VSEPR theory Molecular Polarity Tro Chemistry A Molecular


- Slides: 72

VSEPR theory Molecular Polarity Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Taste • The taste of a food: Interaction between • food molecules and taste cells Factors: Shape of the molecule and charge distribution within the molecule • Food or “spicy” molecule fit snugly into the active site of specialized proteins on the surface of taste cells • When this happens, changes in the protein structure cause a nerve signal Tro: Chemistry: A Molecular Approach, 2/e 2 Copyright 2011 Pearson Education, Inc.

Sweet Taste: Key/Lock model Sugar molecules (“Key”) fit into the active site of taste cell receptors (“Lock”): • When the sugar molecule enters the active site, parts of the taste cell receptor split apart ion channels in the cell membrane to open resulting in nerve signal transmission • Artificial sweeteners also fit into the same receptor, sometimes binding even stronger than sugar (making them “sweeter” than sugar) Tro: Chemistry: A Molecular Approach, 2/e 3 Copyright 2011 Pearson Education, Inc.

Structure Determines Properties! • Properties of molecular substances depend on • the structure of the molecule The structure includes many factors, such as: ü the skeletal arrangement of the atoms ü the kind of bonding between the atoms Øionic, polar covalent, or covalent ü the shape of the molecule • Bonding theory should allow you to predict the shapes of molecules Tro: Chemistry: A Molecular Approach, 2/e 4 Copyright 2011 Pearson Education, Inc.

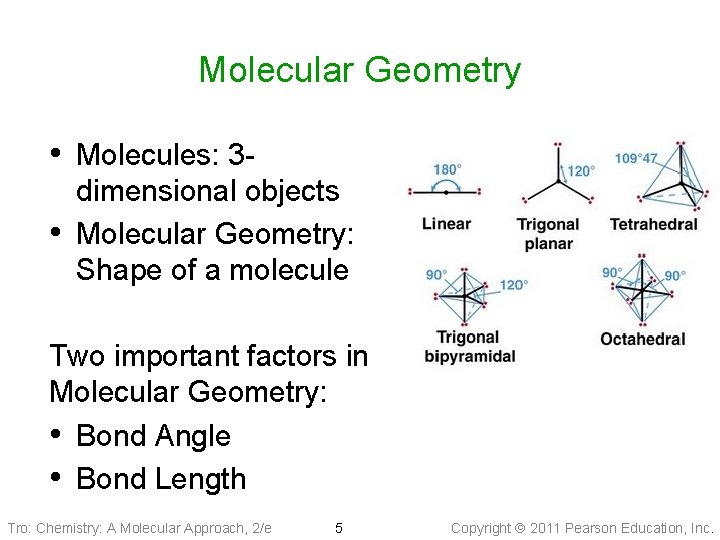
Molecular Geometry • Molecules: 3 • dimensional objects Molecular Geometry: Shape of a molecule Two important factors in Molecular Geometry: • Bond Angle • Bond Length Tro: Chemistry: A Molecular Approach, 2/e 5 Copyright 2011 Pearson Education, Inc.

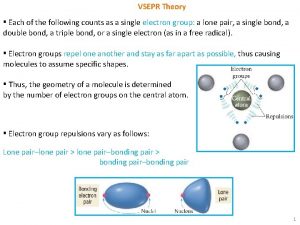
Lewis Theory Predicts Electron Groups • Lewis theory predicts there are regions of • • • electrons in an atom Electron groups (= VSEPR group): Bonding pairs and Lone pairs Bonding pairs: Shared pairs of valence electrons between bonding nuclei Nonbonding pairs (= Lone Pairs): Unshared valence electrons on a single nuclei Tro: Chemistry: A Molecular Approach, 2/e 6 Copyright 2011 Pearson Education, Inc.

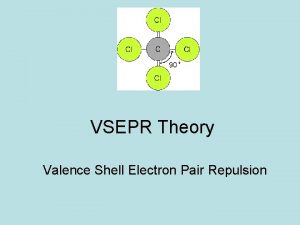
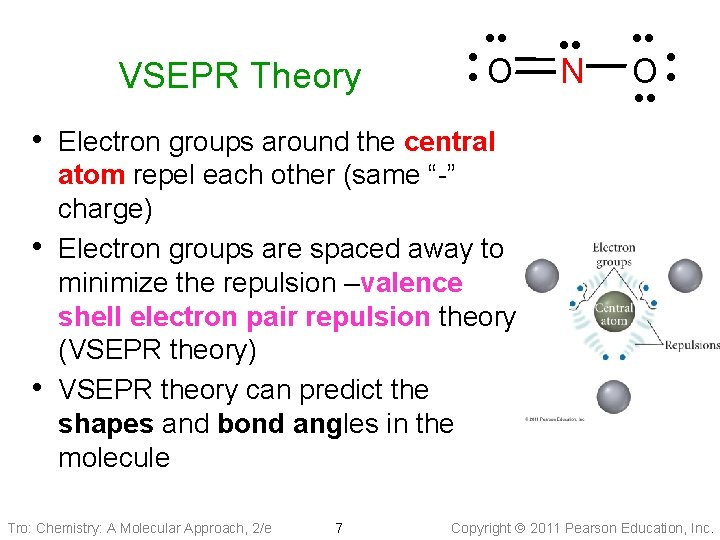
VSEPR Theory • • • O • • • N • • O • • • Electron groups around the central • • atom repel each other (same “-” charge) Electron groups are spaced away to minimize the repulsion –valence shell electron pair repulsion theory (VSEPR theory) VSEPR theory can predict the shapes and bond angles in the molecule Tro: Chemistry: A Molecular Approach, 2/e 7 Copyright 2011 Pearson Education, Inc.

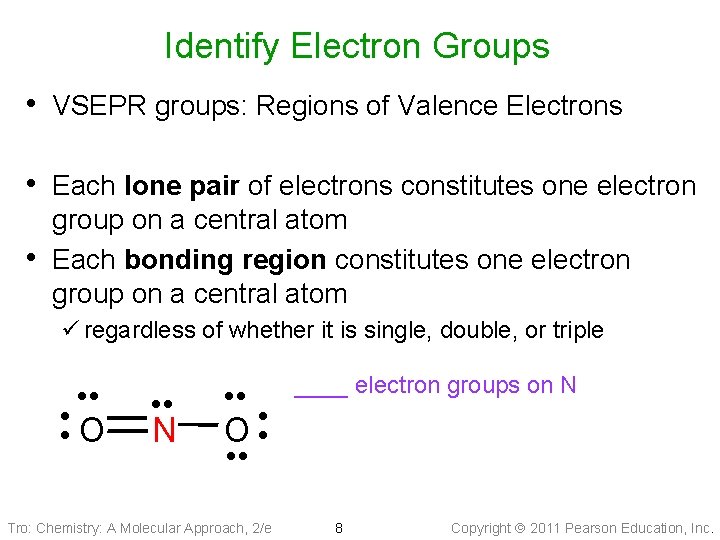
Identify Electron Groups • VSEPR groups: Regions of Valence Electrons • Each lone pair of electrons constitutes one electron • group on a central atom Each bonding region constitutes one electron group on a central atom ü regardless of whether it is single, double, or triple • • • O • • • N • • • O • • • Tro: Chemistry: A Molecular Approach, 2/e ____ electron groups on N 8 Copyright 2011 Pearson Education, Inc.

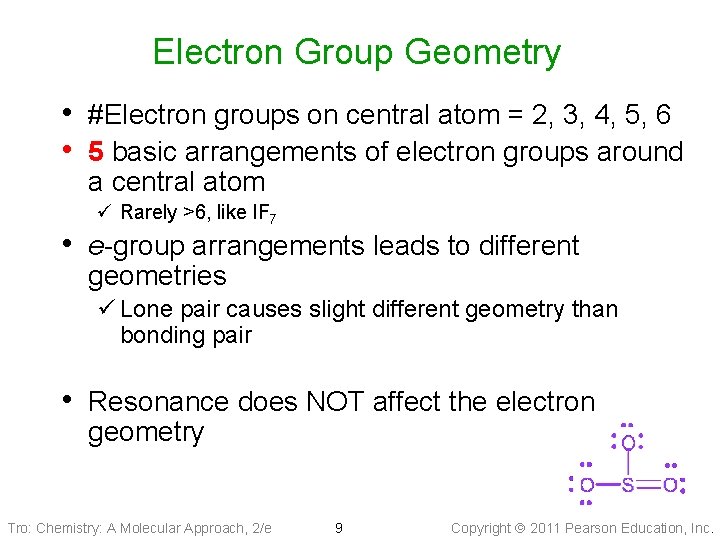
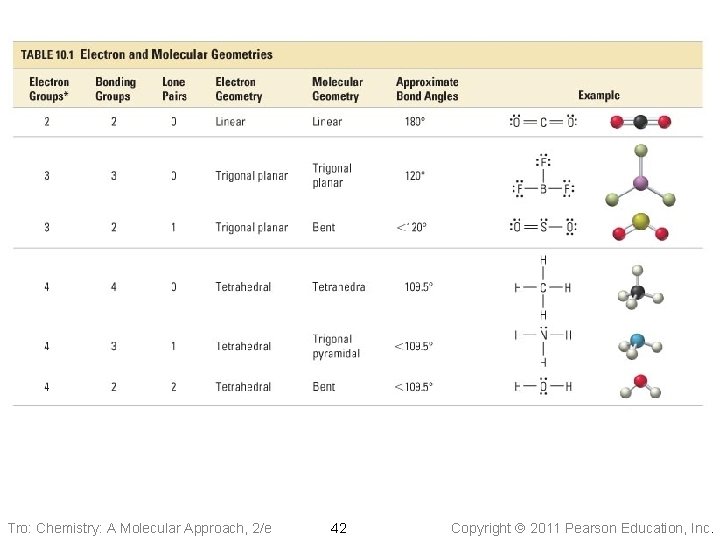
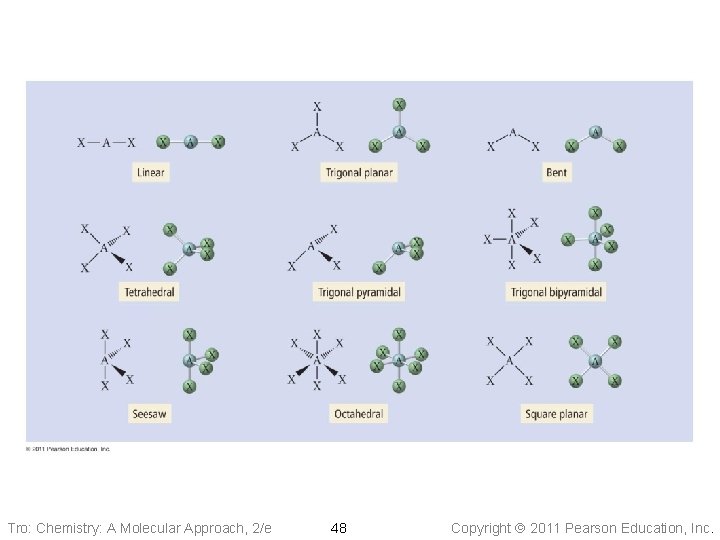
Electron Group Geometry • #Electron groups on central atom = 2, 3, 4, 5, 6 • 5 basic arrangements of electron groups around a central atom ü Rarely >6, like IF 7 • e-group arrangements leads to different geometries ü Lone pair causes slight different geometry than bonding pair • Resonance does NOT affect the electron geometry Tro: Chemistry: A Molecular Approach, 2/e 9 Copyright 2011 Pearson Education, Inc.

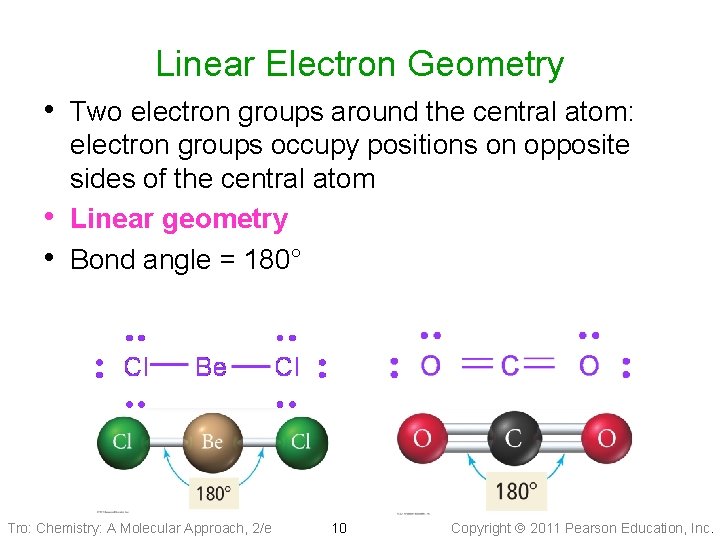
Linear Electron Geometry • Two electron groups around the central atom: • • electron groups occupy positions on opposite sides of the central atom Linear geometry Bond angle = 180° Tro: Chemistry: A Molecular Approach, 2/e 10 Copyright 2011 Pearson Education, Inc.

