Unit 2 4 Rearrangement of Atoms in Chemical



























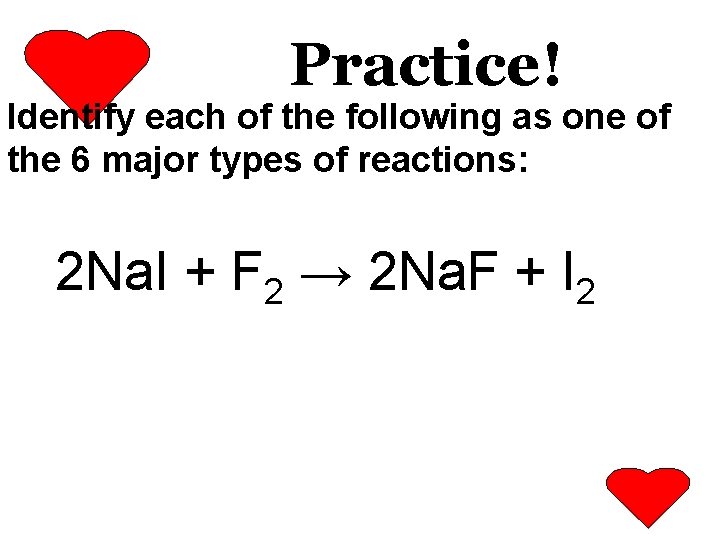





- Slides: 64

Unit 2 -4: Rearrangement of Atoms in Chemical Reactions

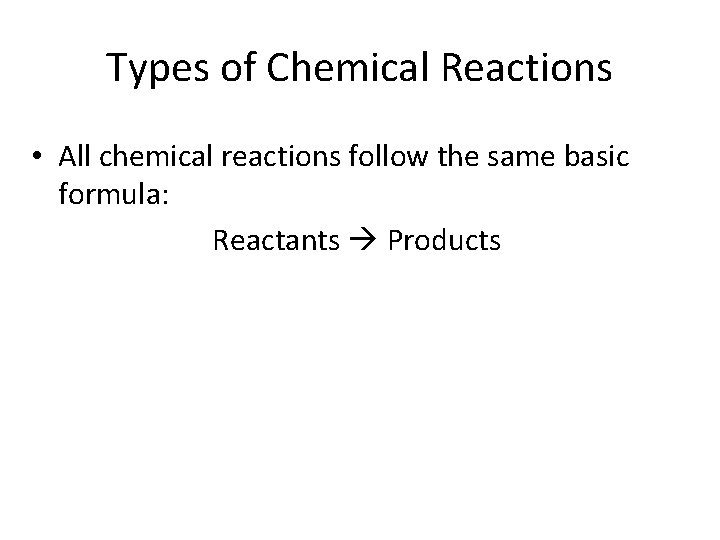
Types of Chemical Reactions • All chemical reactions follow the same basic formula: Reactants Products

In grade 9 you learned that: • If energy is required for a reaction to occur: Reactants + energy Products - Then the reaction is classified as ENDOTHERMIC • If energy is released or produced during a reaction: Reactants Products + energy - Then the reaction is classified as EXOTHERMIC

• Reactions can be further classified based on HOW the reactants combine and HOW the products form • There are 6 common types of reactions that you need to be able to identify!

Classification of Chemical Reactions: The Soap-Opera of Chemistry

6 Main types of reactions • Synthesis- “Love at first sight” • Decomposition- “The big break-up” • Single Replacement- “My best friends girlfriend” • Double Replacement- “Revenge” Also, Neutralization • Combustion- “The player”

Synthesis- “Love at first sight” I Ilike Can I get your Hey your braces hat Harry number Hey Sally Basic equation: A+B AB

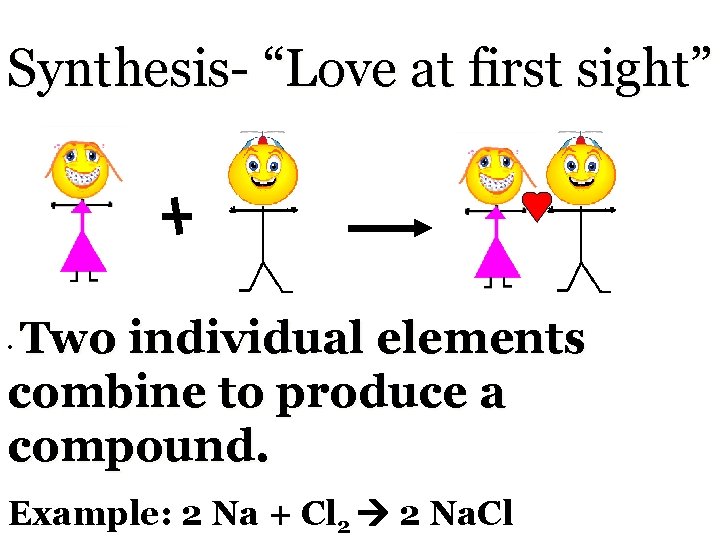
Synthesis- “Love at first sight” Two individual elements combine to produce a compound. • Example: 2 Na + Cl 2 2 Na. Cl

Practice: Mg + N 2

Practice: Al + F 2

Practice: K + O 2

Practice: Cd + I 2

Practice: Cs + P 4

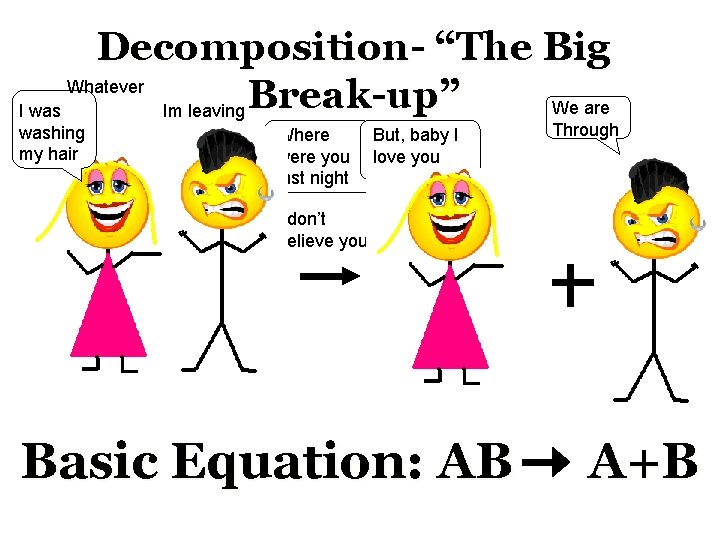
Decomposition- “The Big Whatever Break-up” We are I was Im leaving washing my hair Where were you last night But, baby I love you Through I don’t believe you Basic Equation: AB A+B

