Preparation u We have already covered these methods


- Slides: 26

Preparation u We have already covered these methods • nucleophilic ring opening of epoxides by ammonia and amines. • addition of nitrogen nucleophiles to aldehydes and ketones to form imines • reduction of imines to amines • reduction of amides to amines by Li. Al. H 4 • reduction of nitriles to a 1° amine • nitration of arenes followed by reduction of the NO 2 group to a 1° amine 23 -1

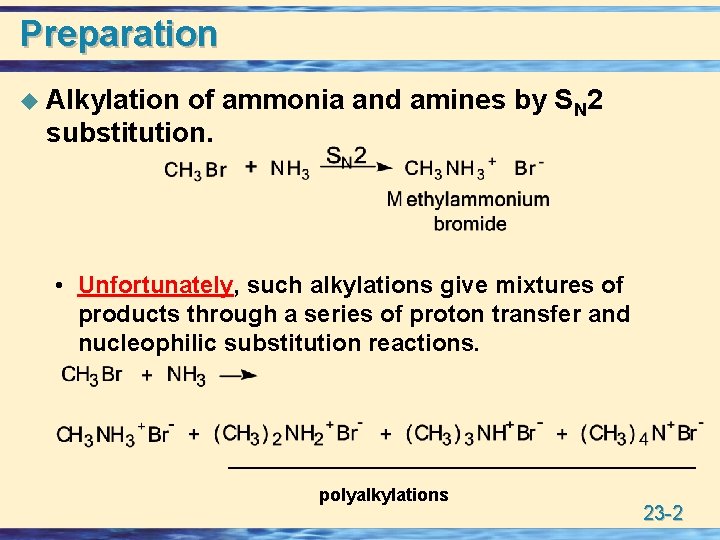
Preparation u Alkylation of ammonia and amines by SN 2 substitution. • Unfortunately, such alkylations give mixtures of products through a series of proton transfer and nucleophilic substitution reactions. polyalkylations 23 -2

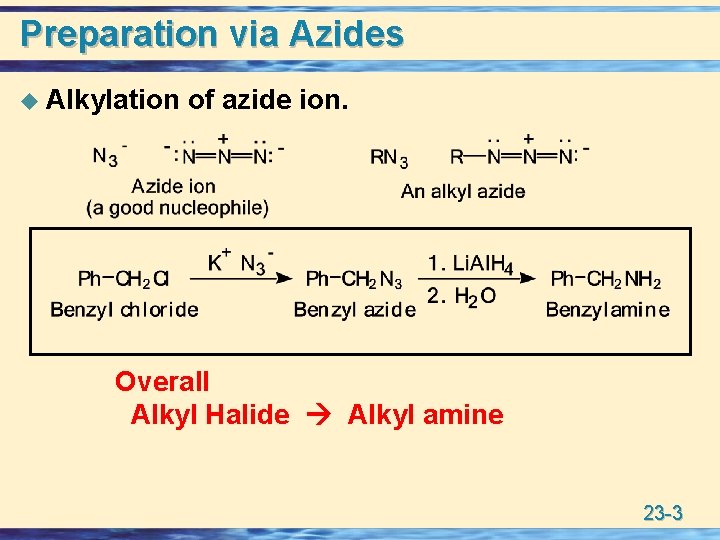
Preparation via Azides u Alkylation of azide ion. Overall Alkyl Halide Alkyl amine 23 -3

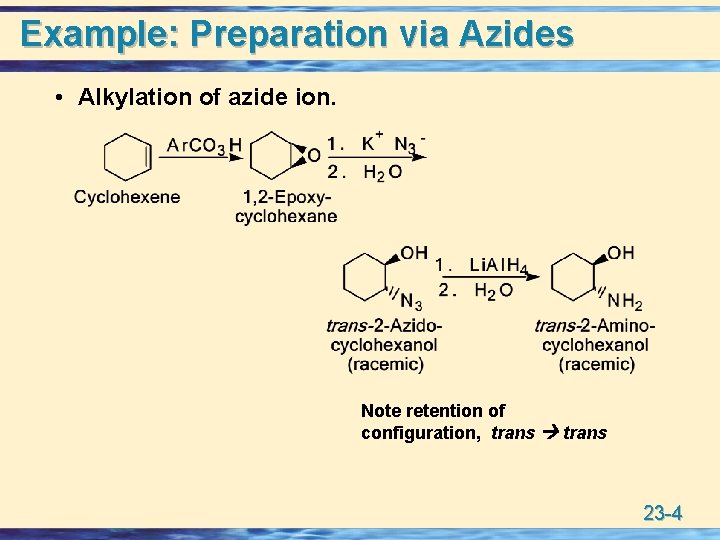
Example: Preparation via Azides • Alkylation of azide ion. Note retention of configuration, trans 23 -4

