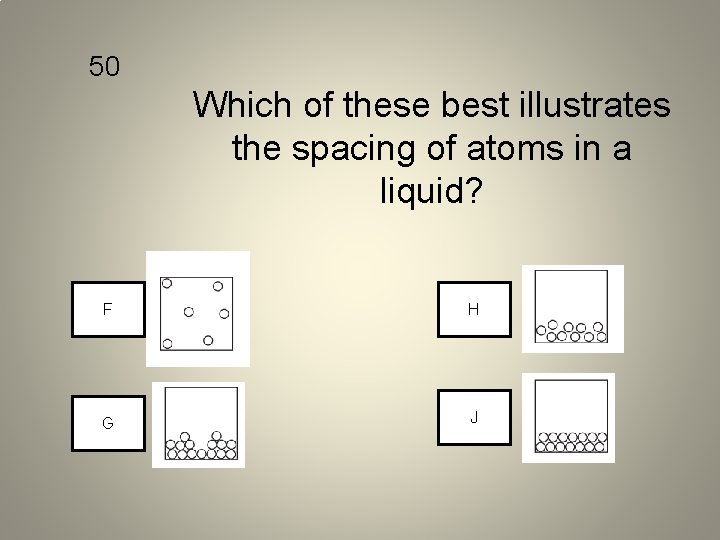
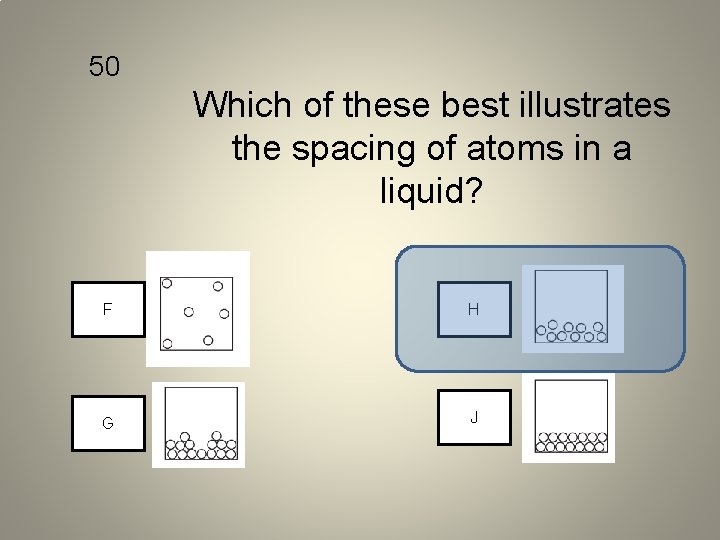
50 Which of these best illustrates the spacing of atoms in a liquid? F H G J

50 Which of these best illustrates the spacing of atoms in a liquid? F H G J

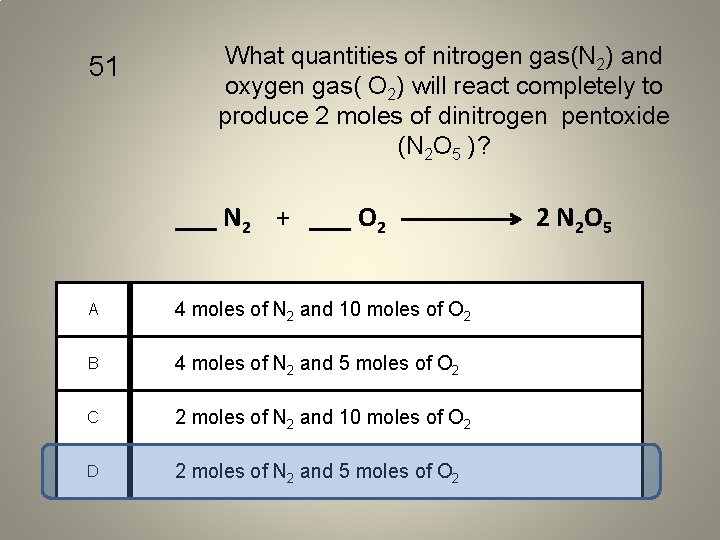
51 What quantities of nitrogen gas(N 2) and oxygen gas( O 2) will react completely to produce 2 moles of dinitrogen pentoxide (N 2 O 5 )? ___ N 2 + ___ O 2 A 4 moles of N 2 and 10 moles of O 2 B 4 moles of N 2 and 5 moles of O 2 C 2 moles of N 2 and 10 moles of O 2 D 2 moles of N 2 and 5 moles of O 2 2 N 2 O 5

51 What quantities of nitrogen gas(N 2) and oxygen gas( O 2) will react completely to produce 2 moles of dinitrogen pentoxide (N 2 O 5 )? ___ N 2 + ___ O 2 A 4 moles of N 2 and 10 moles of O 2 B 4 moles of N 2 and 5 moles of O 2 C 2 moles of N 2 and 10 moles of O 2 D 2 moles of N 2 and 5 moles of O 2 2 N 2 O 5

52 Approximately how many grams of sodium chloride (Na. Cl) are required to prepare 500. m. L of a 3. 00 M solution? A 39. 0 g B 58. 4 g C 87. 8 g D 167 g

