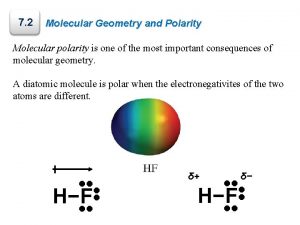
Molecular Polarity Molecular Polarity Depends on 2 factors
- Slides: 14

Molecular Polarity

Molecular Polarity • Depends on 2 factors. – Type of bonds in the molecule – Arrangement of bonds or shape of molecule

Diatomic Molecules • Simplest case: • Bond Polarity and Molecular Polarity are identical • Larger Molecules – Have to look at how the bonds are arranged.

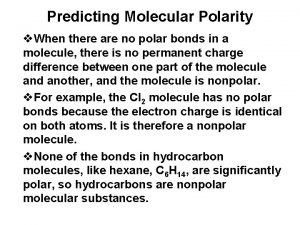
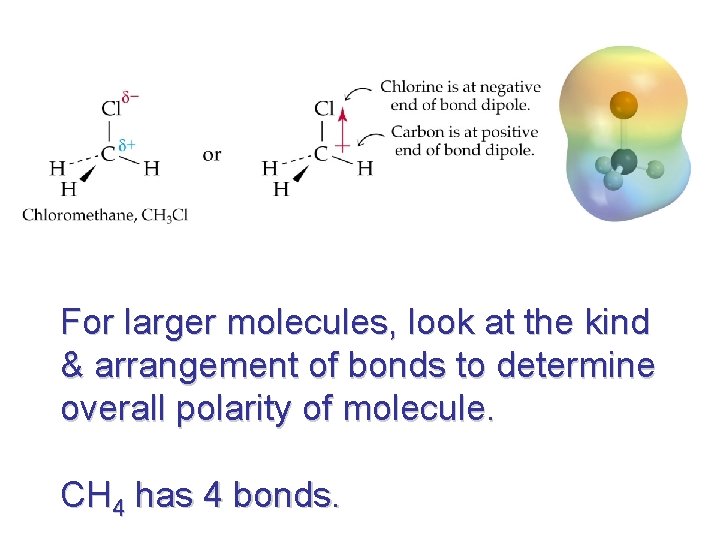
For larger molecules, look at the kind & arrangement of bonds to determine overall polarity of molecule. CH 4 has 4 bonds.

Polarity of Molecules • A molecule may contain polar bonds, but not be polar! Depends on the geometry of the molecule. • If molecule is symmetric, the “pull” of one polar bond is offset by the “pull” of another polar bond. – It’s a tug-of-war that no one can win!

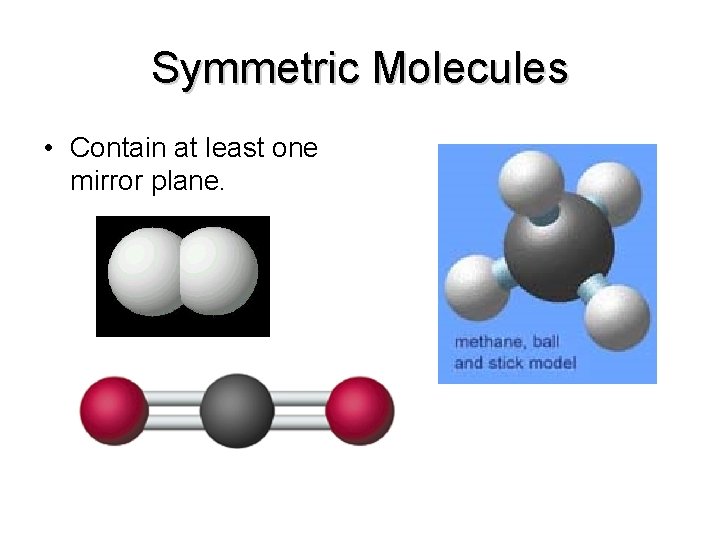
Symmetric Molecules • Contain at least one mirror plane.