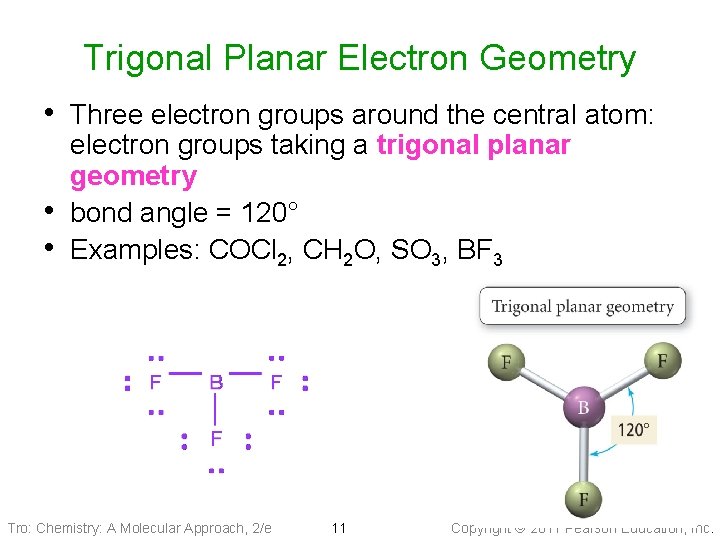
Trigonal Planar Electron Geometry • Three electron groups around the central atom: • • electron groups taking a trigonal planar geometry bond angle = 120° Examples: COCl 2, CH 2 O, SO 3, BF 3 Tro: Chemistry: A Molecular Approach, 2/e 11 Copyright 2011 Pearson Education, Inc.

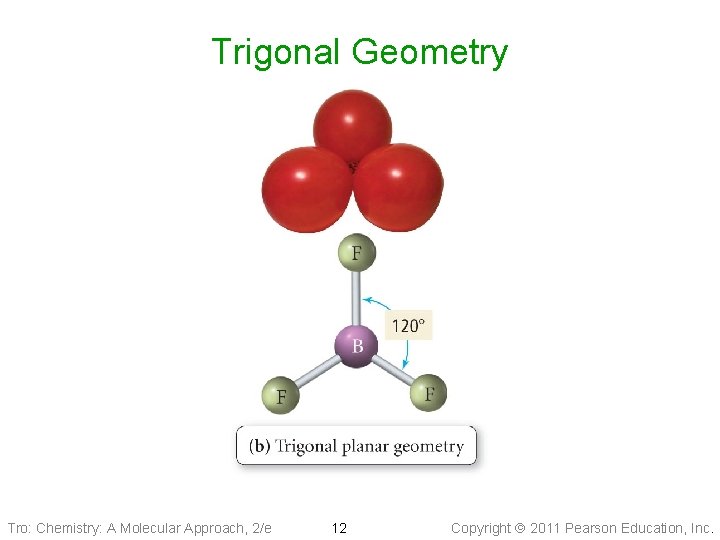
Trigonal Geometry Tro: Chemistry: A Molecular Approach, 2/e 12 Copyright 2011 Pearson Education, Inc.

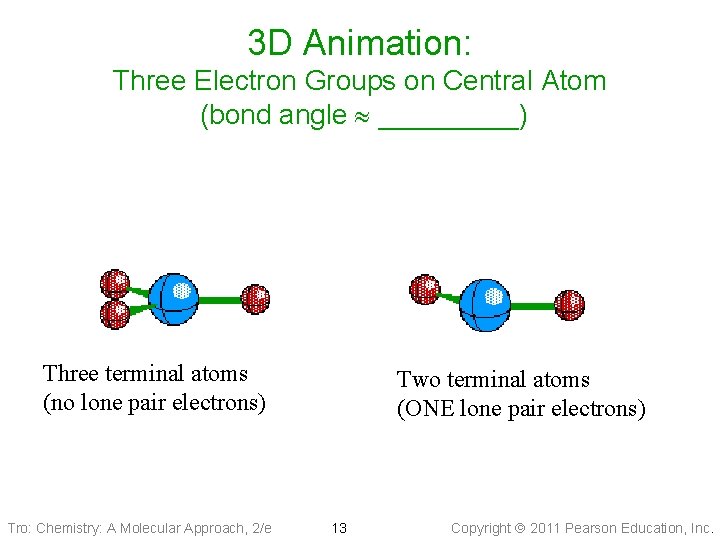
3 D Animation: Three Electron Groups on Central Atom (bond angle _____) Three terminal atoms (no lone pair electrons) Tro: Chemistry: A Molecular Approach, 2/e Two terminal atoms (ONE lone pair electrons) 13 Copyright 2011 Pearson Education, Inc.

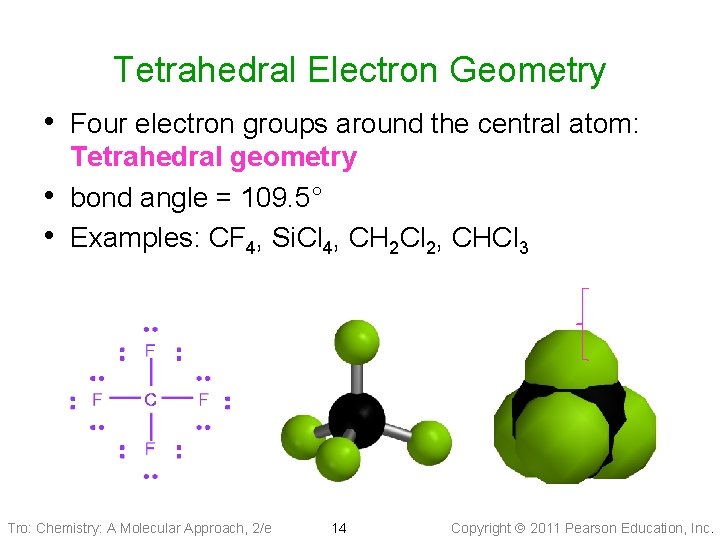
Tetrahedral Electron Geometry • Four electron groups around the central atom: • • Tetrahedral geometry bond angle = 109. 5° Examples: CF 4, Si. Cl 4, CH 2 Cl 2, CHCl 3 Tro: Chemistry: A Molecular Approach, 2/e 14 Copyright 2011 Pearson Education, Inc.

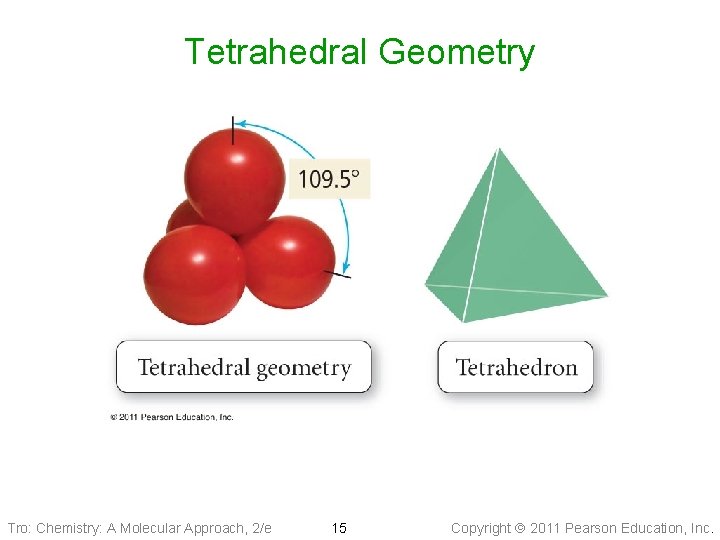
Tetrahedral Geometry Tro: Chemistry: A Molecular Approach, 2/e 15 Copyright 2011 Pearson Education, Inc.

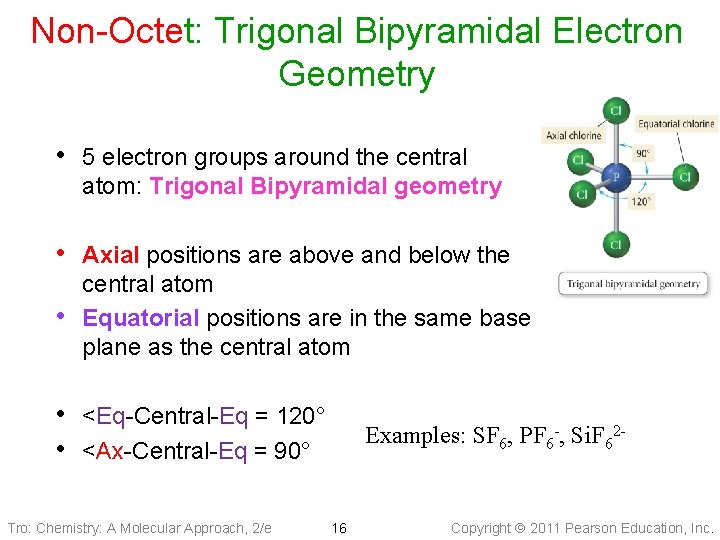
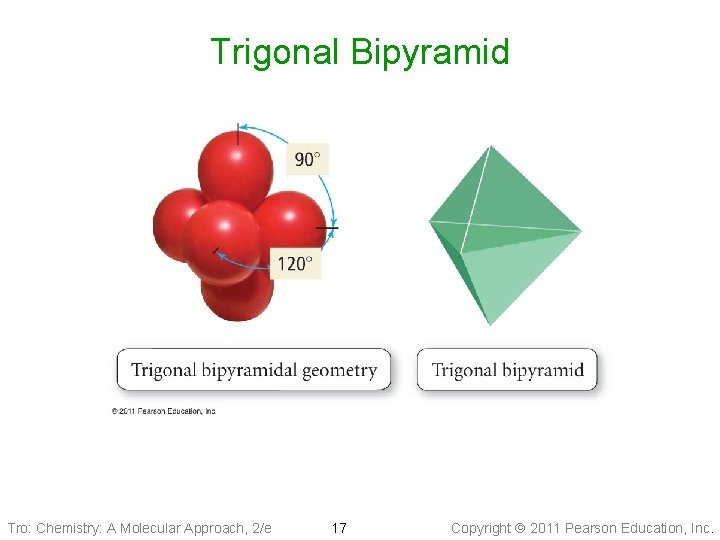
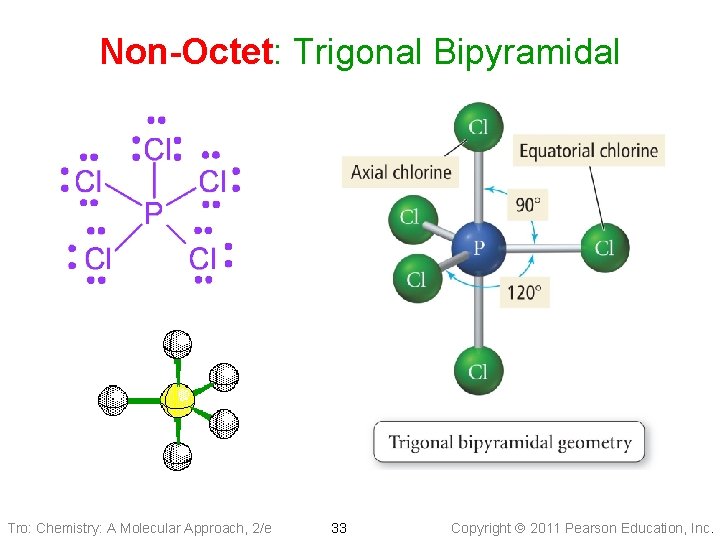
Non-Octet: Trigonal Bipyramidal Electron Geometry • 5 electron groups around the central atom: Trigonal Bipyramidal geometry • Axial positions are above and below the • central atom Equatorial positions are in the same base plane as the central atom • <Eq-Central-Eq = 120° • <Ax-Central-Eq = 90° Tro: Chemistry: A Molecular Approach, 2/e Examples: SF 6, PF 6 -, Si. F 62 - 16 Copyright 2011 Pearson Education, Inc.

Trigonal Bipyramid Tro: Chemistry: A Molecular Approach, 2/e 17 Copyright 2011 Pearson Education, Inc.