52 Approximately how many grams of sodium chloride (Na. Cl) are required to prepare 500. m. L of a 3. 00 M solution? A 39. 0 g B 58. 4 g C 87. 8 g D 167 g

53 According to the quantum mechanical model of the atom, what is the maximum number of electrons that can occupy the second energy level? A 2 B 4 C 8 D 10

53 According to the quantum mechanical model of the atom, what is the maximum number of electrons that can occupy the second energy level? A 2 B 4 C 8 D 10

54 Scientists built a prototype of an electric device that may help predict human reactions to new medicines. Which next step should the scientists take to determine whether the device will be useful? A Test the device B Modify the device C Reevaluate the design of the device D Build the real device for application

54 Scientists built a prototype of an electric device that may help predict human reactions to new medicines. Which next step should the scientists take to determine whether the device will be useful? A Test the device B Modify the device C Reevaluate the design of the device D Build the real device for application

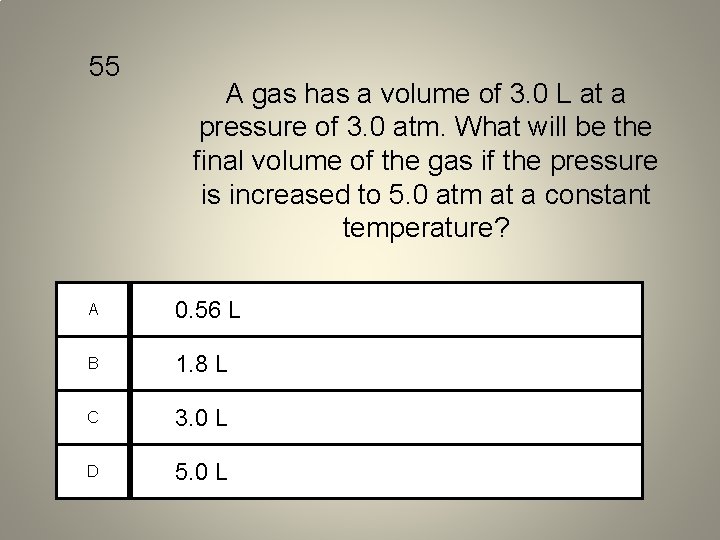
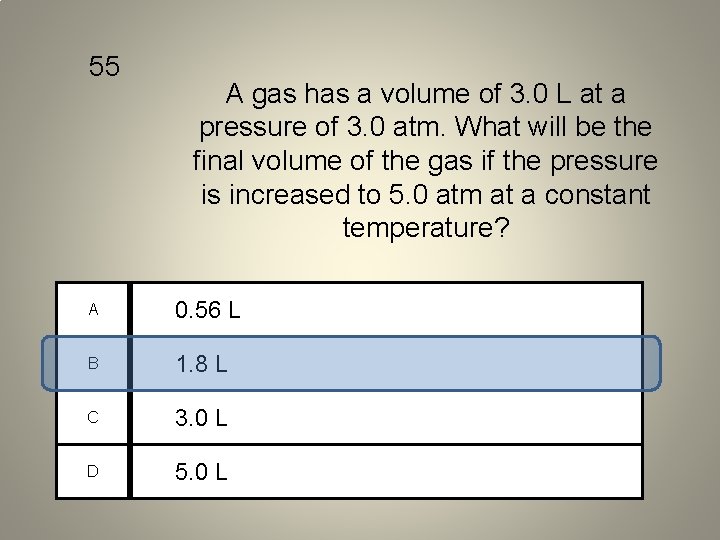
55 A gas has a volume of 3. 0 L at a pressure of 3. 0 atm. What will be the final volume of the gas if the pressure is increased to 5. 0 atm at a constant temperature? A 0. 56 L B 1. 8 L C 3. 0 L D 5. 0 L

55 A gas has a volume of 3. 0 L at a pressure of 3. 0 atm. What will be the final volume of the gas if the pressure is increased to 5. 0 atm at a constant temperature? A 0. 56 L B 1. 8 L C 3. 0 L D 5. 0 L