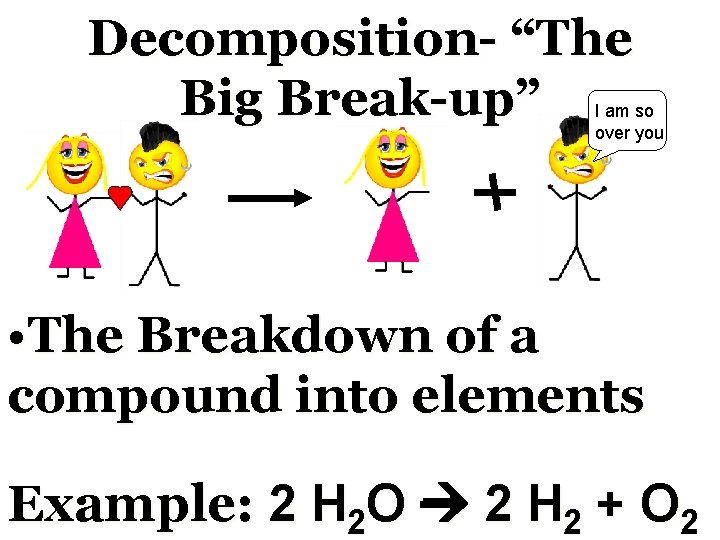
Decomposition- “The Big Break-up” I am so over you • The Breakdown of a compound into elements Example: 2 H 2 O 2 H 2 + O 2

Practice: Au. Cl 3

Practice: K 2 O

Practice: Mg. F 2

Practice: Ca 3 N 2

Practice: Cs. I

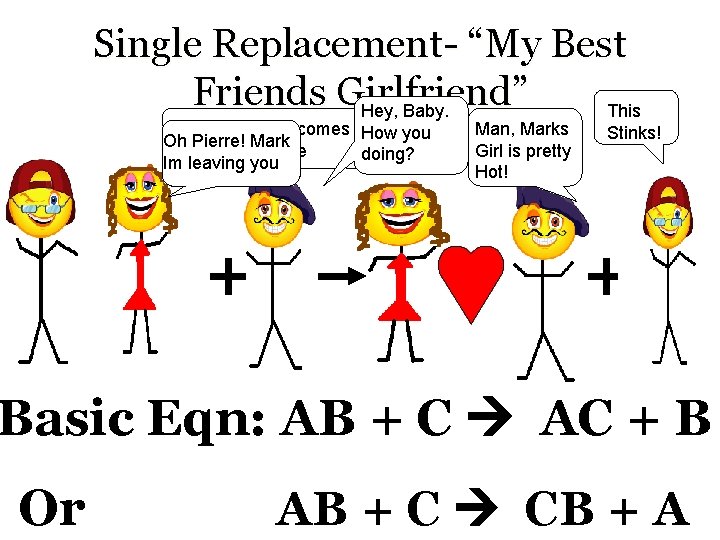
Single Replacement- “My Best Friends Girlfriend” Hey, Baby. This Hey Mark, here comes How you Oh Pierre! Mark your friend Pierre doing? Im leaving you Man, Marks Girl is pretty Hot! Stinks! Basic Eqn: AB + C AC + B Or AB + C CB + A

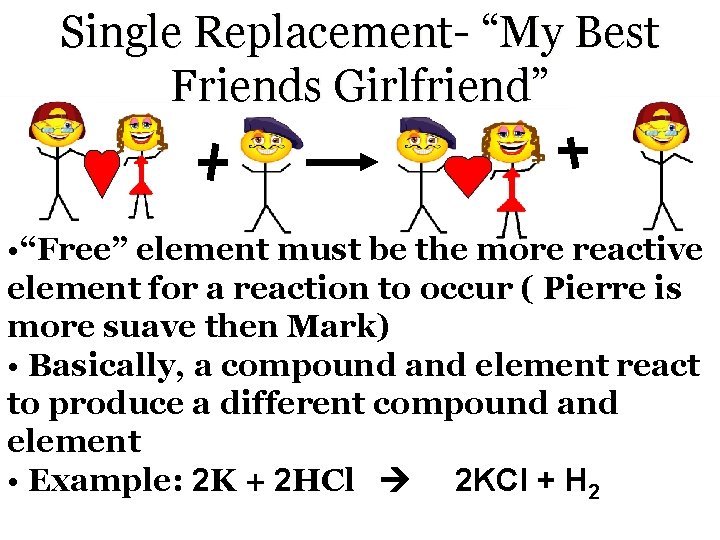
Single Replacement- “My Best Friends Girlfriend” • “Free” element must be the more reactive element for a reaction to occur ( Pierre is more suave then Mark) • Basically, a compound and element react to produce a different compound and element • Example: 2 K + 2 HCl 2 KCl + H 2

Practice: Pb. Cl 4 + Al

Practice: Na + Cu 2 O

Practice: Cu. F 2 + Mg

Practice: Cl 2 + Cs. Br

Practice: Be + Fe(NO 3)2

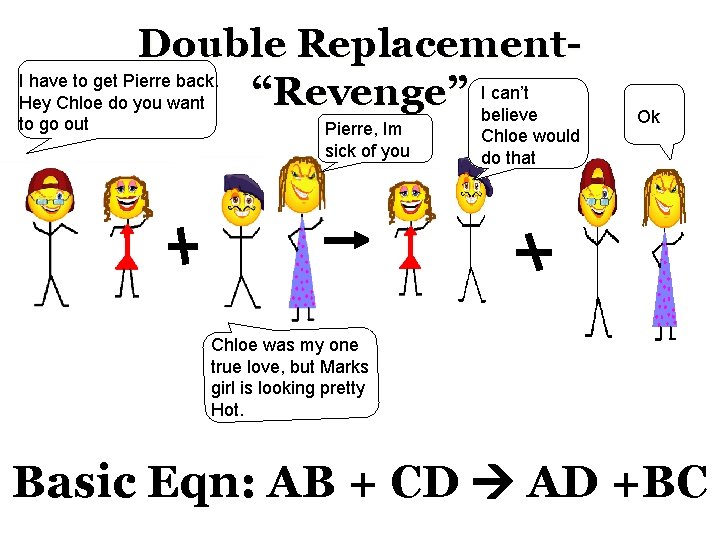
Double Replacement. I have to get Pierre back. can’t “Revenge” Ibelieve Hey Chloe do you want to go out Pierre, Im sick of you Chloe would do that Ok Chloe was my one true love, but Marks girl is looking pretty Hot. Basic Eqn: AB + CD AD +BC