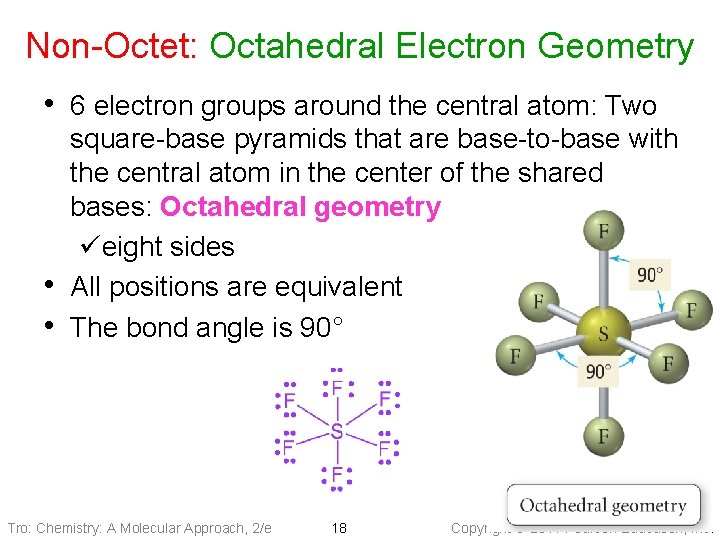
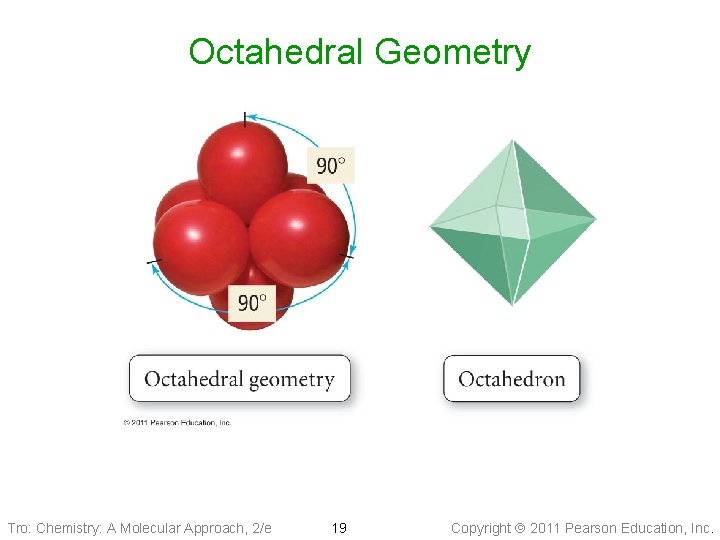
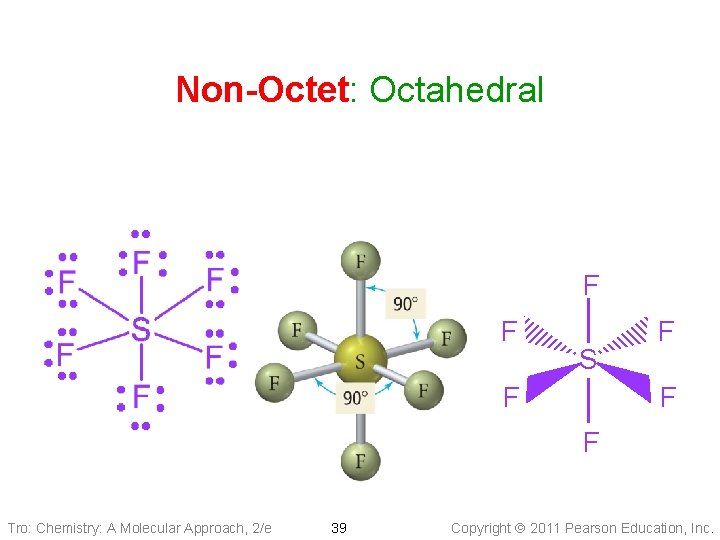
Non-Octet: Octahedral Electron Geometry • 6 electron groups around the central atom: Two • • square-base pyramids that are base-to-base with the central atom in the center of the shared bases: Octahedral geometry üeight sides All positions are equivalent The bond angle is 90° Tro: Chemistry: A Molecular Approach, 2/e 18 Copyright 2011 Pearson Education, Inc.

Octahedral Geometry Tro: Chemistry: A Molecular Approach, 2/e 19 Copyright 2011 Pearson Education, Inc.

Molecular Geometry vs. Electron Geometry The actual geometry of the molecule may be different from the electron geometry when: • The electron groups are attached to atoms of different size, or when the bonding to one atom is different than the bonding to another • Lone pairs occupy more space on the central atom than bonding pairs Tro: Chemistry: A Molecular Approach, 2/e 20 Copyright 2011 Pearson Education, Inc.

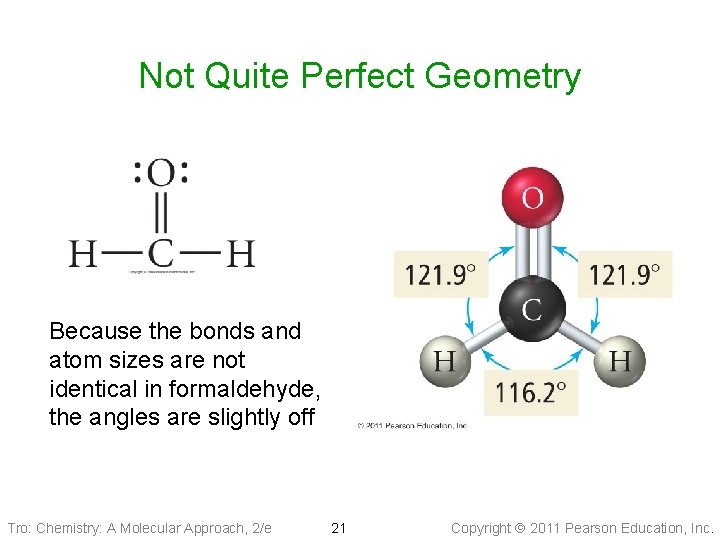
Not Quite Perfect Geometry Because the bonds and atom sizes are not identical in formaldehyde, the angles are slightly off Tro: Chemistry: A Molecular Approach, 2/e 21 Copyright 2011 Pearson Education, Inc.

The Effect of Lone Pairs • <H-O-H in water = 104. 5° < 109. 5°, why? • Lone pair (LP) groups “occupy more space” bonding pair (BP). ü electron density in LP is on the central atom only, not shared like bonding electron groups • Ranking the repulsive force interactions: LP-LP > LP-BP > BP-BP • Stronger repulsive force from LP ____ angles from LP _____ BP-BP angle Tro: Chemistry: A Molecular Approach, 2/e 22 Copyright 2011 Pearson Education, Inc.

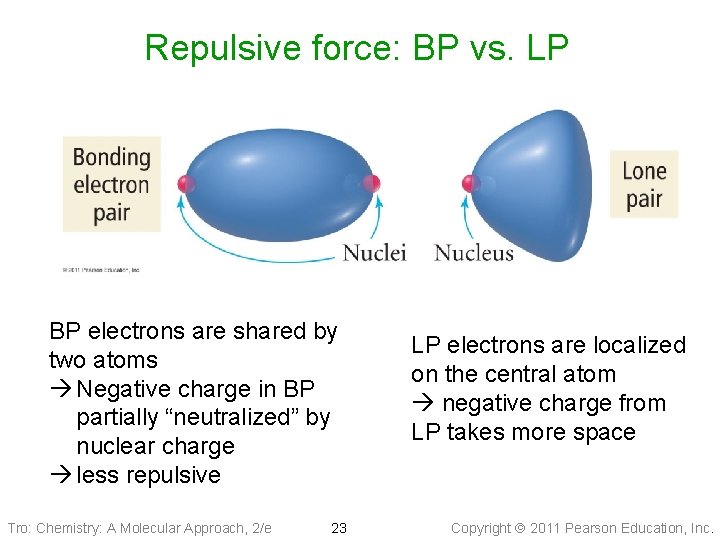
Repulsive force: BP vs. LP BP electrons are shared by two atoms Negative charge in BP partially “neutralized” by nuclear charge less repulsive Tro: Chemistry: A Molecular Approach, 2/e 23 LP electrons are localized on the central atom negative charge from LP takes more space Copyright 2011 Pearson Education, Inc.

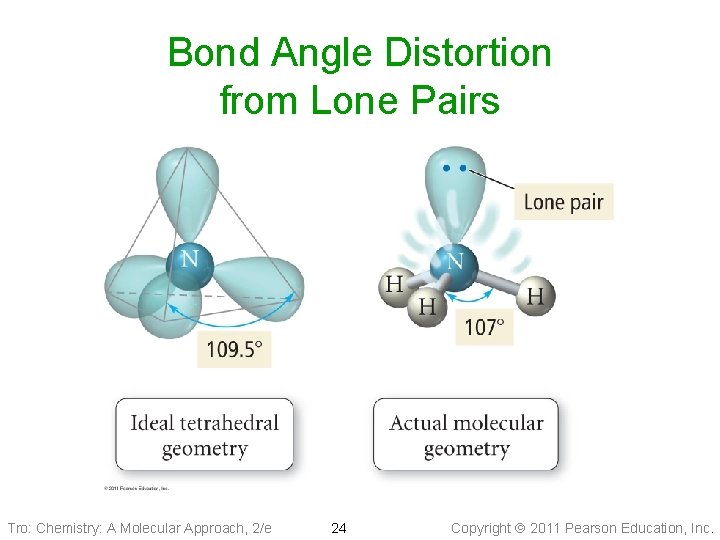
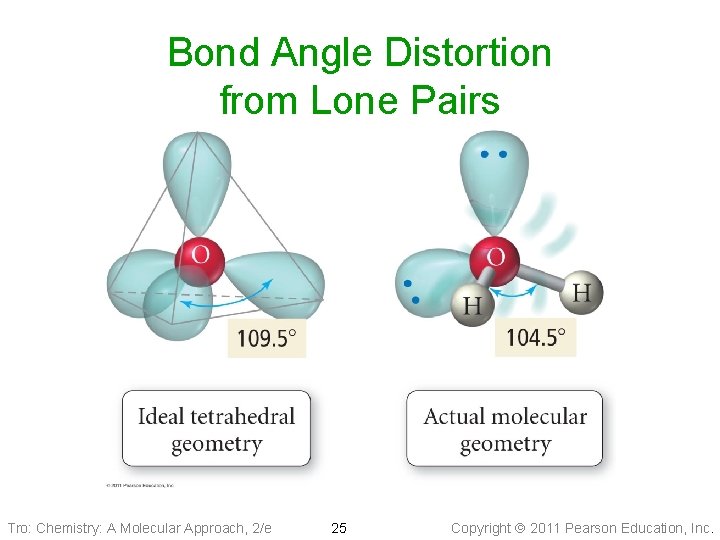
Bond Angle Distortion from Lone Pairs Tro: Chemistry: A Molecular Approach, 2/e 24 Copyright 2011 Pearson Education, Inc.

Bond Angle Distortion from Lone Pairs Tro: Chemistry: A Molecular Approach, 2/e 25 Copyright 2011 Pearson Education, Inc.

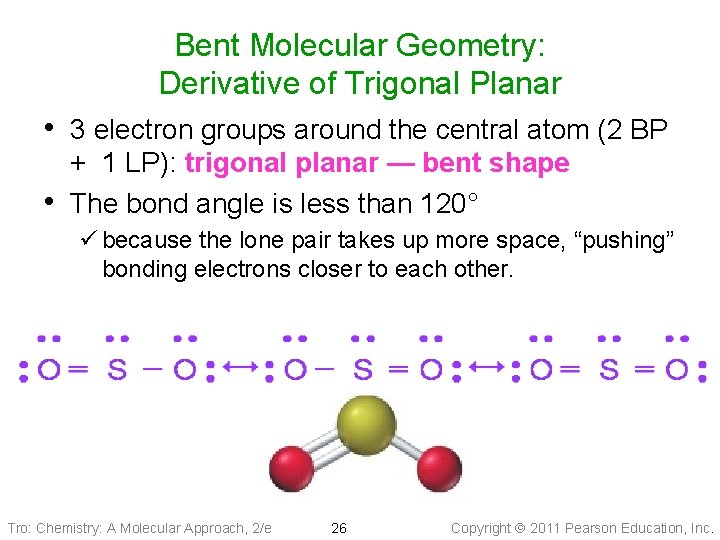
Bent Molecular Geometry: Derivative of Trigonal Planar • 3 electron groups around the central atom (2 BP • + 1 LP): trigonal planar — bent shape The bond angle is less than 120° ü because the lone pair takes up more space, “pushing” bonding electrons closer to each other. Tro: Chemistry: A Molecular Approach, 2/e 26 Copyright 2011 Pearson Education, Inc.