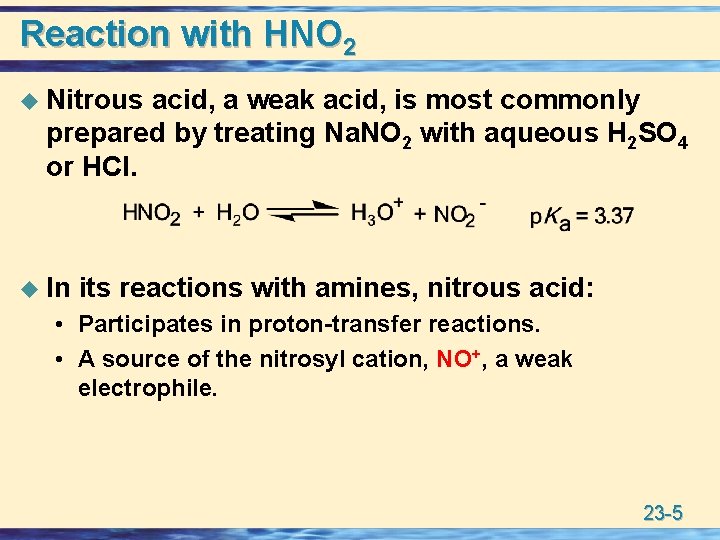
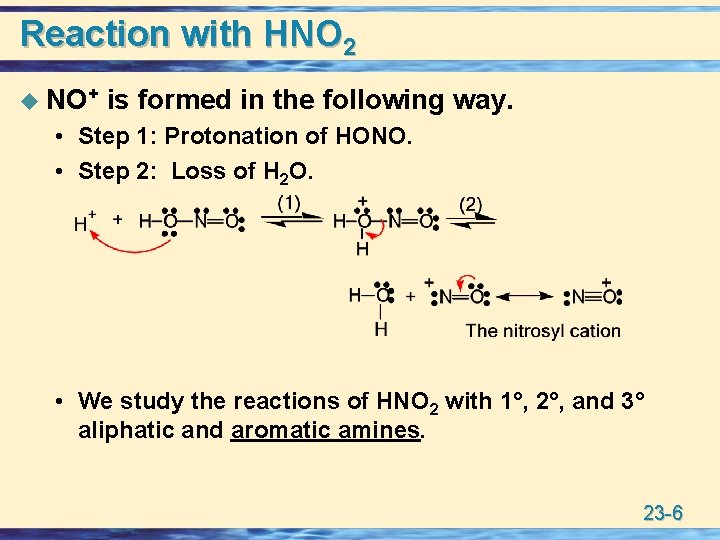
Reaction with HNO 2 u Nitrous acid, a weak acid, is most commonly prepared by treating Na. NO 2 with aqueous H 2 SO 4 or HCl. u In its reactions with amines, nitrous acid: • Participates in proton-transfer reactions. • A source of the nitrosyl cation, NO+, a weak electrophile. 23 -5

Reaction with HNO 2 u NO+ is formed in the following way. • Step 1: Protonation of HONO. • Step 2: Loss of H 2 O. • We study the reactions of HNO 2 with 1°, 2°, and 3° aliphatic and aromatic amines. 23 -6

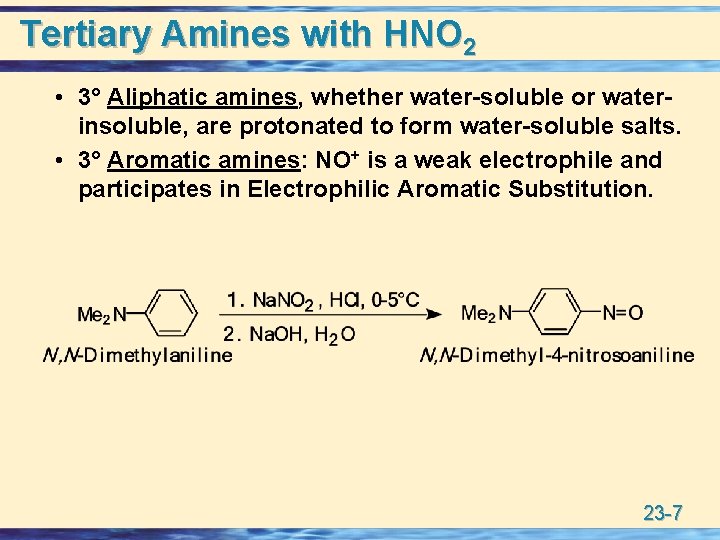
Tertiary Amines with HNO 2 • 3° Aliphatic amines, whether water-soluble or waterinsoluble, are protonated to form water-soluble salts. • 3° Aromatic amines: NO+ is a weak electrophile and participates in Electrophilic Aromatic Substitution. 23 -7