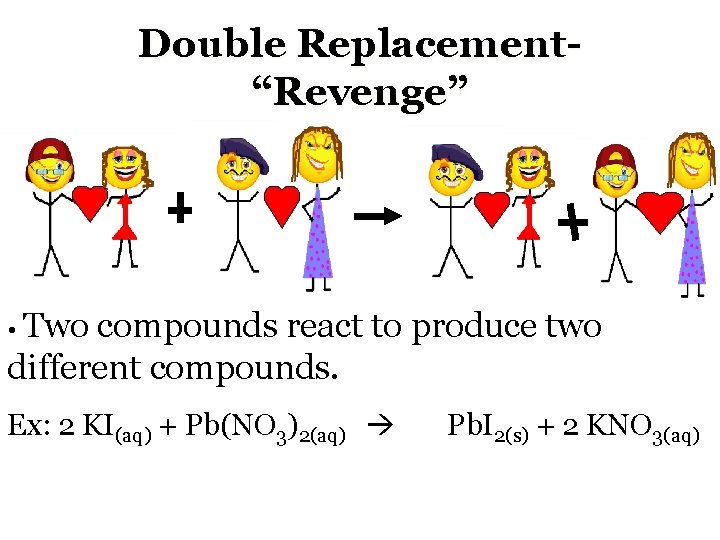
Double Replacement“Revenge” • Two compounds react to produce two different compounds. Ex: 2 KI(aq) + Pb(NO 3)2(aq) Pb. I 2(s) + 2 KNO 3(aq)