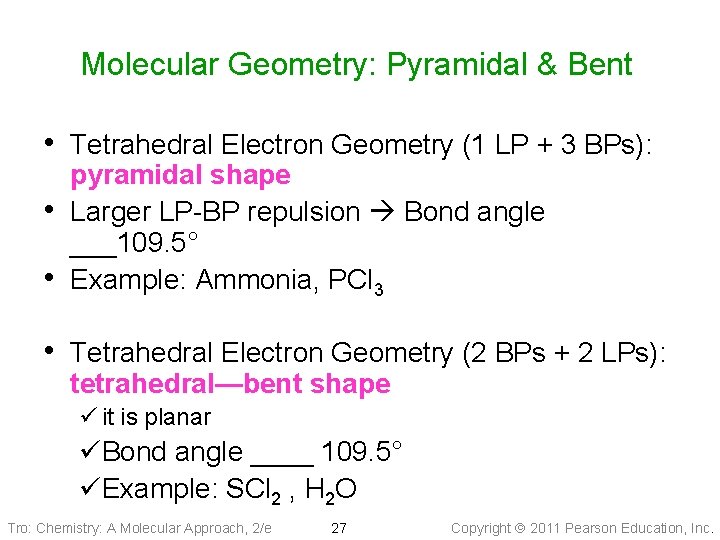
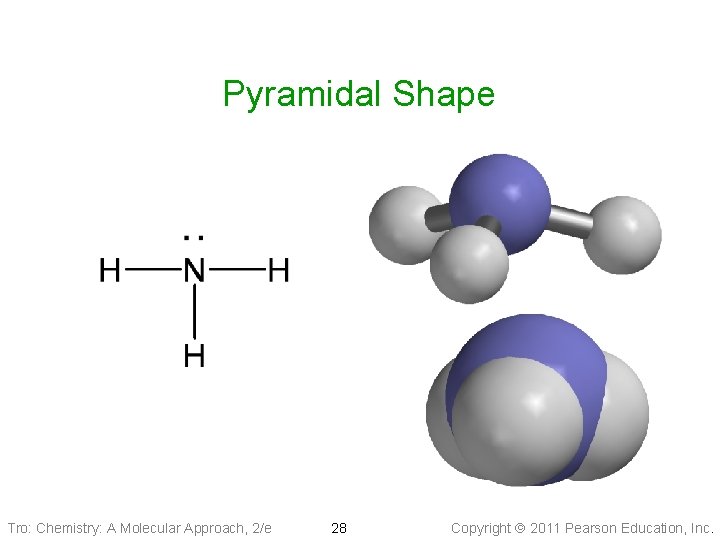
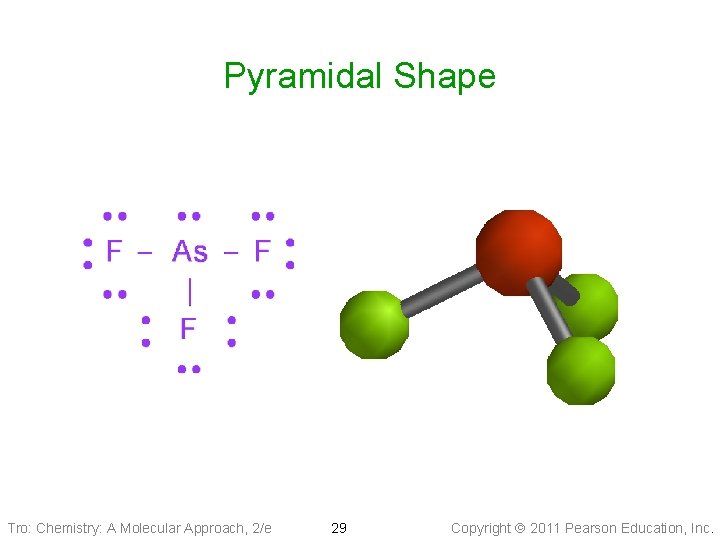
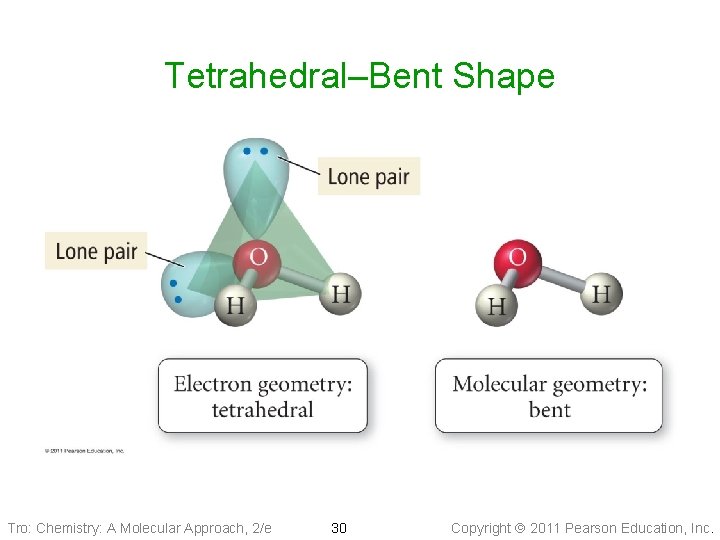
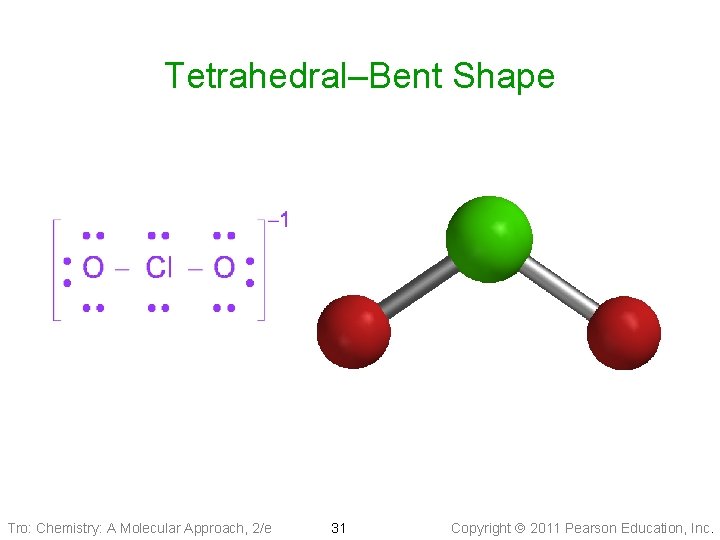
Molecular Geometry: Pyramidal & Bent • Tetrahedral Electron Geometry (1 LP + 3 BPs): • • pyramidal shape Larger LP-BP repulsion Bond angle ___109. 5° Example: Ammonia, PCl 3 • Tetrahedral Electron Geometry (2 BPs + 2 LPs): tetrahedral—bent shape ü it is planar üBond angle ____ 109. 5° üExample: SCl 2 , H 2 O Tro: Chemistry: A Molecular Approach, 2/e 27 Copyright 2011 Pearson Education, Inc.

Pyramidal Shape Tro: Chemistry: A Molecular Approach, 2/e 28 Copyright 2011 Pearson Education, Inc.

Pyramidal Shape Tro: Chemistry: A Molecular Approach, 2/e 29 Copyright 2011 Pearson Education, Inc.

Tetrahedral–Bent Shape Tro: Chemistry: A Molecular Approach, 2/e 30 Copyright 2011 Pearson Education, Inc.

Tetrahedral–Bent Shape Tro: Chemistry: A Molecular Approach, 2/e 31 Copyright 2011 Pearson Education, Inc.

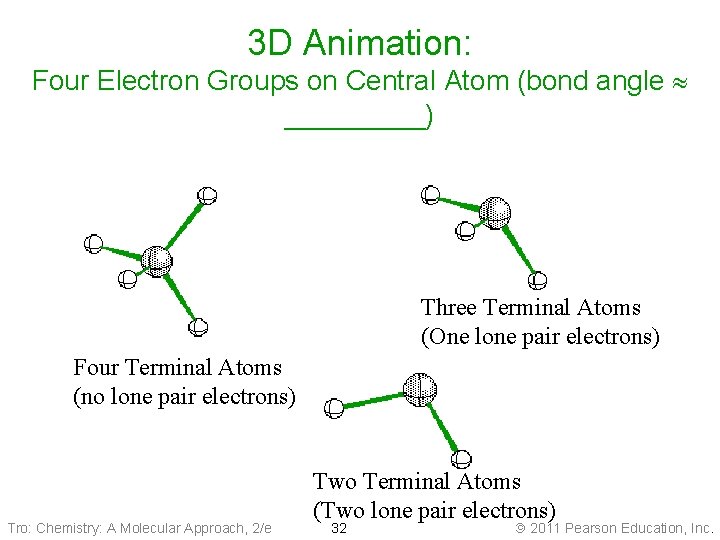
3 D Animation: Four Electron Groups on Central Atom (bond angle _____) Three Terminal Atoms (One lone pair electrons) Four Terminal Atoms (no lone pair electrons) Tro: Chemistry: A Molecular Approach, 2/e Two Terminal Atoms (Two lone pair electrons) 32 Copyright 2011 Pearson Education, Inc.

Non-Octet: Trigonal Bipyramidal Tro: Chemistry: A Molecular Approach, 2/e 33 Copyright 2011 Pearson Education, Inc.

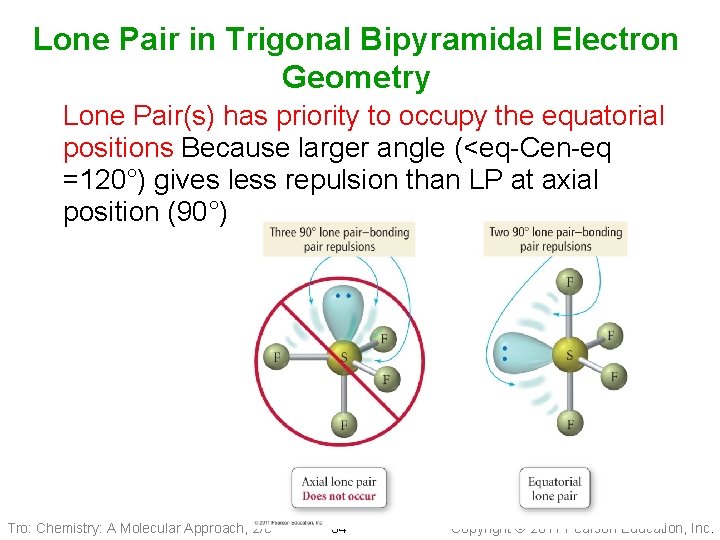
Lone Pair in Trigonal Bipyramidal Electron Geometry Lone Pair(s) has priority to occupy the equatorial positions Because larger angle (<eq-Cen-eq =120°) gives less repulsion than LP at axial position (90°) Tro: Chemistry: A Molecular Approach, 2/e 34 Copyright 2011 Pearson Education, Inc.