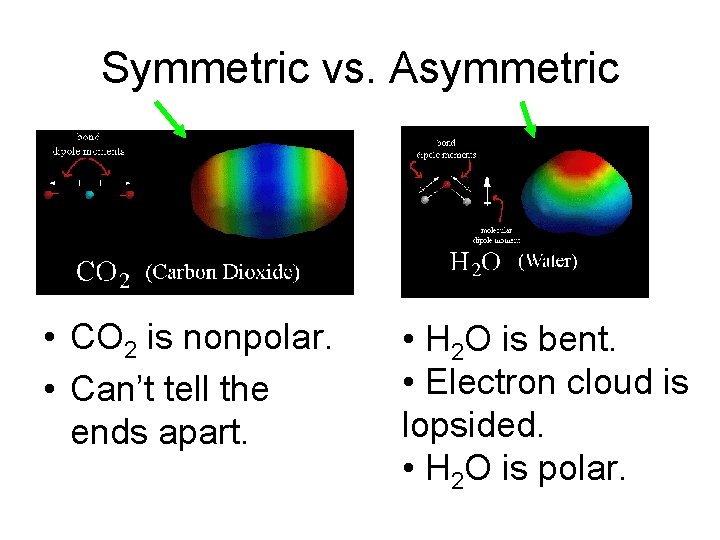
Symmetric vs. Asymmetric • CO 2 is nonpolar. • Can’t tell the ends apart. • H 2 O is bent. • Electron cloud is lopsided. • H 2 O is polar.

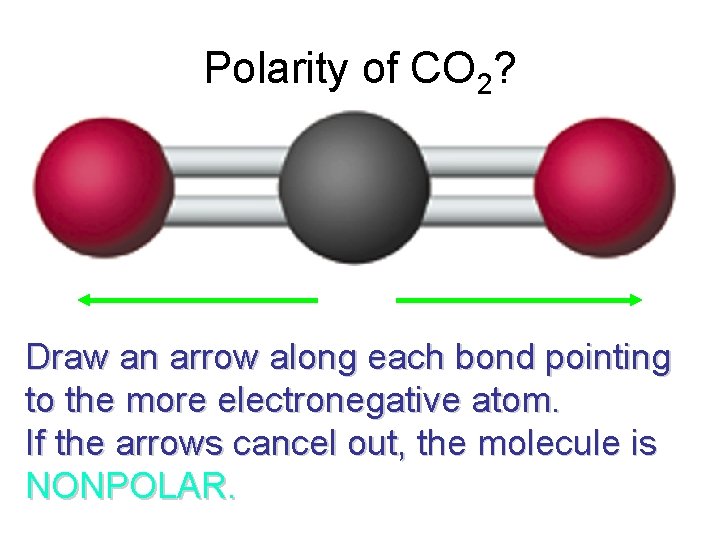
Polarity of CO 2? Draw an arrow along each bond pointing to the more electronegative atom. If the arrows cancel out, the molecule is NONPOLAR.

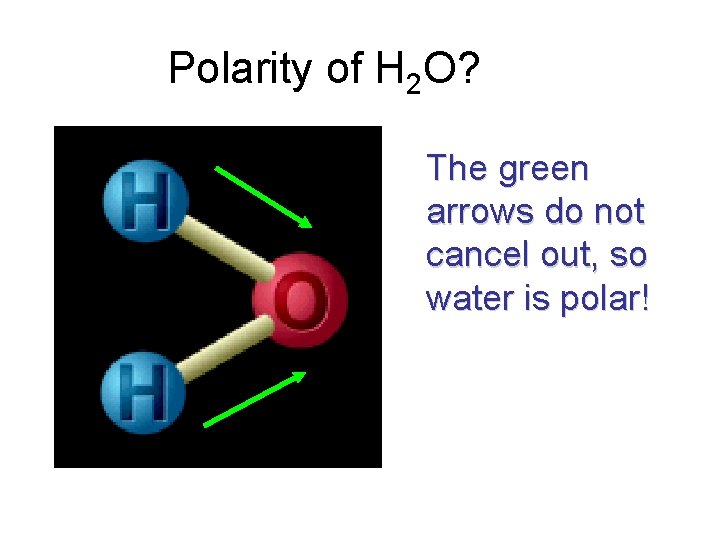
Polarity of H 2 O? The green arrows do not cancel out, so water is polar!