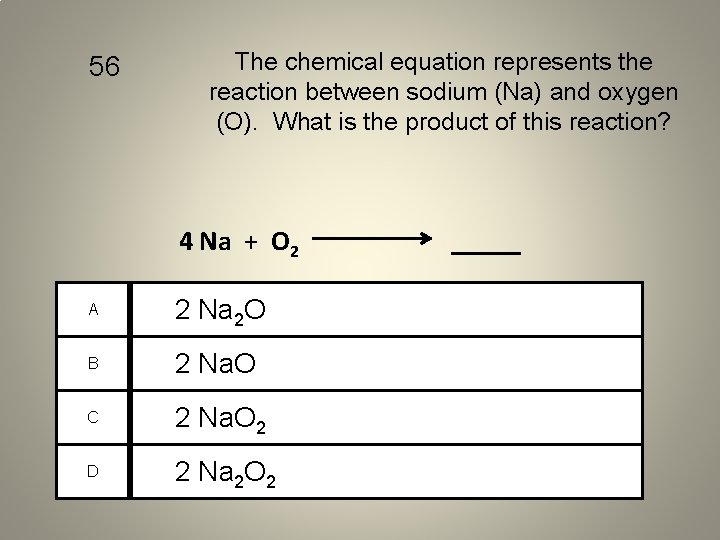
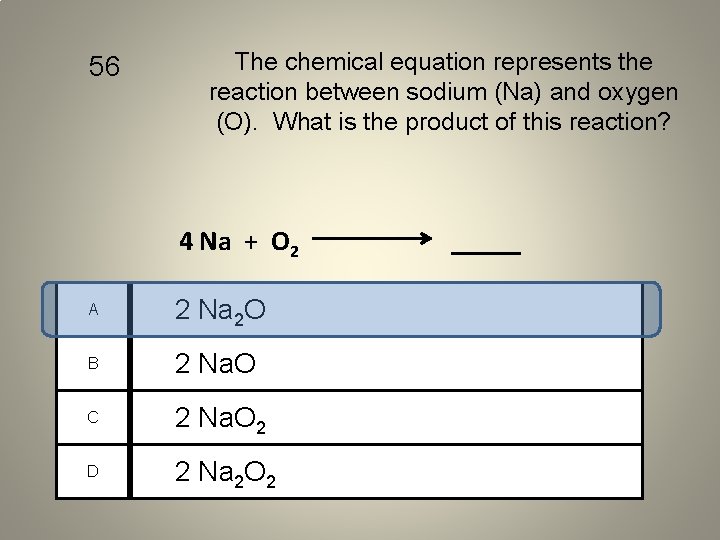
56 The chemical equation represents the reaction between sodium (Na) and oxygen (O). What is the product of this reaction? 4 Na + O 2 A 2 Na 2 O B 2 Na. O C 2 Na. O 2 D 2 Na 2 O 2 _____

56 The chemical equation represents the reaction between sodium (Na) and oxygen (O). What is the product of this reaction? 4 Na + O 2 A 2 Na 2 O B 2 Na. O C 2 Na. O 2 D 2 Na 2 O 2 _____

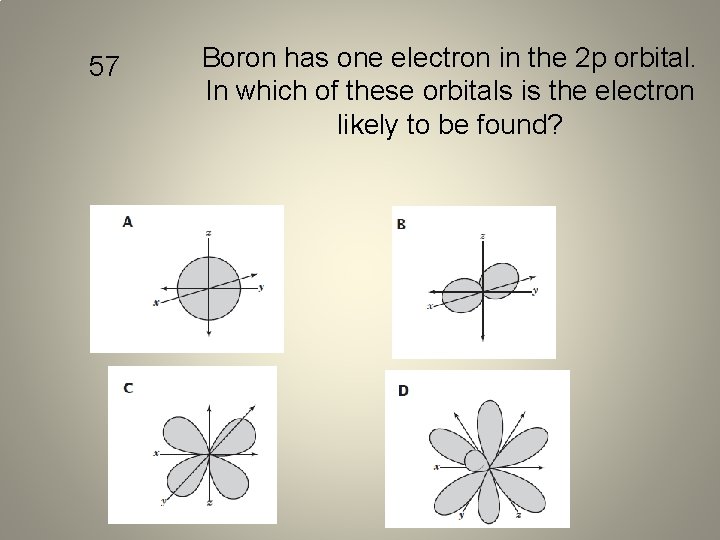
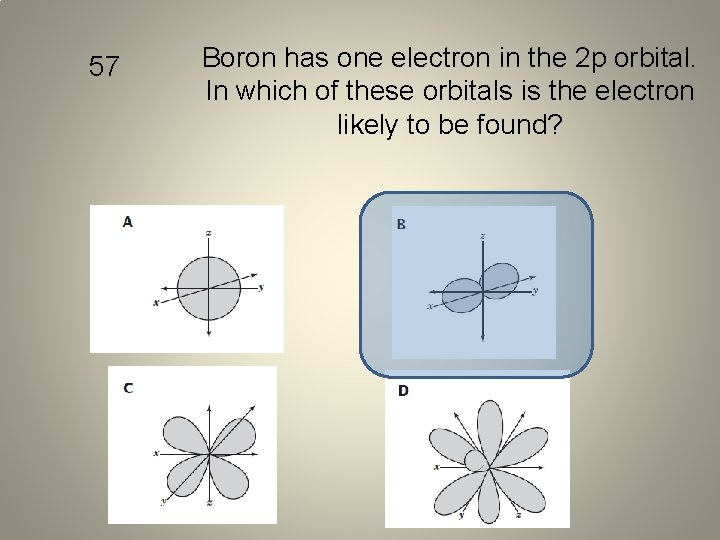
57 Boron has one electron in the 2 p orbital. In which of these orbitals is the electron likely to be found?