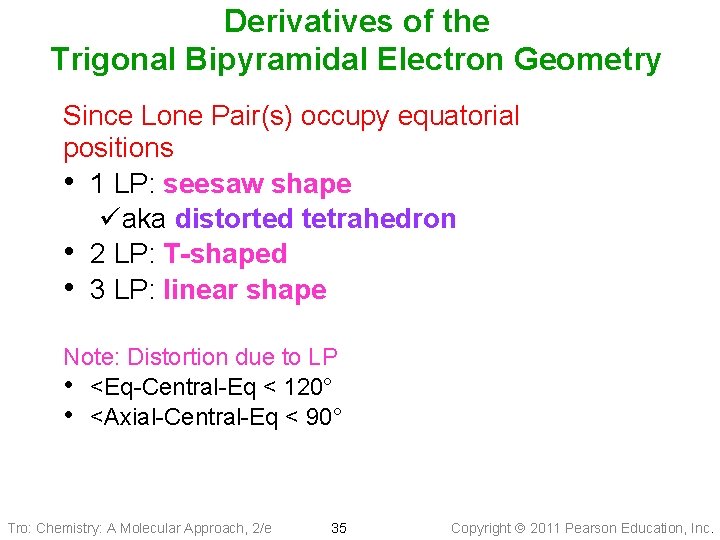
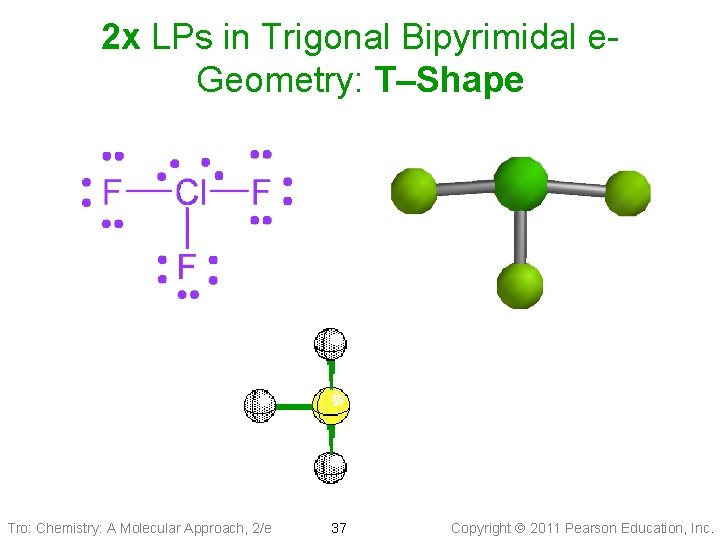
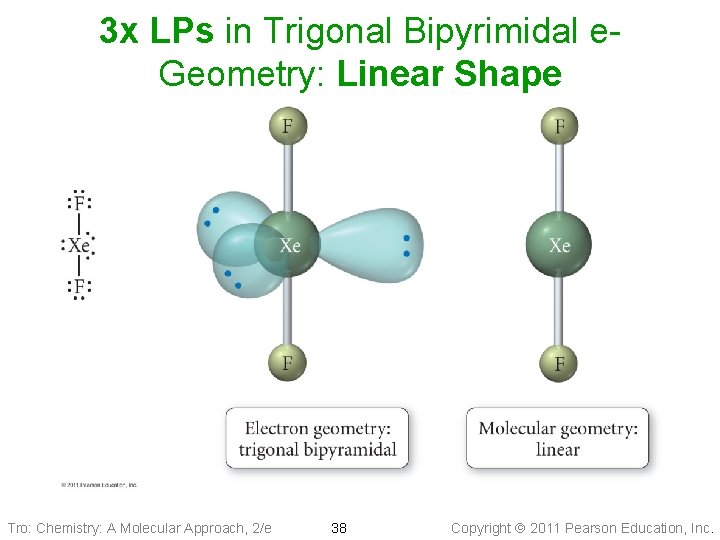
Derivatives of the Trigonal Bipyramidal Electron Geometry Since Lone Pair(s) occupy equatorial positions • 1 LP: seesaw shape üaka distorted tetrahedron • 2 LP: T-shaped • 3 LP: linear shape Note: Distortion due to LP • <Eq-Central-Eq < 120° • <Axial-Central-Eq < 90° Tro: Chemistry: A Molecular Approach, 2/e 35 Copyright 2011 Pearson Education, Inc.

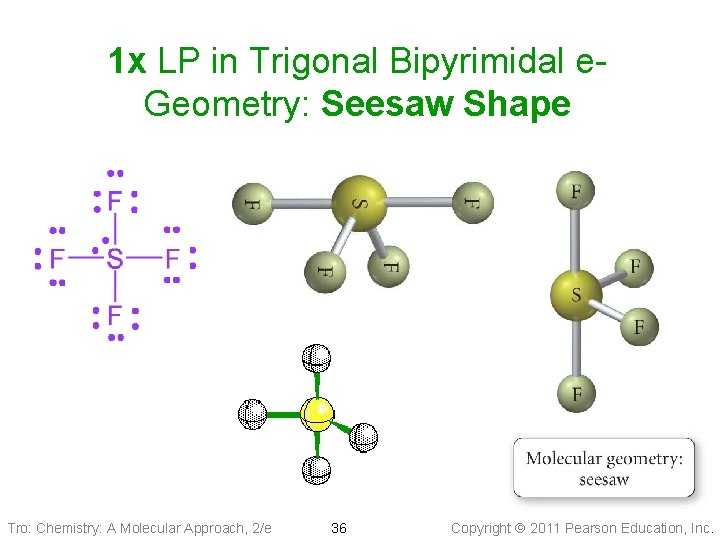
1 x LP in Trigonal Bipyrimidal e. Geometry: Seesaw Shape Tro: Chemistry: A Molecular Approach, 2/e 36 Copyright 2011 Pearson Education, Inc.

2 x LPs in Trigonal Bipyrimidal e. Geometry: T–Shape Tro: Chemistry: A Molecular Approach, 2/e 37 Copyright 2011 Pearson Education, Inc.

3 x LPs in Trigonal Bipyrimidal e. Geometry: Linear Shape Tro: Chemistry: A Molecular Approach, 2/e 38 Copyright 2011 Pearson Education, Inc.

Non-Octet: Octahedral F F S F F Tro: Chemistry: A Molecular Approach, 2/e 39 Copyright 2011 Pearson Education, Inc.

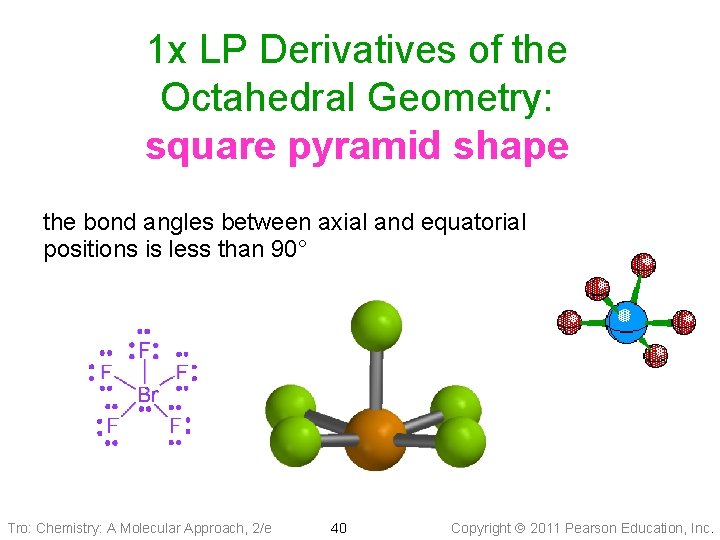
1 x LP Derivatives of the Octahedral Geometry: square pyramid shape the bond angles between axial and equatorial positions is less than 90° Tro: Chemistry: A Molecular Approach, 2/e 40 Copyright 2011 Pearson Education, Inc.

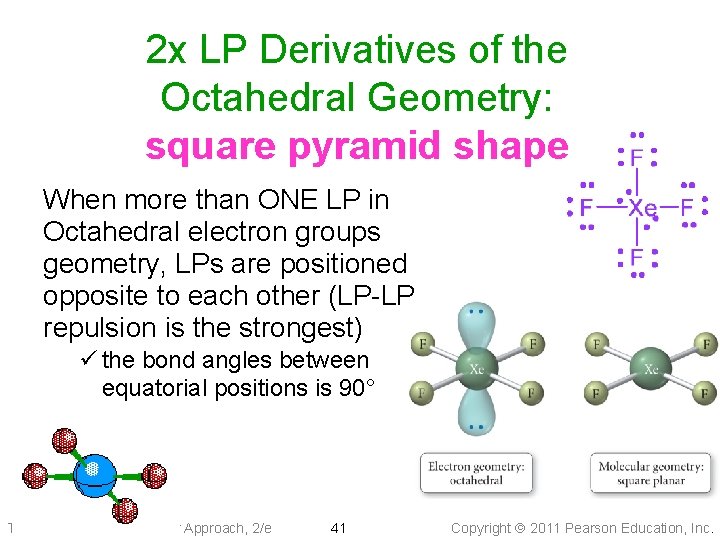
2 x LP Derivatives of the Octahedral Geometry: square pyramid shape When more than ONE LP in Octahedral electron groups geometry, LPs are positioned opposite to each other (LP-LP repulsion is the strongest) ü the bond angles between equatorial positions is 90° Tro: Chemistry: A Molecular Approach, 2/e 41 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 42 Copyright 2011 Pearson Education, Inc.

Predicting the Shapes Around Central Atoms 1. Draw the Lewis structure 2. Determine the number of electron groups around the central atom 3. Classify each electron group as bonding or lone pair, and count each type ü remember, multiple bonds count as one group 4. Determine the shape and bond angles Tro: Chemistry: A Molecular Approach, 2/e 43 Copyright 2011 Pearson Education, Inc.

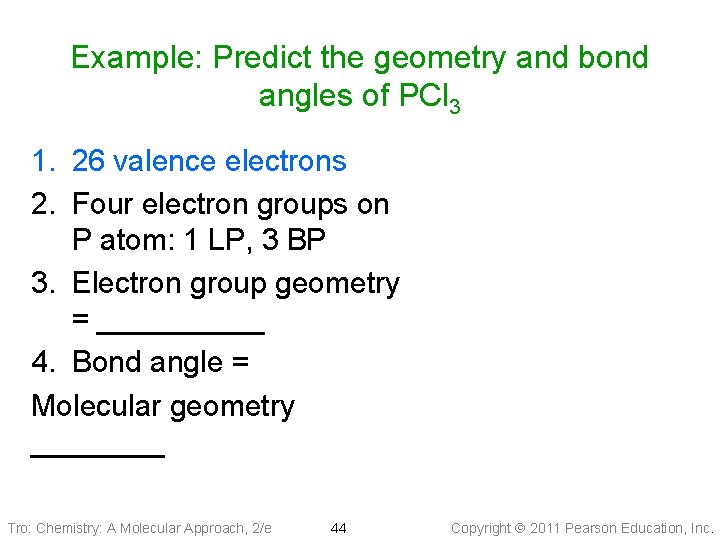
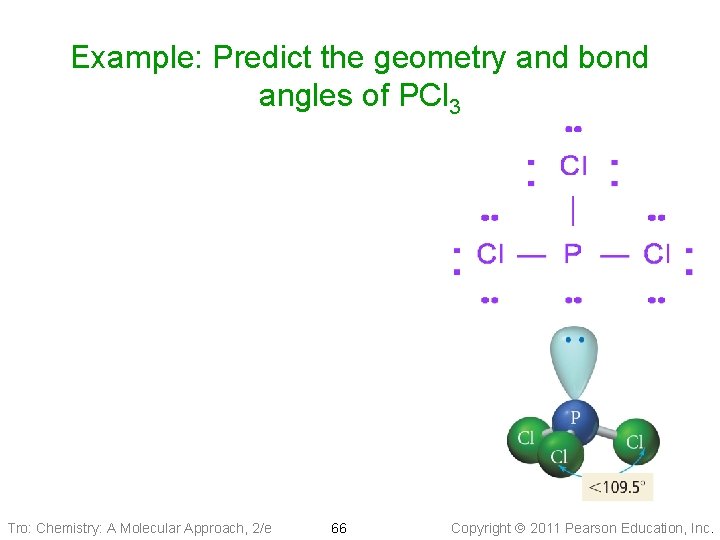
Example: Predict the geometry and bond angles of PCl 3 1. 26 valence electrons 2. Four electron groups on P atom: 1 LP, 3 BP 3. Electron group geometry = _____ 4. Bond angle = Molecular geometry ____ Tro: Chemistry: A Molecular Approach, 2/e 44 Copyright 2011 Pearson Education, Inc.