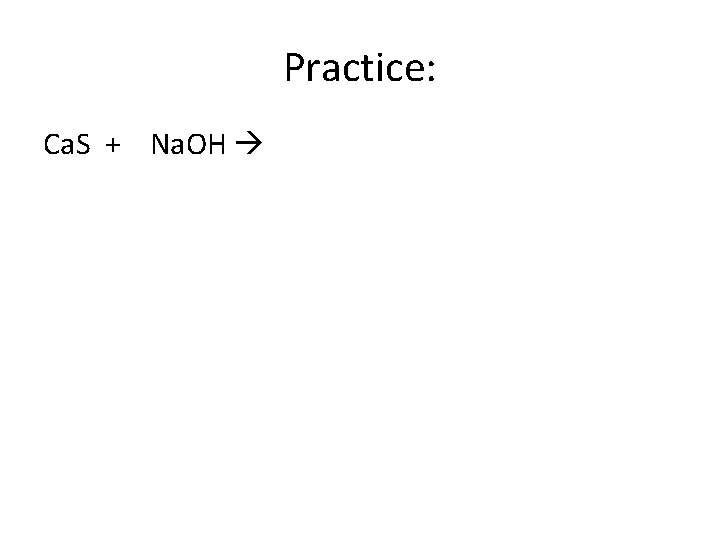
Practice: Ca. S + Na. OH

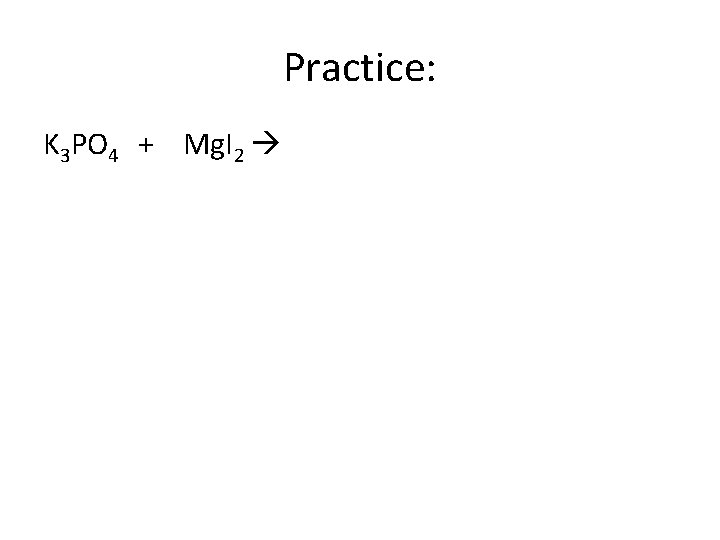
Practice: K 3 PO 4 + Mg. I 2

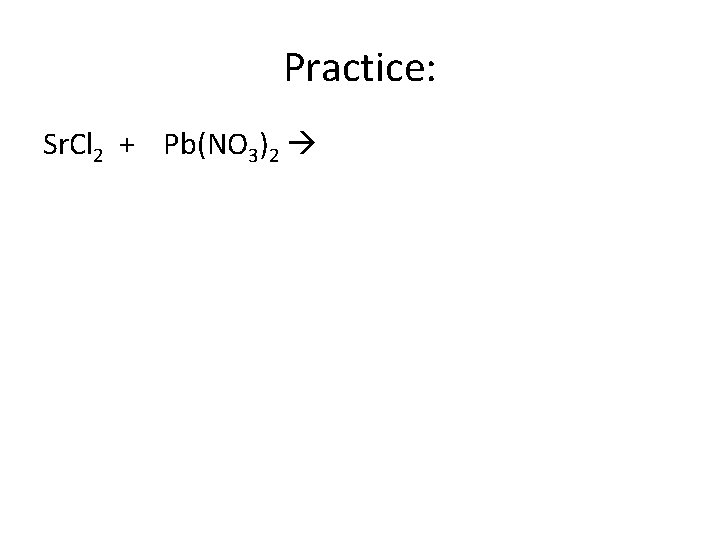
Practice: Sr. Cl 2 + Pb(NO 3)2

Practice: Al. Cl 3 + Cu. NO 3

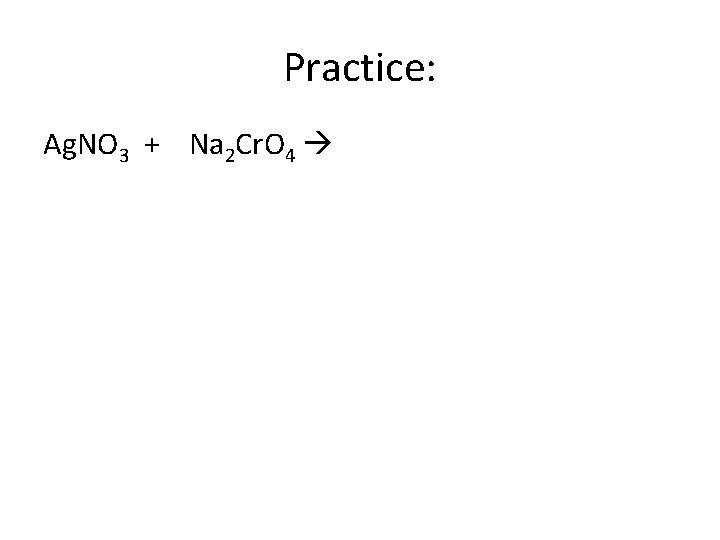
Practice: Ag. NO 3 + Na 2 Cr. O 4

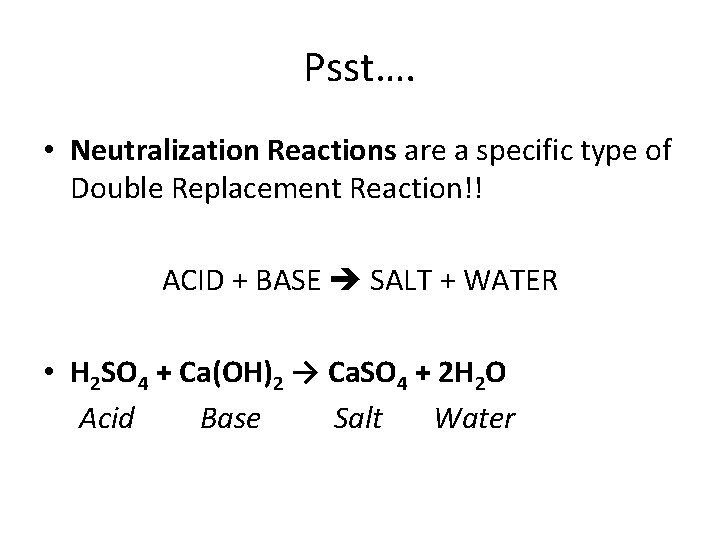
Psst…. • Neutralization Reactions are a specific type of Double Replacement Reaction!! ACID + BASE SALT + WATER • H 2 SO 4 + Ca(OH)2 → Ca. SO 4 + 2 H 2 O Acid Base Salt Water

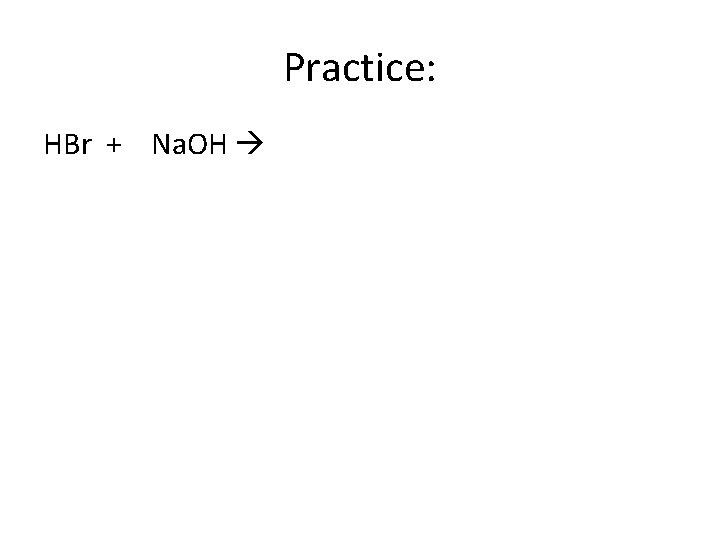
Practice: HBr + Na. OH

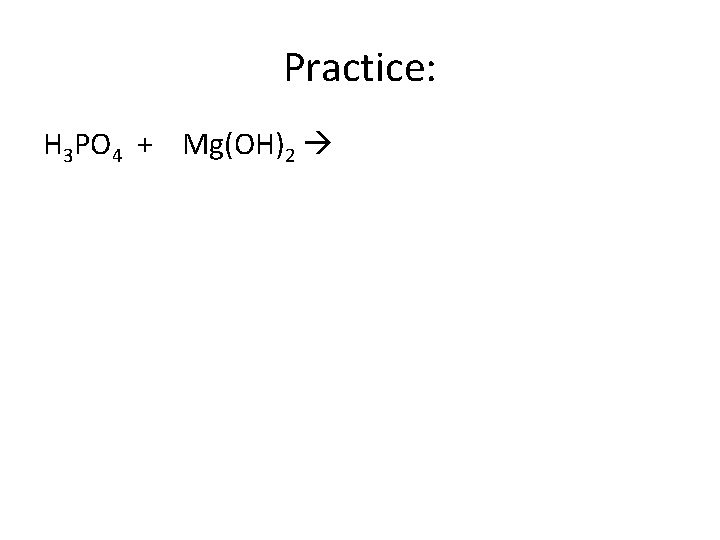
Practice: H 3 PO 4 + Mg(OH)2

Practice: HCl + Pb(OH)2

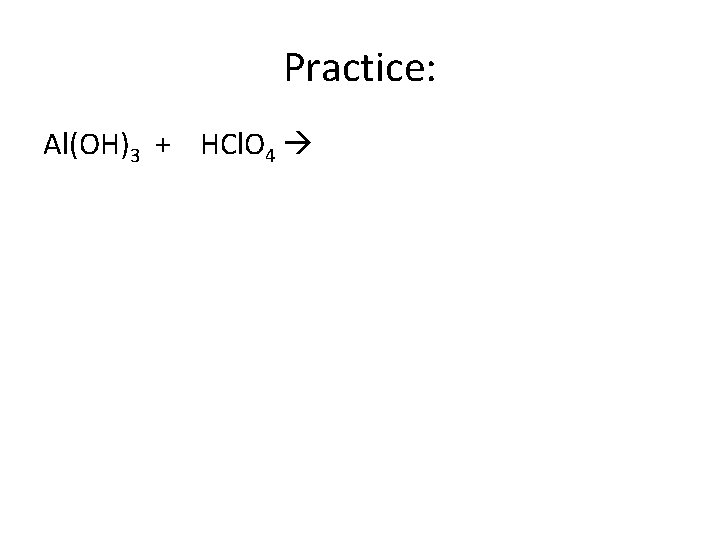
Practice: Al(OH)3 + HCl. O 4

Combustion- “The player” Ashley you’re my BFF Hey Ladies That was a great date Baby, I love you (3 weeks later) Basic Eqn: Cx. Hy + O 2 CO 2 + H 2 O