57 Boron has one electron in the 2 p orbital. In which of these orbitals is the electron likely to be found?

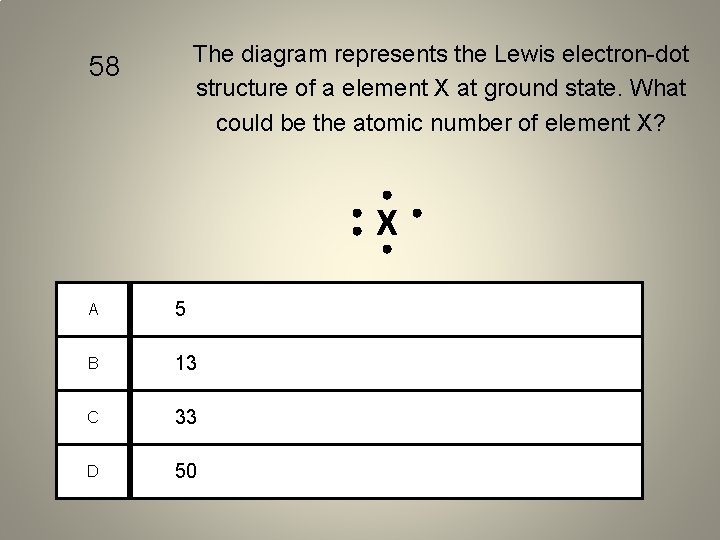
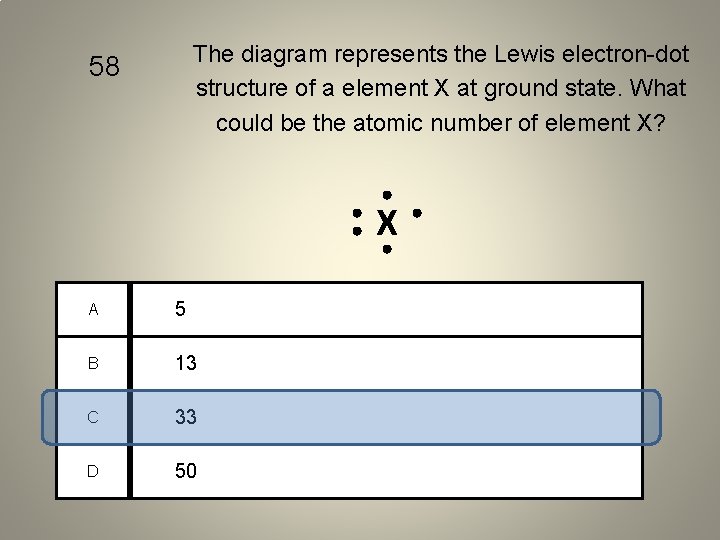
The diagram represents the Lewis electron-dot structure of a element X at ground state. What could be the atomic number of element X? 58 X A 5 B 13 C 33 D 50

The diagram represents the Lewis electron-dot structure of a element X at ground state. What could be the atomic number of element X? 58 X A 5 B 13 C 33 D 50

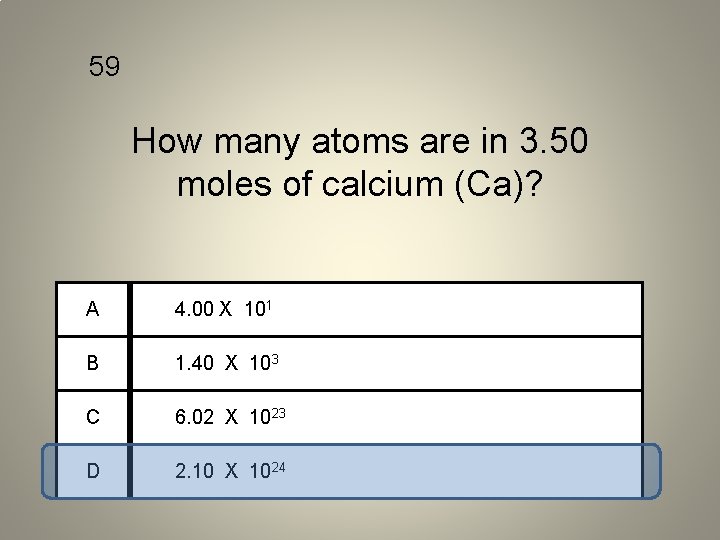
59 How many atoms are in 3. 50 moles of calcium (Ca)? A 4. 00 X 101 B 1. 40 X 103 C 6. 02 X 1023 D 2. 10 X 1024