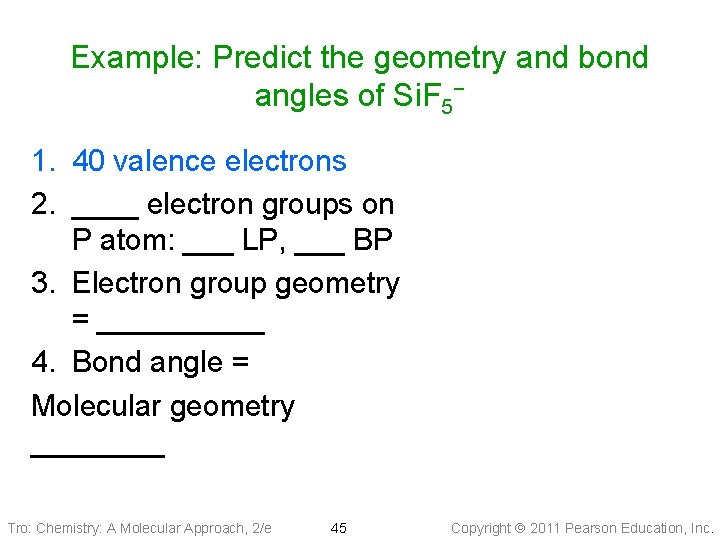
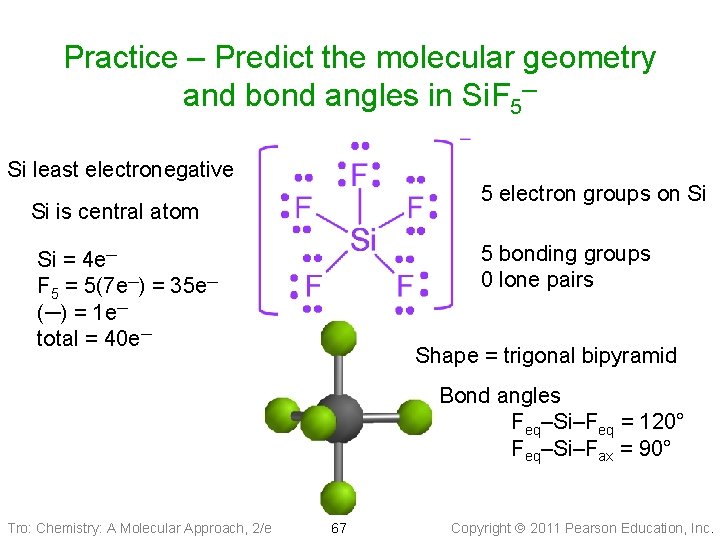
Example: Predict the geometry and bond angles of Si. F 5− 1. 40 valence electrons 2. ____ electron groups on P atom: ___ LP, ___ BP 3. Electron group geometry = _____ 4. Bond angle = Molecular geometry ____ Tro: Chemistry: A Molecular Approach, 2/e 45 Copyright 2011 Pearson Education, Inc.

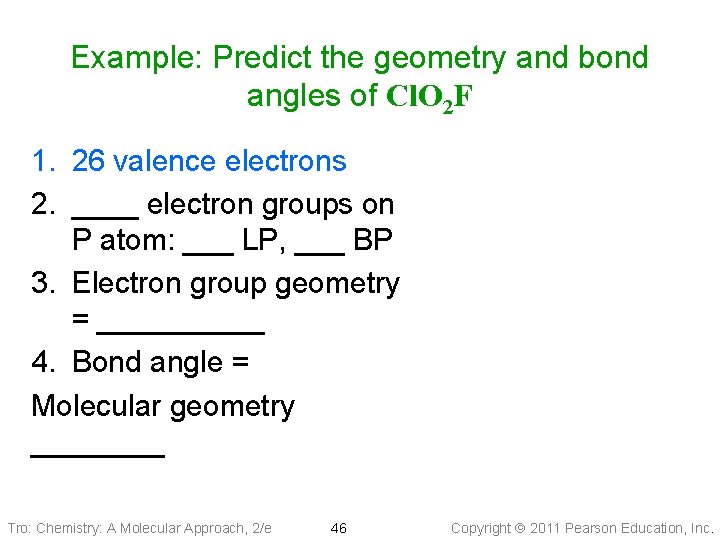
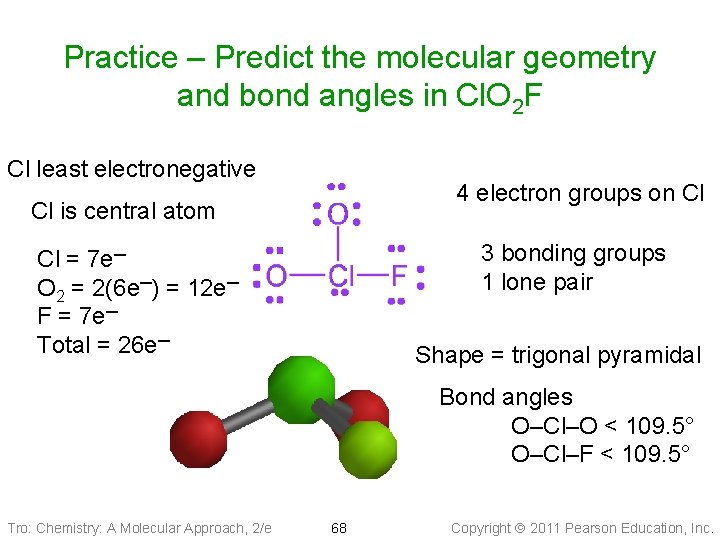
Example: Predict the geometry and bond angles of Cl. O 2 F 1. 26 valence electrons 2. ____ electron groups on P atom: ___ LP, ___ BP 3. Electron group geometry = _____ 4. Bond angle = Molecular geometry ____ Tro: Chemistry: A Molecular Approach, 2/e 46 Copyright 2011 Pearson Education, Inc.

Representing 3 -Dimensional Shapes on a 2 -Dimensional Surface General guideline: • The central atom is put in the plane of the paper • Put as many other atoms as possible in the same plane and indicate with a straight line • Atoms in front of the plane: solid wedge • Atoms behind the plane: hashed wedge Tro: Chemistry: A Molecular Approach, 2/e 47 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 48 Copyright 2011 Pearson Education, Inc.

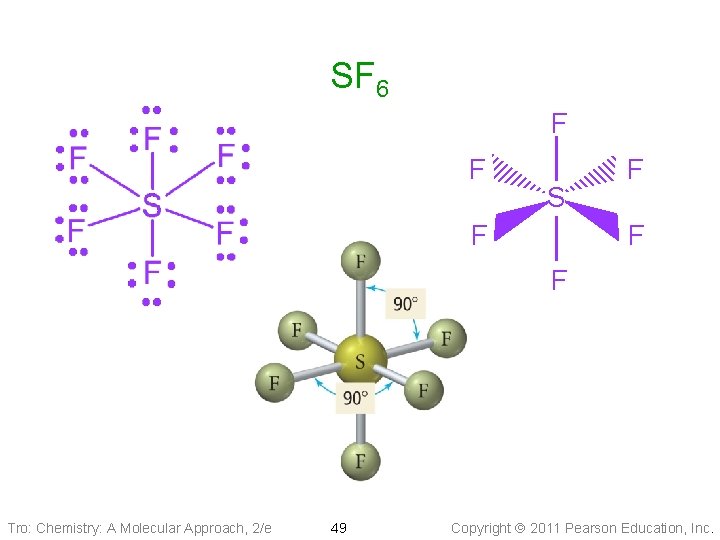
SF 6 F F S F F Tro: Chemistry: A Molecular Approach, 2/e 49 Copyright 2011 Pearson Education, Inc.

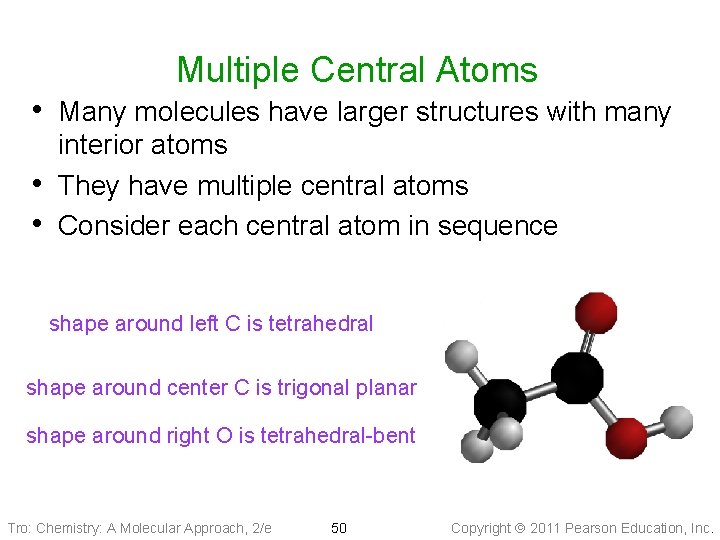
Multiple Central Atoms • Many molecules have larger structures with many • • interior atoms They have multiple central atoms Consider each central atom in sequence shape around left C is tetrahedral shape around center C is trigonal planar shape around right O is tetrahedral-bent Tro: Chemistry: A Molecular Approach, 2/e 50 Copyright 2011 Pearson Education, Inc.

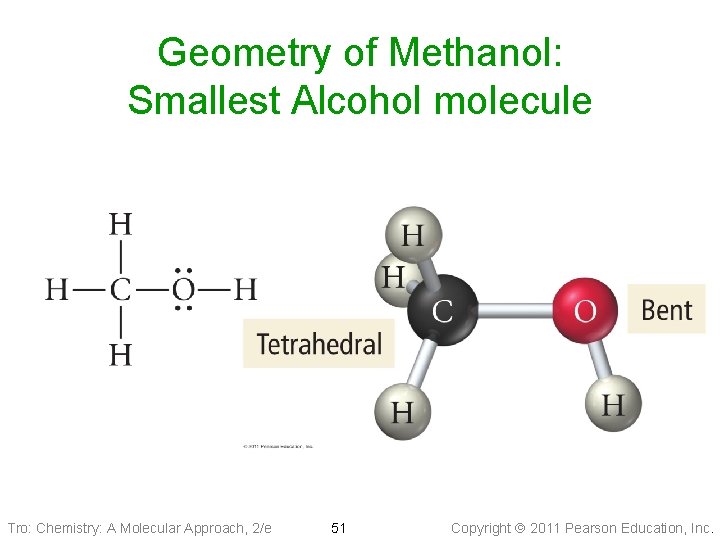
Geometry of Methanol: Smallest Alcohol molecule Tro: Chemistry: A Molecular Approach, 2/e 51 Copyright 2011 Pearson Education, Inc.

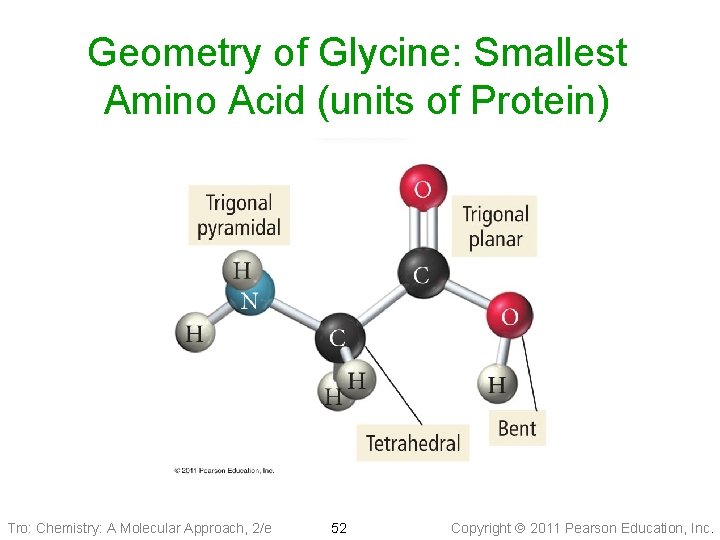
Geometry of Glycine: Smallest Amino Acid (units of Protein) Tro: Chemistry: A Molecular Approach, 2/e 52 Copyright 2011 Pearson Education, Inc.

Practice – Predict the molecular geometries in H 3 BO 3 Tro: Chemistry: A Molecular Approach, 2/e 53 Copyright 2011 Pearson Education, Inc.

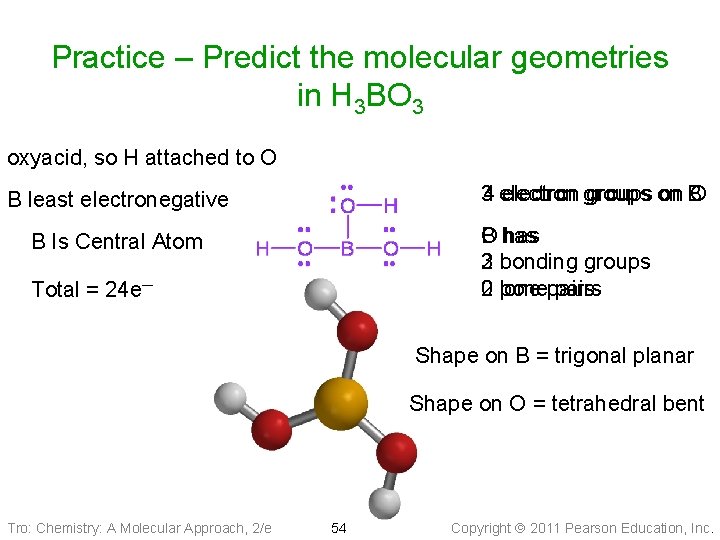
Practice – Predict the molecular geometries in H 3 BO 3 oxyacid, so H attached to O 34 electron groups on on B O B least electronegative O has B has 3 bonding groups 2 0 lone 2 ponepairs B Is Central Atom Total = 24 e─ Shape on B = trigonal planar Shape on O = tetrahedral bent Tro: Chemistry: A Molecular Approach, 2/e 54 Copyright 2011 Pearson Education, Inc.