Combustion- “The player” Honey, Why is Ashley’s number on your phone? A hydrocarbon reacts with oxygen to produce carbon dioxide, water and energy (very exothermic!) •

Practice: C 3 H 8 + O 2

Practice: C 4 H 10 + O 2

Practice: C 2 H 4 + O 2

Practice: C 6 H 12 O 6 + O 2

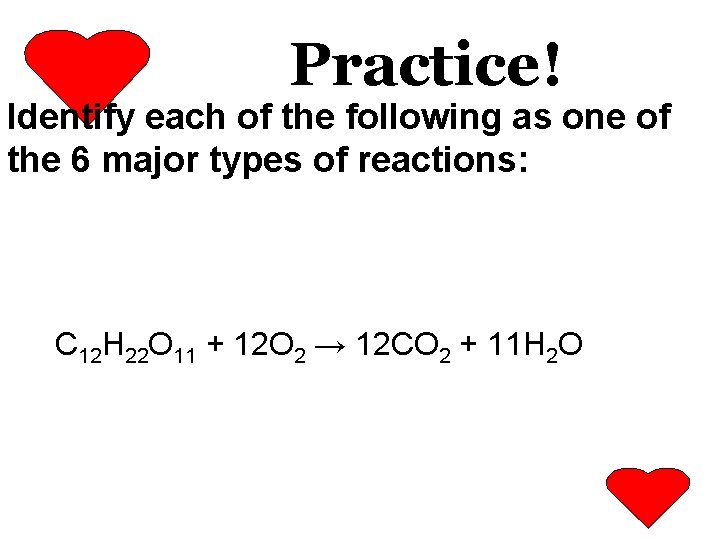
Practice: C 12 H 22 O 11 + O 2

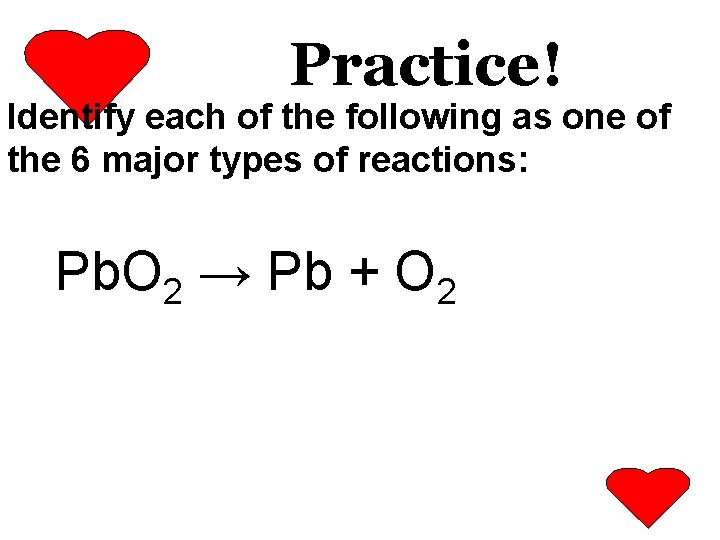
Practice! Identify each of the following as one of the 6 major types of reactions: Pb. O 2 → Pb + O 2

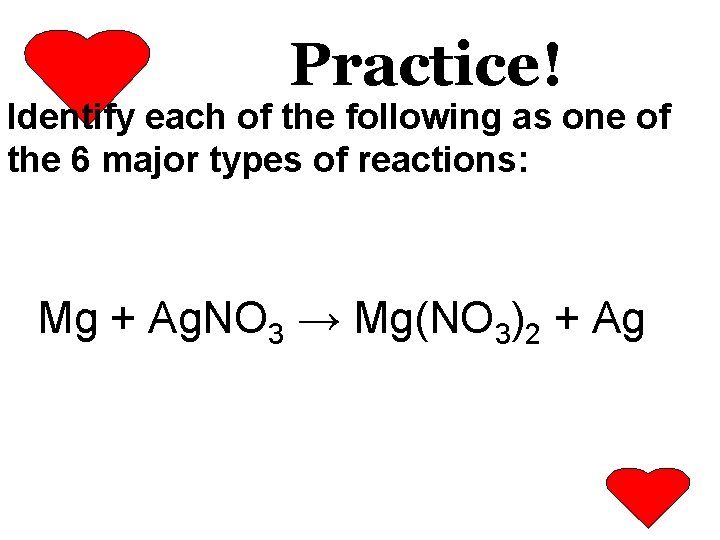
Practice! Identify each of the following as one of the 6 major types of reactions: Mg + Ag. NO 3 → Mg(NO 3)2 + Ag