59 How many atoms are in 3. 50 moles of calcium (Ca)? A 4. 00 X 101 B 1. 40 X 103 C 6. 02 X 1023 D 2. 10 X 1024

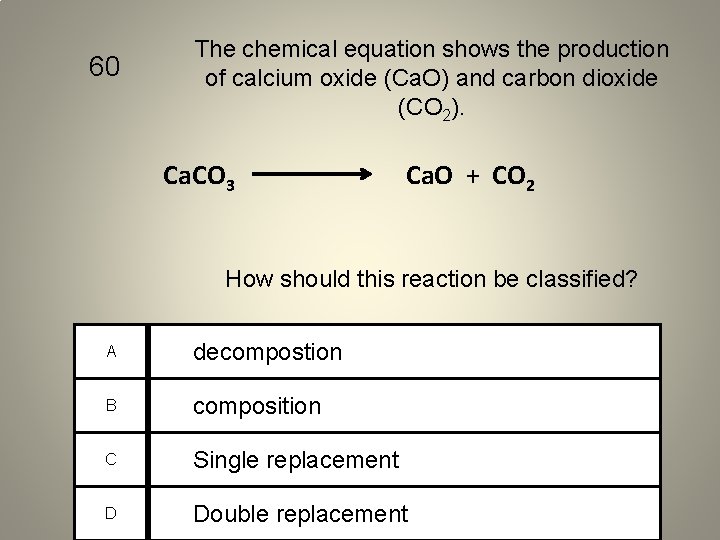
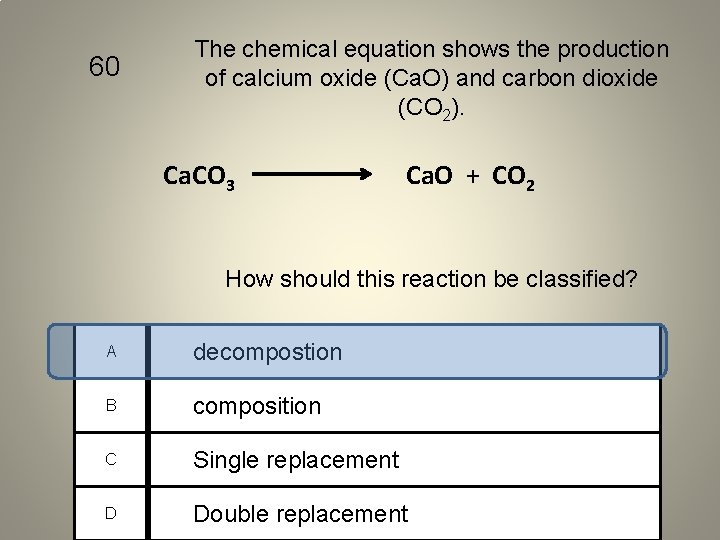
60 The chemical equation shows the production of calcium oxide (Ca. O) and carbon dioxide (CO 2). Ca. CO 3 Ca. O + CO 2 How should this reaction be classified? A decompostion B composition C Single replacement D Double replacement

60 The chemical equation shows the production of calcium oxide (Ca. O) and carbon dioxide (CO 2). Ca. CO 3 Ca. O + CO 2 How should this reaction be classified? A decompostion B composition C Single replacement D Double replacement

61 Which of these gives an example of a chemical change? A burning a piece of wood B cracking an egg C folding a piece of paper D melting an ice cube

61 Which of these gives an example of a chemical change? A burning a piece of wood B cracking an egg C folding a piece of paper D melting an ice cube

62 Which of these describes how gallium forms a 3+ ion? A The gallium loses 3 electrons. B The gallium loses 3 protons. C The gallium gains 3 electrons. D The gallium gains 3 protons.

62 Which of these describes how gallium forms a 3+ ion? A The gallium loses 3 electrons. B The gallium loses 3 protons. C The gallium gains 3 electrons. D The gallium gains 3 protons.

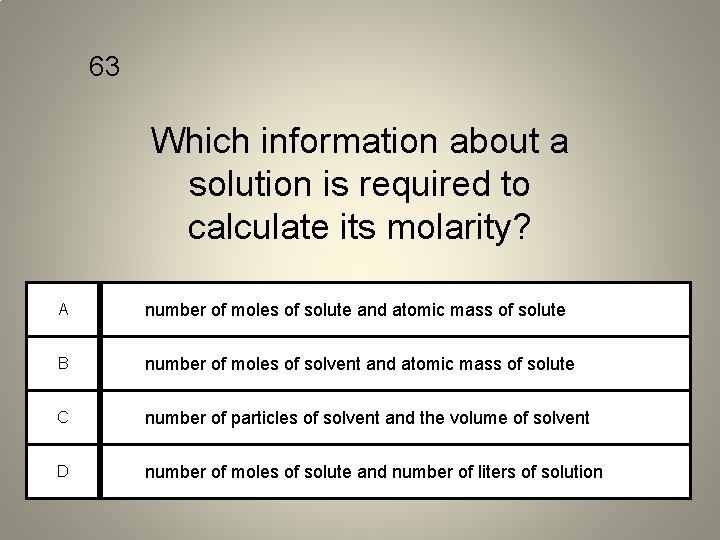
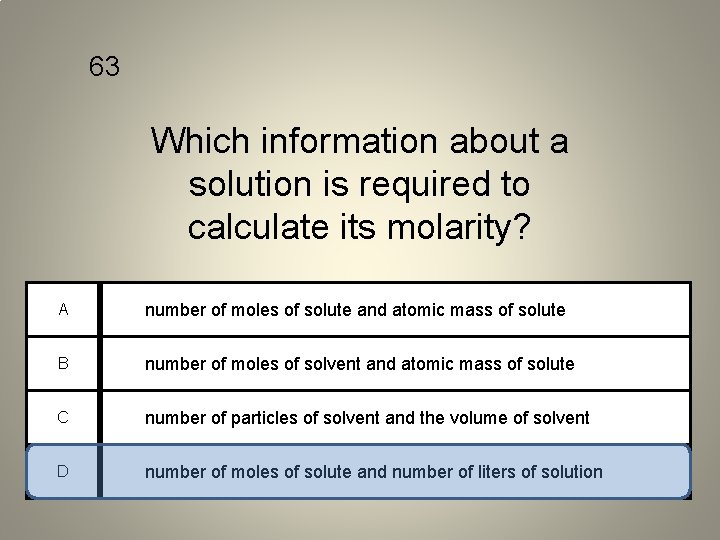
63 Which information about a solution is required to calculate its molarity? A number of moles of solute and atomic mass of solute B number of moles of solvent and atomic mass of solute C number of particles of solvent and the volume of solvent D number of moles of solute and number of liters of solution