Polarity of Molecules • For a molecule to be polar it must 1. have polar bonds Ø electronegativity difference - theory Ø bond dipole moments - measured 2. have an unsymmetrical shape Ø vector addition • Polarity affects the intermolecular forces of attraction ü therefore boiling points and solubilities Ø like dissolves like • Nonbonding pairs affect molecular polarity, strong pull in its direction Tro: Chemistry: A Molecular Approach, 2/e 55 Copyright 2011 Pearson Education, Inc.

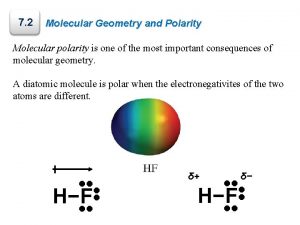
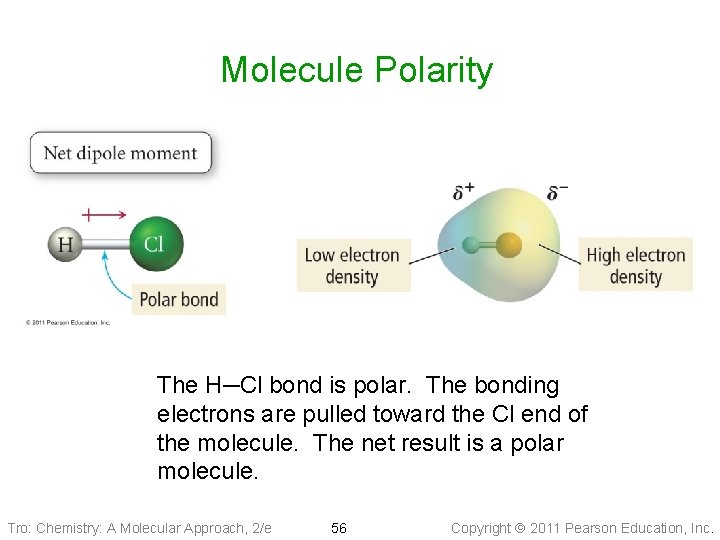
Molecule Polarity The H─Cl bond is polar. The bonding electrons are pulled toward the Cl end of the molecule. The net result is a polar molecule. Tro: Chemistry: A Molecular Approach, 2/e 56 Copyright 2011 Pearson Education, Inc.

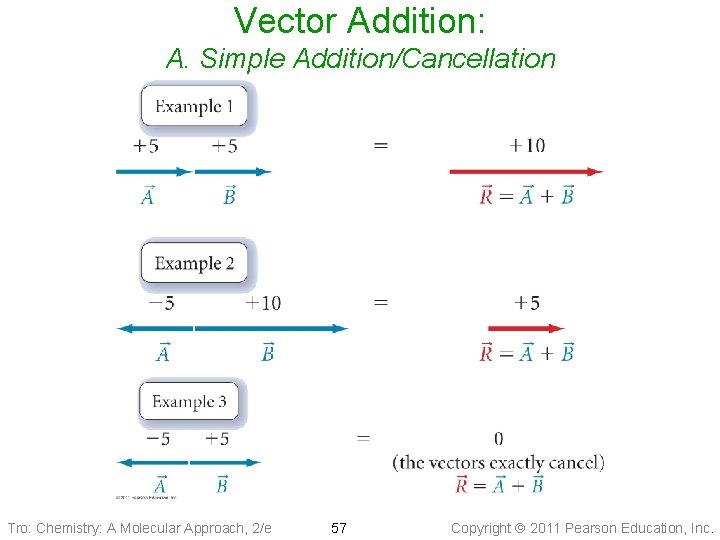
Vector Addition: A. Simple Addition/Cancellation Tro: Chemistry: A Molecular Approach, 2/e 57 Copyright 2011 Pearson Education, Inc.

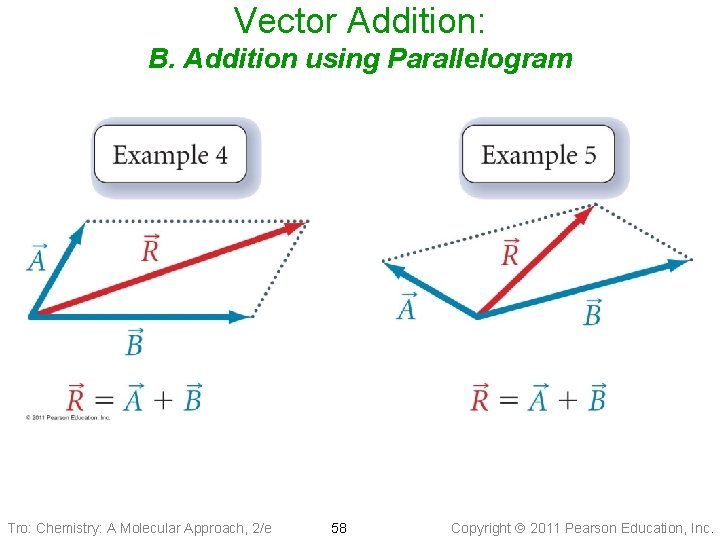
Vector Addition: B. Addition using Parallelogram Tro: Chemistry: A Molecular Approach, 2/e 58 Copyright 2011 Pearson Education, Inc.

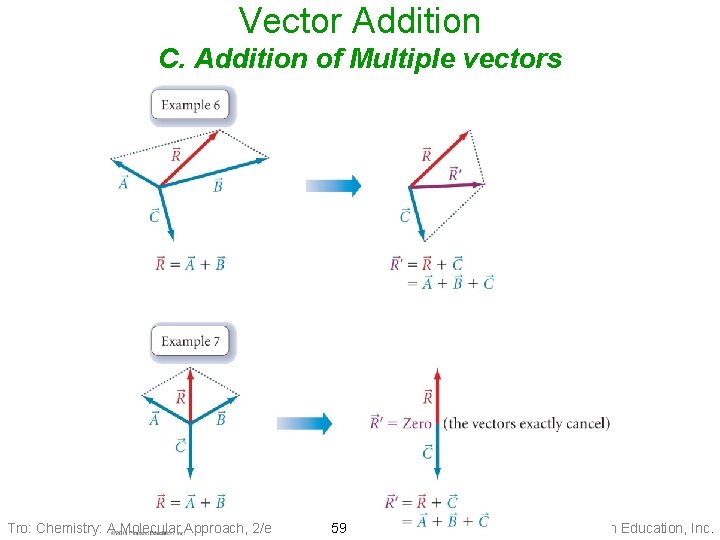
Vector Addition C. Addition of Multiple vectors Tro: Chemistry: A Molecular Approach, 2/e 59 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 60 Copyright 2011 Pearson Education, Inc.

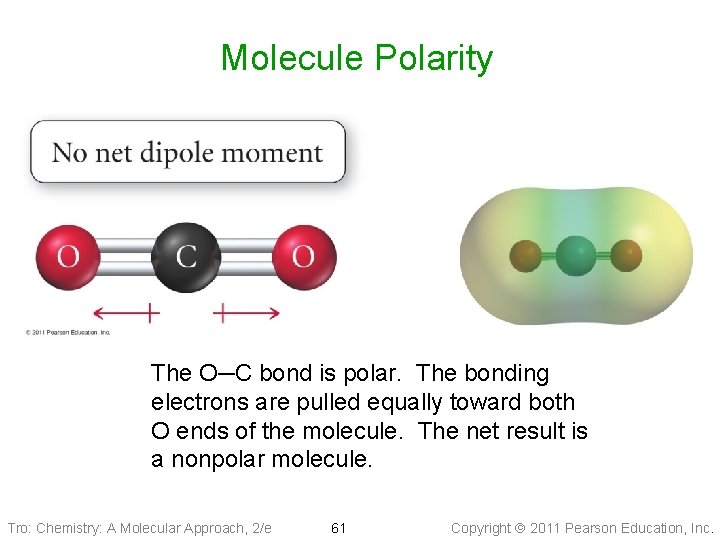
Molecule Polarity The O─C bond is polar. The bonding electrons are pulled equally toward both O ends of the molecule. The net result is a nonpolar molecule. Tro: Chemistry: A Molecular Approach, 2/e 61 Copyright 2011 Pearson Education, Inc.

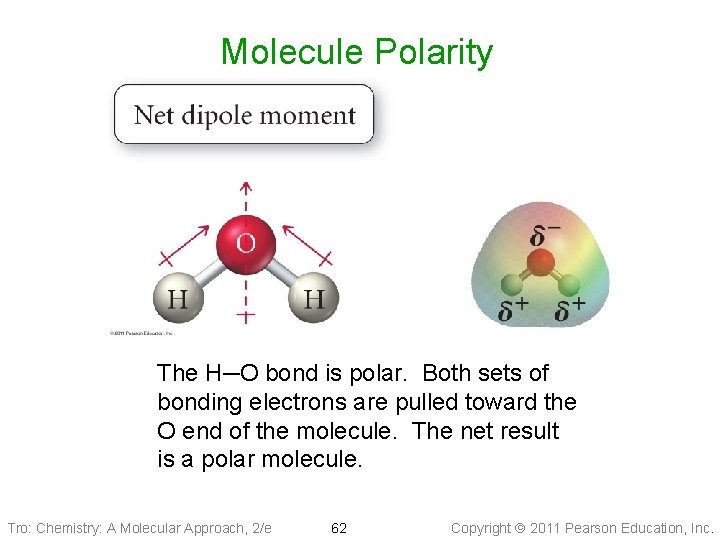
Molecule Polarity The H─O bond is polar. Both sets of bonding electrons are pulled toward the O end of the molecule. The net result is a polar molecule. Tro: Chemistry: A Molecular Approach, 2/e 62 Copyright 2011 Pearson Education, Inc.

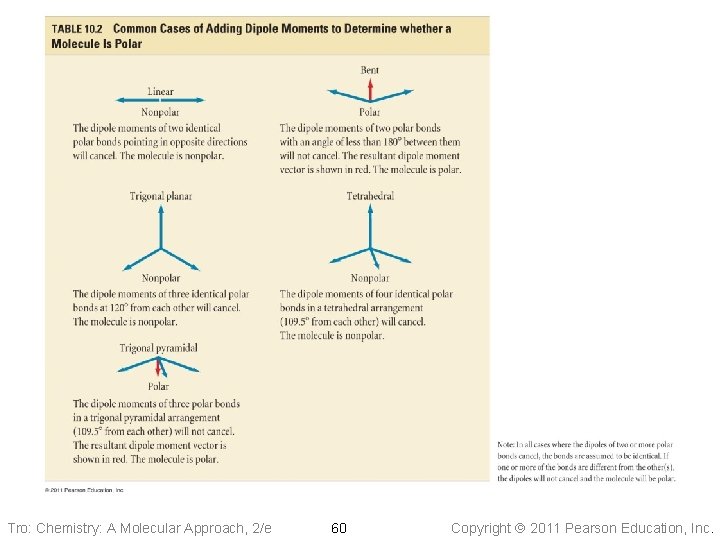
Predicting Polarity of Molecules 1. Draw the Lewis structure and determine the molecular geometry. Bent, Trig. Pyr. , Seesaw, T-shape: always polar!!! 2. Determine if bonds are polar if there are no polar bonds (DEN = 0), the molecule is nonpolar 3. Determine whether the polar bonds add together to give a net dipole moment Tro: Chemistry: A Molecular Approach, 2/e 63 Copyright 2011 Pearson Education, Inc.

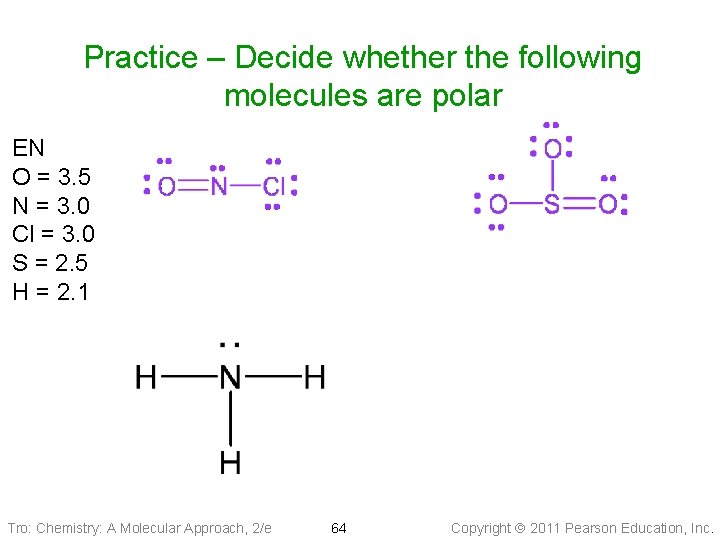
Practice – Decide whether the following molecules are polar EN O = 3. 5 N = 3. 0 Cl = 3. 0 S = 2. 5 H = 2. 1 Tro: Chemistry: A Molecular Approach, 2/e 64 Copyright 2011 Pearson Education, Inc.