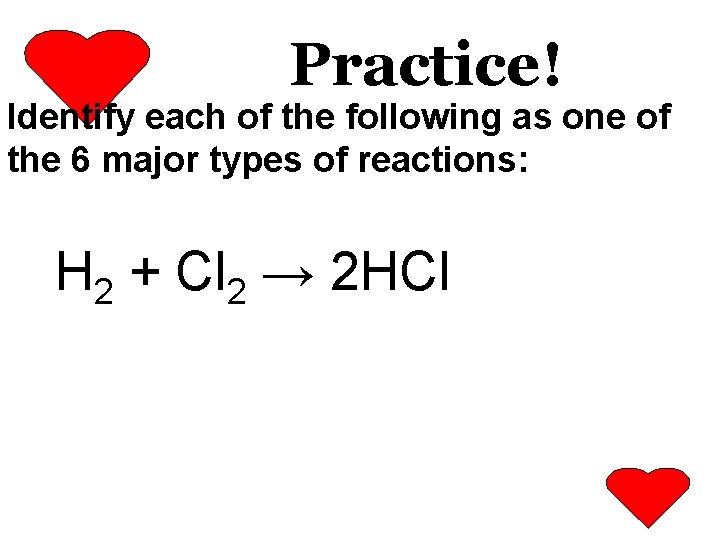
Practice! Identify each of the following as one of the 6 major types of reactions: H 2 + Cl 2 → 2 HCl
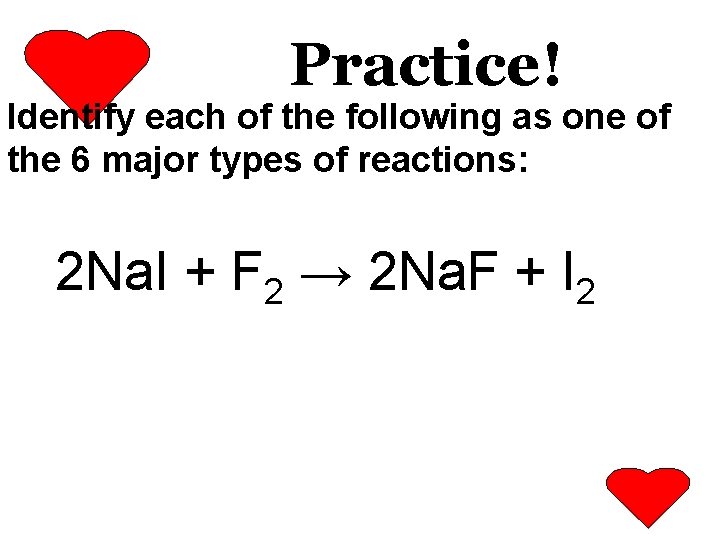
Practice! Identify each of the following as one of the 6 major types of reactions: 2 Na. I + F 2 → 2 Na. F + I 2

Practice! Identify each of the following as one of the 6 major types of reactions: C 12 H 22 O 11 + 12 O 2 → 12 CO 2 + 11 H 2 O

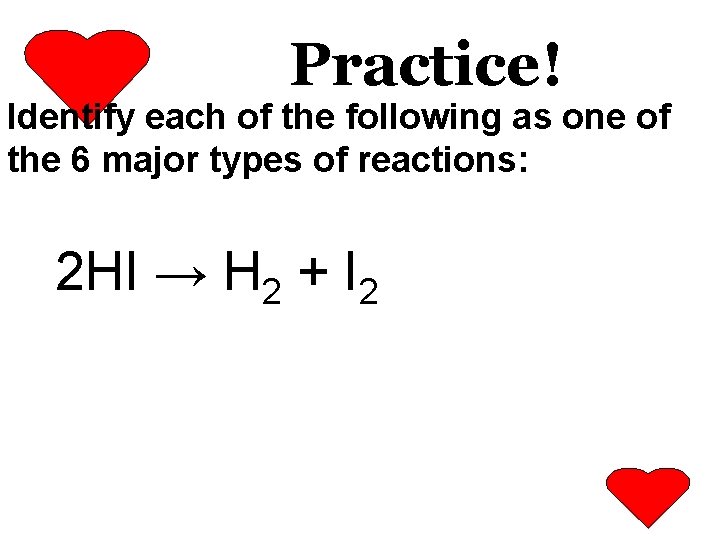
Practice! Identify each of the following as one of the 6 major types of reactions: 2 HI → H 2 + I 2

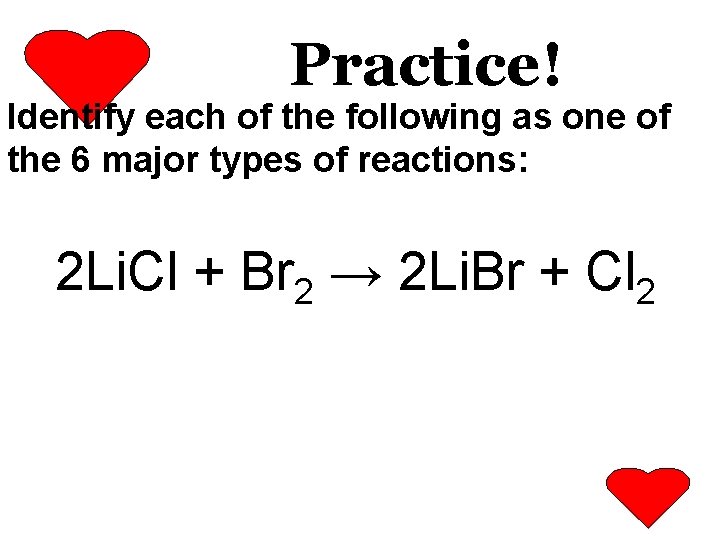
Practice! Identify each of the following as one of the 6 major types of reactions: 2 Li. Cl + Br 2 → 2 Li. Br + Cl 2

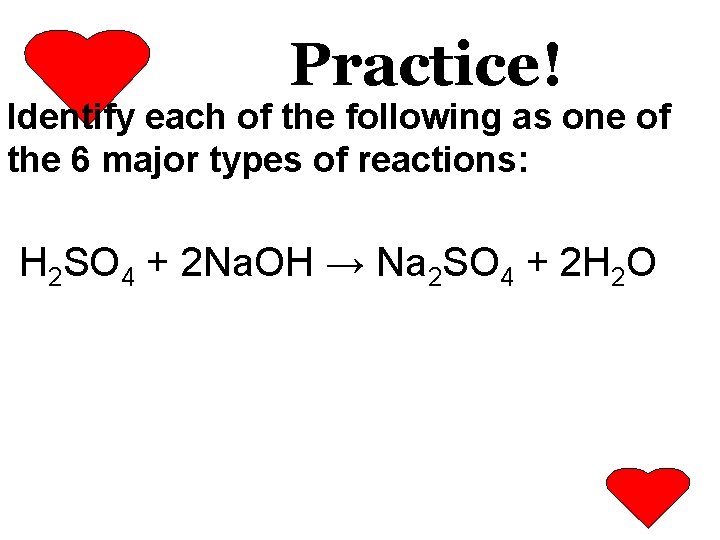
Practice! Identify each of the following as one of the 6 major types of reactions: H 2 SO 4 + 2 Na. OH → Na 2 SO 4 + 2 H 2 O

Practice! Identify each of the following as one of the 6 major types of reactions: Silver + chlorine → silver chloride

Practice! Identify each of the following as one of the 6 major types of reactions: Sulfur dioxide → sulfur + oxygen