63 Which information about a solution is required to calculate its molarity? A number of moles of solute and atomic mass of solute B number of moles of solvent and atomic mass of solute C number of particles of solvent and the volume of solvent D number of moles of solute and number of liters of solution

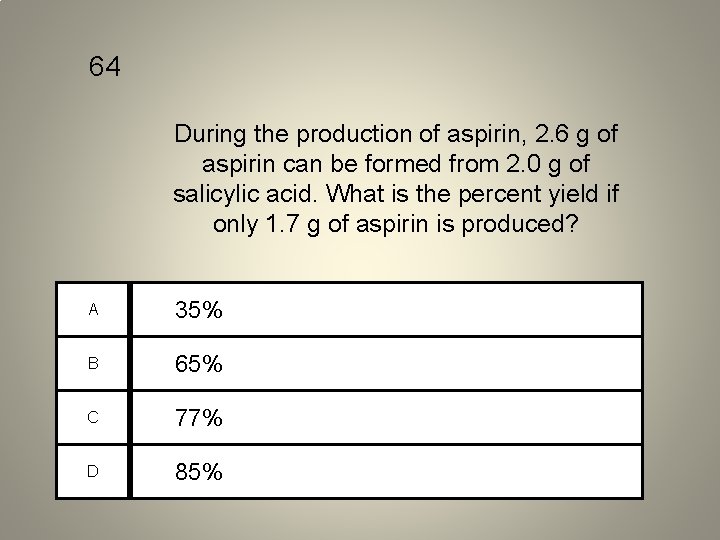
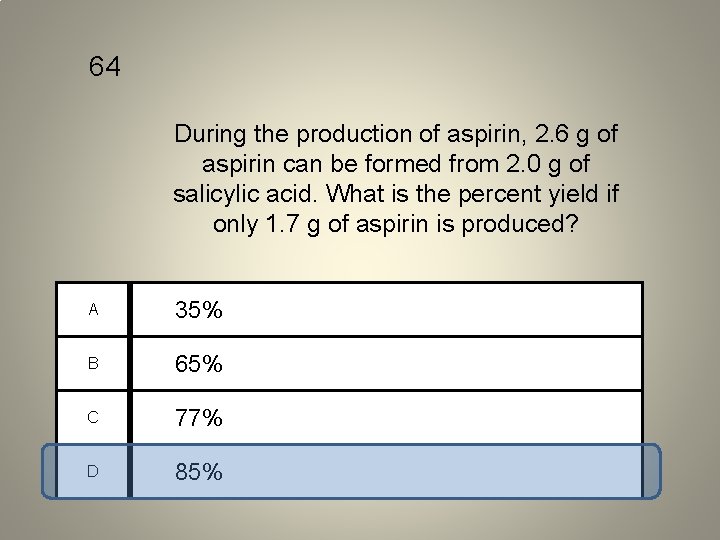
64 During the production of aspirin, 2. 6 g of aspirin can be formed from 2. 0 g of salicylic acid. What is the percent yield if only 1. 7 g of aspirin is produced? A 35% B 65% C 77% D 85%

64 During the production of aspirin, 2. 6 g of aspirin can be formed from 2. 0 g of salicylic acid. What is the percent yield if only 1. 7 g of aspirin is produced? A 35% B 65% C 77% D 85%

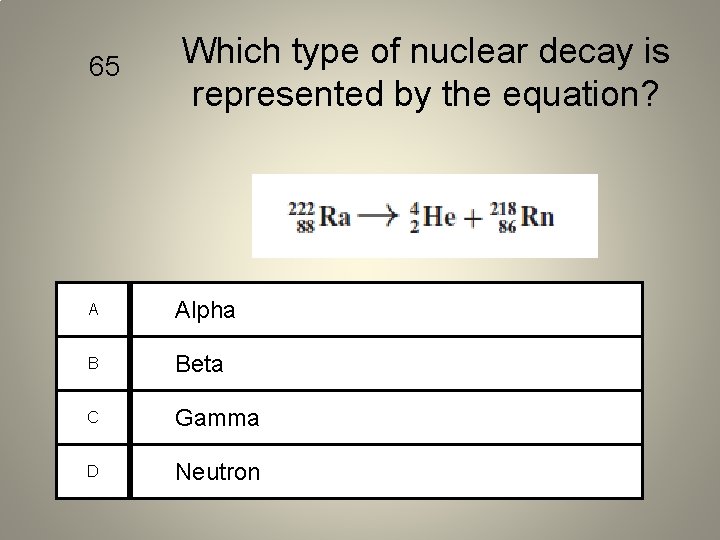
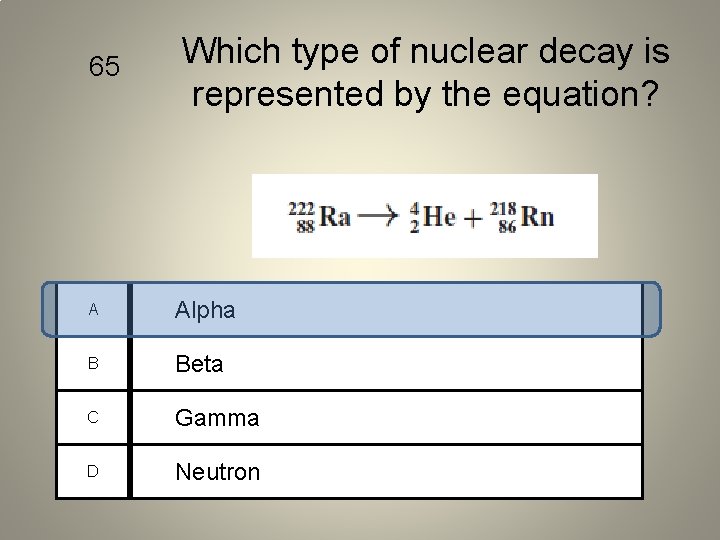
65 Which type of nuclear decay is represented by the equation? A Alpha B Beta C Gamma D Neutron