Molecular Polarity Affects Solubility in Water • Polar molecules are attracted • to other polar molecules Because water is a polar molecule, other polar molecules dissolve well in water ü and ionic compounds as well • Some molecules have both polar and nonpolar parts Tro: Chemistry: A Molecular Approach, 2/e 65 Copyright 2011 Pearson Education, Inc.

Example: Predict the geometry and bond angles of PCl 3 Tro: Chemistry: A Molecular Approach, 2/e 66 Copyright 2011 Pearson Education, Inc.

Practice – Predict the molecular geometry and bond angles in Si. F 5─ Si least electronegative 5 electron groups on Si Si is central atom 5 bonding groups 0 lone pairs Si = 4 e─ F 5 = 5(7 e─) = 35 e─ (─) = 1 e─ total = 40 e─ Shape = trigonal bipyramid Bond angles Feq–Si–Feq = 120° Feq–Si–Fax = 90° Tro: Chemistry: A Molecular Approach, 2/e 67 Copyright 2011 Pearson Education, Inc.

Practice – Predict the molecular geometry and bond angles in Cl. O 2 F Cl least electronegative 4 electron groups on Cl Cl is central atom 3 bonding groups 1 lone pair Cl = 7 e─ O 2 = 2(6 e─) = 12 e─ F = 7 e─ Total = 26 e─ Shape = trigonal pyramidal Bond angles O–Cl–O < 109. 5° O–Cl–F < 109. 5° Tro: Chemistry: A Molecular Approach, 2/e 68 Copyright 2011 Pearson Education, Inc.

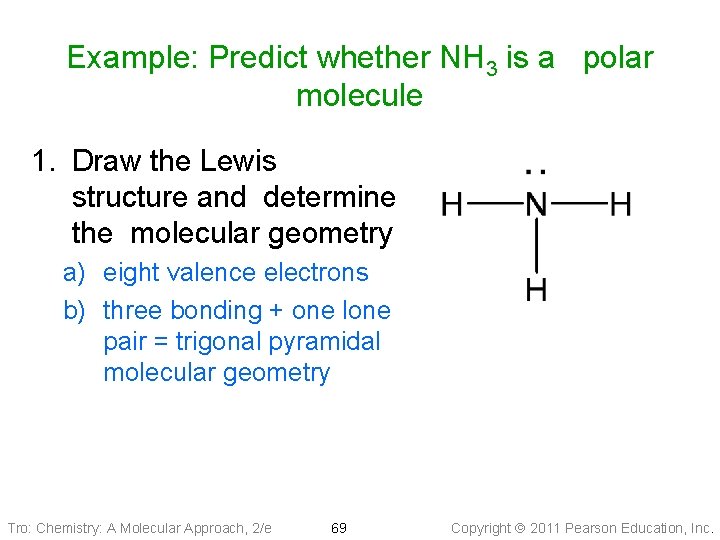
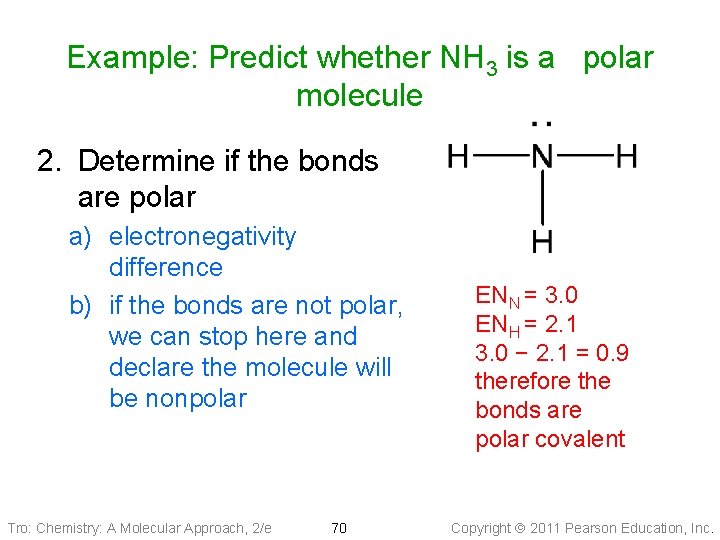
Example: Predict whether NH 3 is a polar molecule 1. Draw the Lewis structure and determine the molecular geometry a) eight valence electrons b) three bonding + one lone pair = trigonal pyramidal molecular geometry Tro: Chemistry: A Molecular Approach, 2/e 69 Copyright 2011 Pearson Education, Inc.

Example: Predict whether NH 3 is a polar molecule 2. Determine if the bonds are polar a) electronegativity difference b) if the bonds are not polar, we can stop here and declare the molecule will be nonpolar Tro: Chemistry: A Molecular Approach, 2/e 70 ENN = 3. 0 ENH = 2. 1 3. 0 − 2. 1 = 0. 9 therefore the bonds are polar covalent Copyright 2011 Pearson Education, Inc.

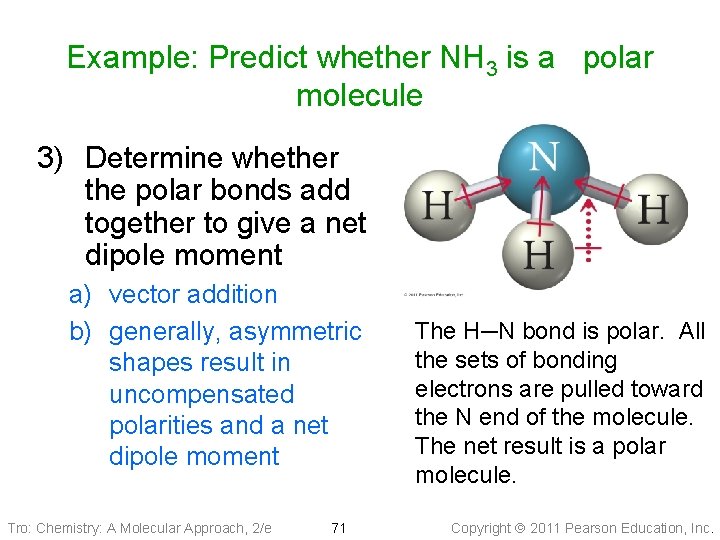
Example: Predict whether NH 3 is a polar molecule 3) Determine whether the polar bonds add together to give a net dipole moment a) vector addition b) generally, asymmetric shapes result in uncompensated polarities and a net dipole moment Tro: Chemistry: A Molecular Approach, 2/e 71 The H─N bond is polar. All the sets of bonding electrons are pulled toward the N end of the molecule. The net result is a polar molecule. Copyright 2011 Pearson Education, Inc.

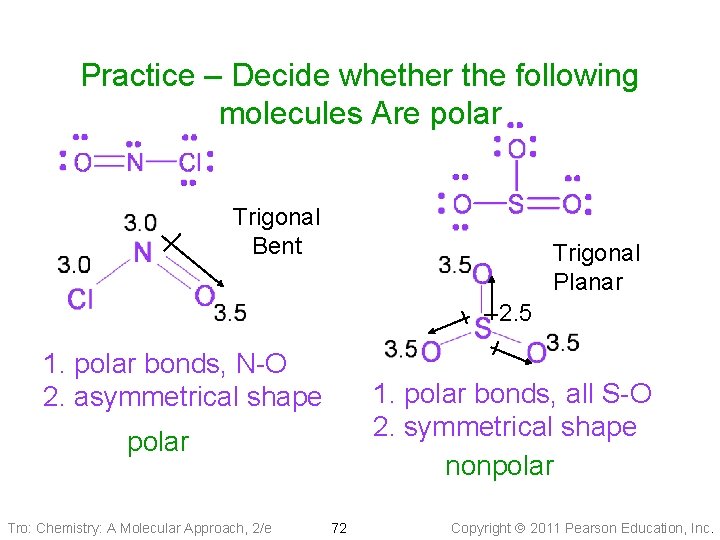
Practice – Decide whether the following molecules Are polar Trigonal Bent Trigonal Planar 2. 5 1. polar bonds, N-O 2. asymmetrical shape 1. polar bonds, all S-O 2. symmetrical shape nonpolar Tro: Chemistry: A Molecular Approach, 2/e 72 Copyright 2011 Pearson Education, Inc.
 Co shape and polarity
Co shape and polarity Bond polarity and molecular polarity
Bond polarity and molecular polarity How to memorize molecular geometry
How to memorize molecular geometry Vsepr theory project
Vsepr theory project Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition answers
Introductory chemistry 5th edition answers Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Polarity worksheet
Polarity worksheet Molecular polarity
Molecular polarity Molecular shapes
Molecular shapes Predicting molecular polarity
Predicting molecular polarity H h c h h formula
H h c h h formula Molecular polarity
Molecular polarity Molecular geometry polarity
Molecular geometry polarity Molecular geometry and bonding theories
Molecular geometry and bonding theories Vsepr stands for :
Vsepr stands for : Chcch3 lewis structure
Chcch3 lewis structure Vsepr metoda
Vsepr metoda Vsepr formulas
Vsepr formulas Vsepr theory angles
Vsepr theory angles Vsepr theory assignment
Vsepr theory assignment Vserp pia
Vserp pia Bond angle
Bond angle 5 bond pairs 1 lone pair
5 bond pairs 1 lone pair Importance of molecular geometry
Importance of molecular geometry What do scientists use vsepr theory for
What do scientists use vsepr theory for Vbt and mot
Vbt and mot Valence bond theory vs molecular orbital theory
Valence bond theory vs molecular orbital theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Theories of covalent bonding
Theories of covalent bonding Bond order of li2
Bond order of li2 Notice tro
Notice tro Tra tre tri tro tru imagenes
Tra tre tri tro tru imagenes Nêu vai trò của nghề làm vườn
Nêu vai trò của nghề làm vườn Hỡi người hãy nhớ mình là bụi tro
Hỡi người hãy nhớ mình là bụi tro Tò vò mà nuôi con nhện phương thức biểu đạt
Tò vò mà nuôi con nhện phương thức biểu đạt Con trỏ sql
Con trỏ sql Trò chơi khuông nhạc bàn tay
Trò chơi khuông nhạc bàn tay Trò chơi xem kịch câm
Trò chơi xem kịch câm Trò chơi chữ cái u ư chủ đề nghề nghiệp
Trò chơi chữ cái u ư chủ đề nghề nghiệp Valence bond formula
Valence bond formula Tro
Tro Biblical definition of humility
Biblical definition of humility Bài tập về nhà
Bài tập về nhà Bài hát ước mơ lớp 5
Bài hát ước mơ lớp 5 Tro
Tro Vai trò của thực vật đối với động vật
Vai trò của thực vật đối với động vật Vai trò thực tiễn của lớp giáp xác
Vai trò thực tiễn của lớp giáp xác Aftaleindgåelse
Aftaleindgåelse ơn phù trợ chúng ta ở nơi danh chúa
ơn phù trợ chúng ta ở nơi danh chúa Trả lời câu hỏi nghĩa thầy trò
Trả lời câu hỏi nghĩa thầy trò Covalent bond boiling point
Covalent bond boiling point Ionic covalent metallic
Ionic covalent metallic Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Ap chemistry molecular geometry
Ap chemistry molecular geometry Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Polarity of water
Polarity of water Polarity of pollen grain
Polarity of pollen grain Polarity analysis
Polarity analysis Reference polarity
Reference polarity Bond order formula
Bond order formula Chcl3 bond polarity
Chcl3 bond polarity Different polarities
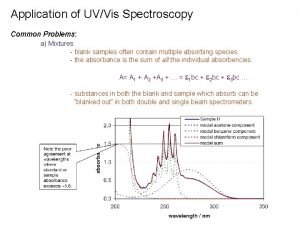
Different polarities Effect of solvent polarity on uv absorption
Effect of solvent polarity on uv absorption Ch3 nh ch ch3 twice
Ch3 nh ch ch3 twice Polarity electronegativity
Polarity electronegativity Hades do bonds do anything
Hades do bonds do anything Covalent bond comic strip
Covalent bond comic strip Why is water polar
Why is water polar Hei and eis are examples of
Hei and eis are examples of Polarity trend
Polarity trend