Practice Problems Page 265 Check Your Understanding Page 271 #1 -6

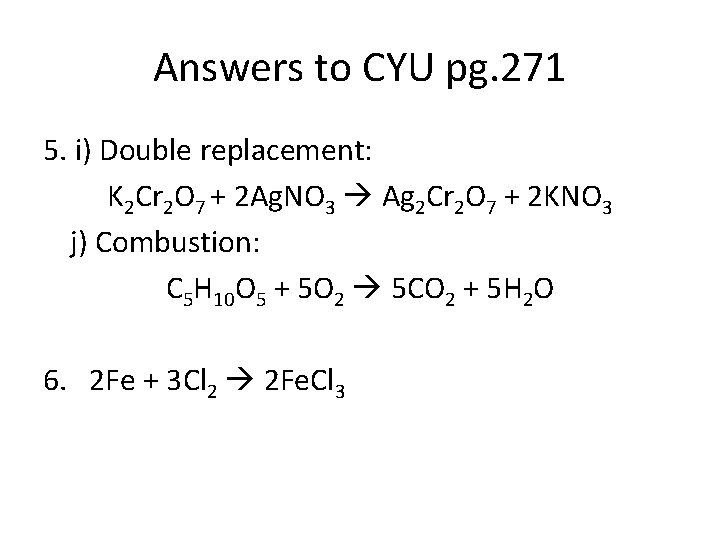
Answers to CYU pg. 271 1. a) Neutralization b) Synthesis c) Double replacement d) Synthesis e) Combustion f) Double replacement g) Single replacement (metal) h) Neutralization i) Decomposition j) Single replacement (metal)

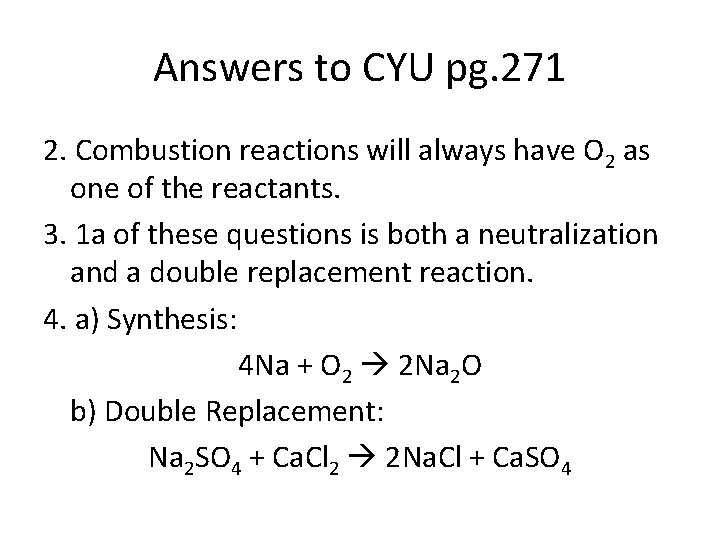
Answers to CYU pg. 271 2. Combustion reactions will always have O 2 as one of the reactants. 3. 1 a of these questions is both a neutralization and a double replacement reaction. 4. a) Synthesis: 4 Na + O 2 2 Na 2 O b) Double Replacement: Na 2 SO 4 + Ca. Cl 2 2 Na. Cl + Ca. SO 4

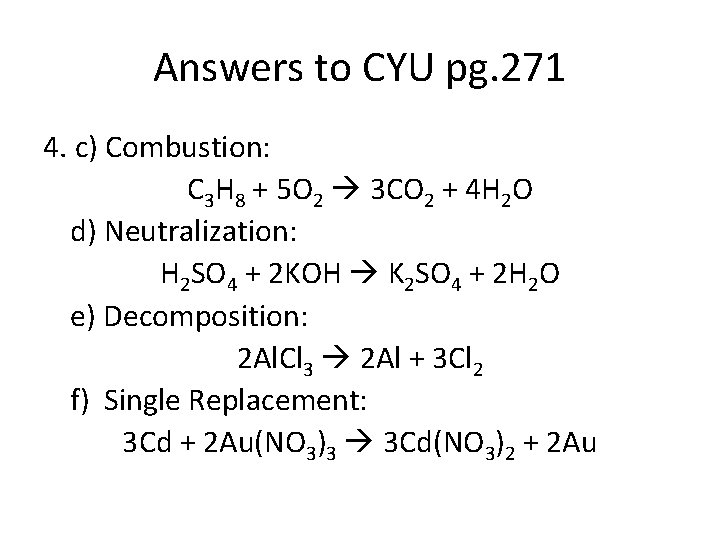
Answers to CYU pg. 271 4. c) Combustion: C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O d) Neutralization: H 2 SO 4 + 2 KOH K 2 SO 4 + 2 H 2 O e) Decomposition: 2 Al. Cl 3 2 Al + 3 Cl 2 f) Single Replacement: 3 Cd + 2 Au(NO 3)3 3 Cd(NO 3)2 + 2 Au

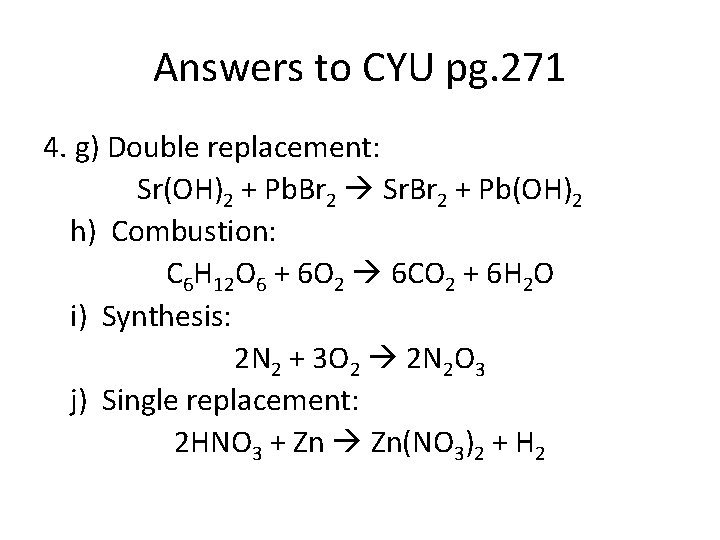
Answers to CYU pg. 271 4. g) Double replacement: Sr(OH)2 + Pb. Br 2 Sr. Br 2 + Pb(OH)2 h) Combustion: C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O i) Synthesis: 2 N 2 + 3 O 2 2 N 2 O 3 j) Single replacement: 2 HNO 3 + Zn Zn(NO 3)2 + H 2