65 Which type of nuclear decay is represented by the equation? A Alpha B Beta C Gamma D Neutron
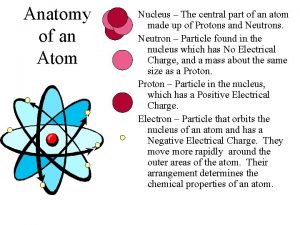
 The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Atom menurut democritus
Atom menurut democritus Which part of earth contains most of its mass
Which part of earth contains most of its mass Skinny pacs
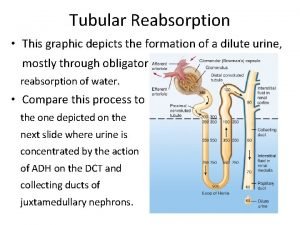
Skinny pacs Reabsorption
Reabsorption Absolute charge
Absolute charge Big things come in small packages answers
Big things come in small packages answers Henry moseley periodic table contribution
Henry moseley periodic table contribution Part whole model subtraction
Part whole model subtraction Unit ratio definition
Unit ratio definition Brainpop ratios
Brainpop ratios What is a technical description?
What is a technical description? Basic bar layout
Basic bar layout The part of a shadow surrounding the darkest part
The part of a shadow surrounding the darkest part 미니탭 gage r&r 해석
미니탭 gage r&r 해석 Most important part of the body
Most important part of the body The most complex part of tls is the
The most complex part of tls is the The most obvious part of a flower
The most obvious part of a flower Most streams carry the largest part of their load
Most streams carry the largest part of their load Which atom contains exactly 15 protons
Which atom contains exactly 15 protons Bonds broken minus bonds formed
Bonds broken minus bonds formed Write the full ground state electron configuration of o+
Write the full ground state electron configuration of o+ Most general to most specific classification
Most general to most specific classification Most general to most specific classification
Most general to most specific classification In the name of allah most gracious most merciful
In the name of allah most gracious most merciful The most
The most Beneficent pronunciation
Beneficent pronunciation In the name of allah the most beneficent the most merciful
In the name of allah the most beneficent the most merciful Organisms taxonomy
Organisms taxonomy The arrangement chapter 9
The arrangement chapter 9 In the name of god most gracious most merciful
In the name of god most gracious most merciful In the name of allah the most gracious the most merciful
In the name of allah the most gracious the most merciful In the name of allah the most
In the name of allah the most In the name of god most gracious most merciful
In the name of god most gracious most merciful Aqeedah
Aqeedah In the name of god most gracious prayer
In the name of god most gracious prayer Allah the most beneficent
Allah the most beneficent Which post holds up the greater part of the load
Which post holds up the greater part of the load Trans pecos erosion
Trans pecos erosion Which part of the poem you like best why
Which part of the poem you like best why The visible light we see from our sun comes from which part
The visible light we see from our sun comes from which part Why plant breeding is an art
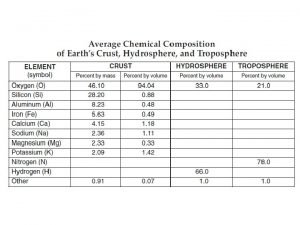
Why plant breeding is an art Hydrosphere includes all the on earth
Hydrosphere includes all the on earth What produces pollen
What produces pollen Part to whole examples
Part to whole examples Name the basic parts of the nail unit
Name the basic parts of the nail unit Which part of the cell contains genetic material
Which part of the cell contains genetic material Which part of the cell contains genetic material
Which part of the cell contains genetic material Objective summary define
Objective summary define Red flag bit is a part of which register
Red flag bit is a part of which register Attention to in letter
Attention to in letter Fluid mechanics chapter 4
Fluid mechanics chapter 4 Which is not part of a store’s strategy mix
Which is not part of a store’s strategy mix Which part of the earth is the hottest?
Which part of the earth is the hottest? Withdrawal of status respect is a theory of entrepreneurial
Withdrawal of status respect is a theory of entrepreneurial The treasure of lemon brown analysis
The treasure of lemon brown analysis Which part of the neuron serves as the protective coating?
Which part of the neuron serves as the protective coating? The dome pictured below is part of which structure?
The dome pictured below is part of which structure? One body many parts meaning
One body many parts meaning Specify which part of the brain includes calliculis
Specify which part of the brain includes calliculis Which location is the flattest part of the ocean
Which location is the flattest part of the ocean What is alimentary canal
What is alimentary canal Which is not part of the alimentary canal
Which is not part of the alimentary canal Which sentence contains an infinitive?
Which sentence contains an infinitive? Good is which part of speech
Good is which part of speech A bean seed and its parts
A bean seed and its parts The tool used for making internal threads is called as
The tool used for making internal threads is called as A bright object with a long tail of glowing gases