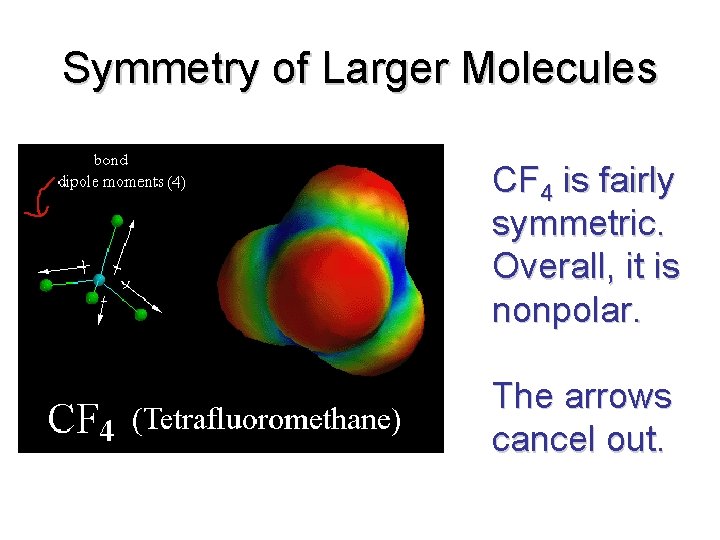
Symmetry of Larger Molecules CF 4 is fairly symmetric. Overall, it is nonpolar. The arrows cancel out.

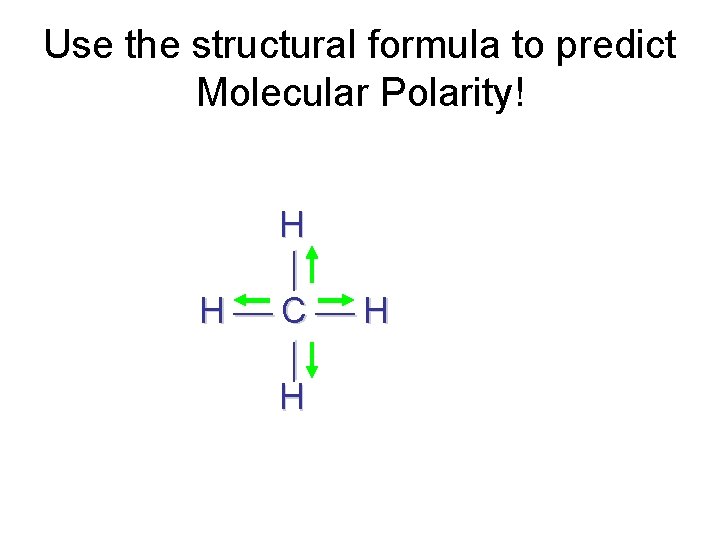
Use the structural formula to predict Molecular Polarity! H H C H H