Answers to CYU pg. 271 5. a) Synthesis: 6 Na + N 2 2 Na 3 N b) Decomposition: 2 Al. F 3 2 Al + 3 F 2 c) Single Replacement: 3 Cu. SO 4 + 2 Al Al 2(SO 4)3 + 3 Cu d) Double Replacement: Ca. I 2 + Pb(NO 3)2 Ca(NO 3)2 + Pb. I 2

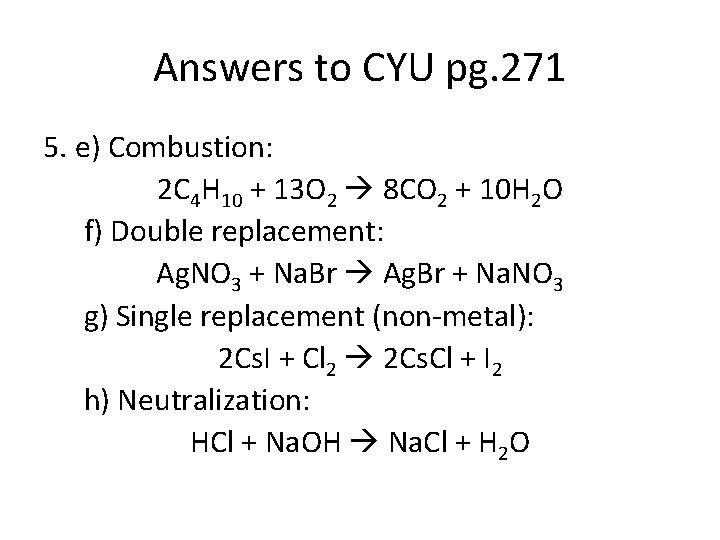
Answers to CYU pg. 271 5. e) Combustion: 2 C 4 H 10 + 13 O 2 8 CO 2 + 10 H 2 O f) Double replacement: Ag. NO 3 + Na. Br Ag. Br + Na. NO 3 g) Single replacement (non-metal): 2 Cs. I + Cl 2 2 Cs. Cl + I 2 h) Neutralization: HCl + Na. OH Na. Cl + H 2 O

Answers to CYU pg. 271 5. i) Double replacement: K 2 Cr 2 O 7 + 2 Ag. NO 3 Ag 2 Cr 2 O 7 + 2 KNO 3 j) Combustion: C 5 H 10 O 5 + 5 O 2 5 CO 2 + 5 H 2 O 6. 2 Fe + 3 Cl 2 2 Fe. Cl 3
 Rearrangement of atoms
Rearrangement of atoms Periodic table of elements regents
Periodic table of elements regents What is sine and cosine rule
What is sine and cosine rule Demjanov rearrangement
Demjanov rearrangement Carbonium ion rearrangement
Carbonium ion rearrangement Openreach line plant rearrangement
Openreach line plant rearrangement Migratory aptitude is greater for
Migratory aptitude is greater for Rearrange the sentences in correct order online
Rearrange the sentences in correct order online Semipinacol rearrangement
Semipinacol rearrangement Diels alder
Diels alder What is mclafferty rearrangement
What is mclafferty rearrangement Application of beckmann rearrangement
Application of beckmann rearrangement Enediol rearrangement
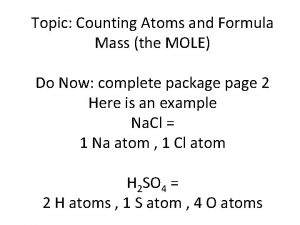
Enediol rearrangement Counting atoms examples
Counting atoms examples Is lighter fluid burning a physical change
Is lighter fluid burning a physical change Chemical bonds form when atoms *
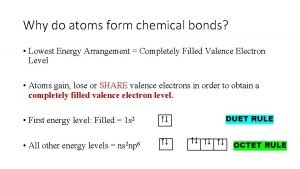
Chemical bonds form when atoms * Why do atoms form bonds? *
Why do atoms form bonds? * Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Why do most atoms form chemical bonds
Why do most atoms form chemical bonds In chemical reactions atoms are rearranged
In chemical reactions atoms are rearranged Are atoms the smallest unit of matter
Are atoms the smallest unit of matter Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Love chemical formula
Love chemical formula Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Unit 5 chemical reactions
Unit 5 chemical reactions Unit 13 biological cultural and chemical control of pests
Unit 13 biological cultural and chemical control of pests Unit 11 chemical reactions
Unit 11 chemical reactions Unit chemical bonding forming ionic compounds ws 2
Unit chemical bonding forming ionic compounds ws 2 Types of reactions grade 11
Types of reactions grade 11 Unit chemical bonding molecular geometry
Unit chemical bonding molecular geometry Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Unit chemical bonding molecular geometry
Unit chemical bonding molecular geometry Unit: chemical bonding “bonding basics” – ws #1
Unit: chemical bonding “bonding basics” – ws #1 Unit chemical quantities the mole 1 step
Unit chemical quantities the mole 1 step C3h5 name
C3h5 name What is the relationship between atoms and elements
What is the relationship between atoms and elements What are atoms?
What are atoms? Kesler science atoms answer key
Kesler science atoms answer key The atoms family atomic math challenge answer key
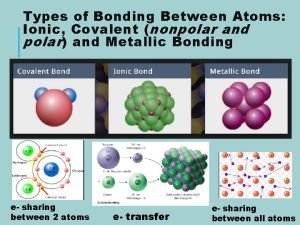
The atoms family atomic math challenge answer key Nonpolar polar and ionic bonds
Nonpolar polar and ionic bonds Gfm formula
Gfm formula Atoms in increasing size
Atoms in increasing size The smallest building block of matter
The smallest building block of matter Matterville worksheet
Matterville worksheet What's inside the nucleus
What's inside the nucleus Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Atoms with 4 valence electrons
Atoms with 4 valence electrons Atoms with unpaired electrons are called diamagnetic.
Atoms with unpaired electrons are called diamagnetic. Periodic reference table
Periodic reference table Grams to mols
Grams to mols Amu vs molar mass
Amu vs molar mass Metallic bonding occurs between atoms of
Metallic bonding occurs between atoms of Atoms escape room digital locks answers
Atoms escape room digital locks answers Mri hydrogen atoms
Mri hydrogen atoms Fcc linear density 111
Fcc linear density 111 Whats ionic bonding
Whats ionic bonding How does a positive ion form
How does a positive ion form