A bright object with a long tail of glowing gases How many blue rhombuses are in one yellow hexagon
How many blue rhombuses are in one yellow hexagon Reproductive system of pila
Reproductive system of pila Indications for suspension therapy
Indications for suspension therapy A unicellular protist is part of which domain?
A unicellular protist is part of which domain? Disadvantages of programmable logic devices
Disadvantages of programmable logic devices Which part of the plant carries and protects the seed
Which part of the plant carries and protects the seed Photosynthesis equation
Photosynthesis equation Epithelium which protects inner part of the body is
Epithelium which protects inner part of the body is Excited part of speech
Excited part of speech Which statement best describes this part of the story?
Which statement best describes this part of the story? Which of the following is not part of overall biodiversity?
Which of the following is not part of overall biodiversity? Smallest part of a cell
Smallest part of a cell Arthromegaly
Arthromegaly Which part of this passage is a flashback?
Which part of this passage is a flashback? Payroll cycle flowchart
Payroll cycle flowchart What profession would you like to choose
What profession would you like to choose A primary reason that geographers study maps is to
A primary reason that geographers study maps is to Thermal energy states of matter
Thermal energy states of matter Mrp
Mrp Which sentence uses the most precise language?
Which sentence uses the most precise language? Chapter 6 electronic structure of atoms
Chapter 6 electronic structure of atoms Which is the primary sales tool in most operations?
Which is the primary sales tool in most operations? Which shoes create the most pressure
Which shoes create the most pressure Unsaturated solubility
Unsaturated solubility Which solution is the most concentrated
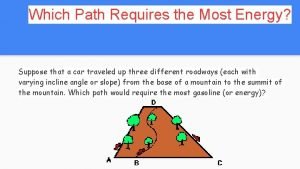
Which solution is the most concentrated Which path requires the most energy
Which path requires the most energy Which layer of earth is most likely made of solid metals
Which layer of earth is most likely made of solid metals Knack maneuver
Knack maneuver Which graph correctly represents the three most abundant
Which graph correctly represents the three most abundant Lydia minatoya
Lydia minatoya Which rock weathers most rapidly when exposed to acid rain
Which rock weathers most rapidly when exposed to acid rain Which athlete has the most olympic gold medals
Which athlete has the most olympic gold medals Which of the following gases will effuse the most rapidly
Which of the following gases will effuse the most rapidly Which graph correctly represents the three most abundant
Which graph correctly represents the three most abundant Venus’s surface shows all of the following except
Venus’s surface shows all of the following except Andrea yates cognitive perspective
Andrea yates cognitive perspective Which is the most enduring free trade area in the world?
Which is the most enduring free trade area in the world? Which of these headlines most involves a due process right?
Which of these headlines most involves a due process right? Which joint is the most complex diarthrosis in the body?
Which joint is the most complex diarthrosis in the body? Which of the promotion methods is the most precise?
Which of the promotion methods is the most precise? What is your favourite...
What is your favourite... A liaison is a thickener made from a mixture of *
A liaison is a thickener made from a mixture of * How does the clause that which was most sad and lamentable
How does the clause that which was most sad and lamentable Which country has won the most olympic medals
Which country has won the most olympic medals Which object has the most mass?
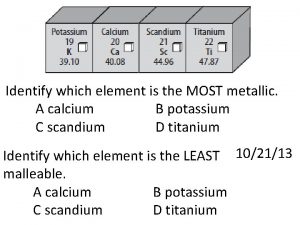
Which object has the most mass? Which element is the most metallic
Which element is the most metallic Where the agricultural sangam was developed
Where the agricultural sangam was developed What is the most abundant biomolecule on earth
What is the most abundant biomolecule on earth Which word most strongly appeals to pathos?
Which word most strongly appeals to pathos? With whom does napoleon play cards with
With whom does napoleon play cards with Regents
Regents Which of the following therapists would most likely
Which of the following therapists would most likely Chapter 19 the giver
Chapter 19 the giver Applications of size separation
Applications of size separation Which is the most common type of precipitation?
Which is the most common type of precipitation? Which of these business situations would worry you most
Which of these business situations would worry you most Answer
Answer Reactivity series anagram
Reactivity series anagram Lr parsing example
Lr parsing example Which cell that was viewed is most likely a prokaryote
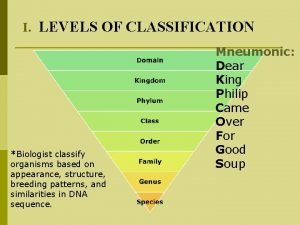
Which cell that was viewed is most likely a prokaryote Which level of classification is the most specific?
Which level of classification is the most specific? Ilike broccoli
Ilike broccoli