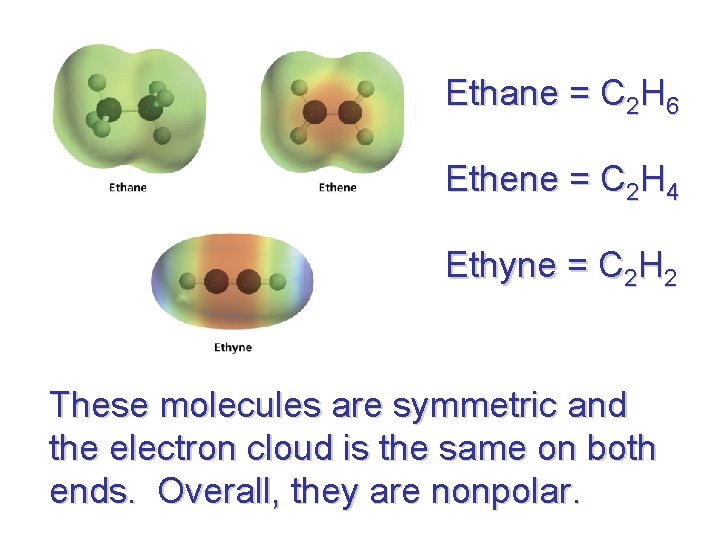
Ethane = C 2 H 6 Ethene = C 2 H 4 Ethyne = C 2 H 2 These molecules are symmetric and the electron cloud is the same on both ends. Overall, they are nonpolar.

Molecular Polarity • If you know the shape, you can use the arrow technique to determine the polarity. • So how do you get the shape?

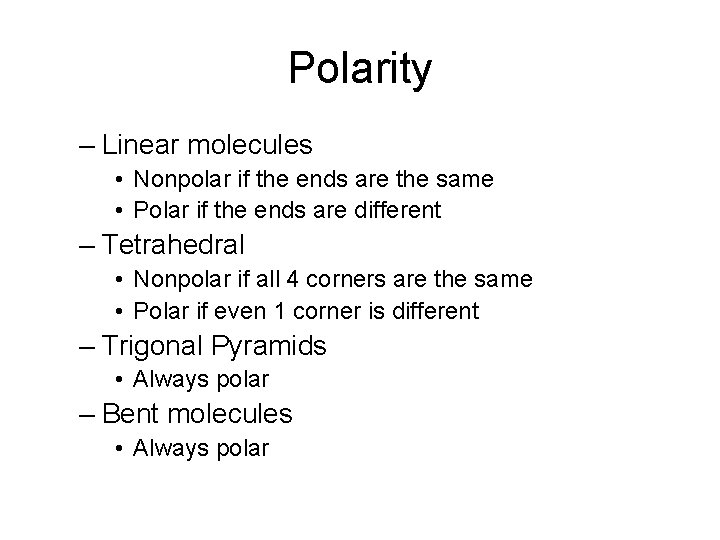
Polarity – Linear molecules • Nonpolar if the ends are the same • Polar if the ends are different – Tetrahedral • Nonpolar if all 4 corners are the same • Polar if even 1 corner is different – Trigonal Pyramids • Always polar – Bent molecules • Always polar
 Shape and polarity
Shape and polarity Bond polarity and molecular polarity
Bond polarity and molecular polarity Momentum is affected by what two factors
Momentum is affected by what two factors Predicting molecular polarity
Predicting molecular polarity Molecular shape worksheet
Molecular shape worksheet H h c h h formula
H h c h h formula Molecular polarity
Molecular polarity Molecular polarity
Molecular polarity Color by number molecular geometry and polarity
Color by number molecular geometry and polarity Molecular geometry polarity
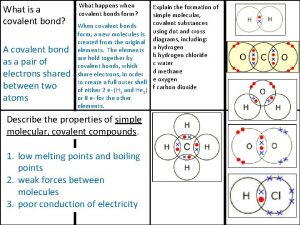
Molecular geometry polarity What is a covalent bond simple definition
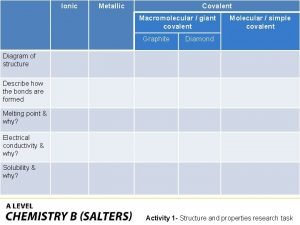
What is a covalent bond simple definition Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Site and situation factors
Site and situation factors What is site vs situation
What is site vs situation