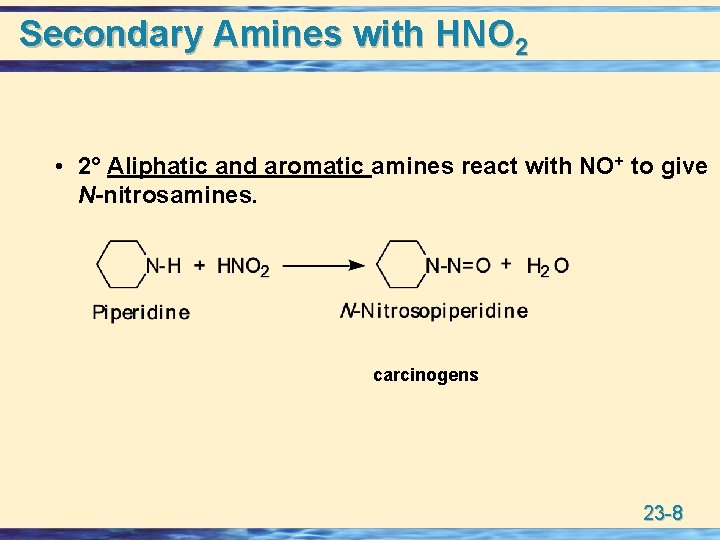
Secondary Amines with HNO 2 • 2° Aliphatic and aromatic amines react with NO+ to give N-nitrosamines. carcinogens 23 -8

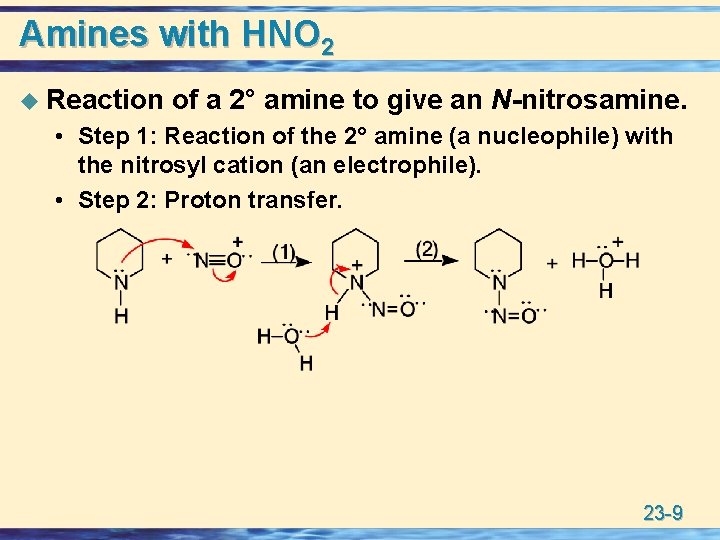
Amines with HNO 2 u Reaction of a 2° amine to give an N-nitrosamine. • Step 1: Reaction of the 2° amine (a nucleophile) with the nitrosyl cation (an electrophile). • Step 2: Proton transfer. 23 -9

RNH 2 with HNO 2 u 1° aliphatic amines give a mixture of unrearranged and rearranged substitution and elimination products, all of which are produced by way of a diazonium ion and its loss of N 2 to give a carbocation. u Diazonium ion: An RN 2+ or Ar. N 2+ ion 23 -10

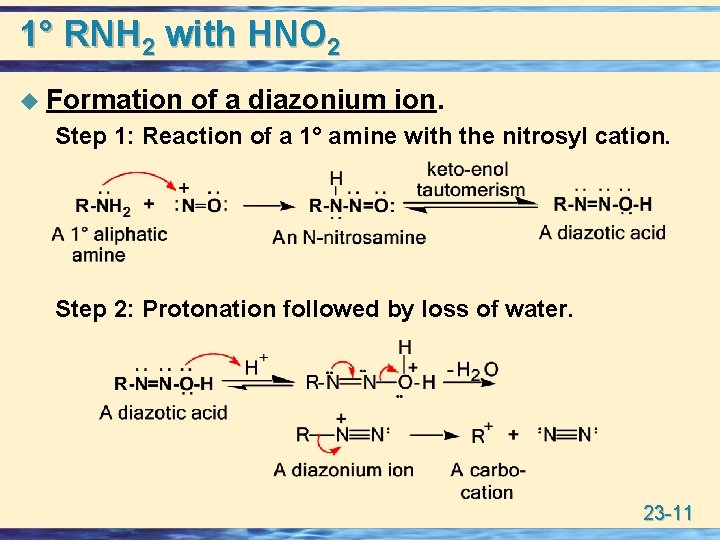
1° RNH 2 with HNO 2 u Formation of a diazonium ion. Step 1: Reaction of a 1° amine with the nitrosyl cation. Step 2: Protonation followed by loss of water. 23 -11

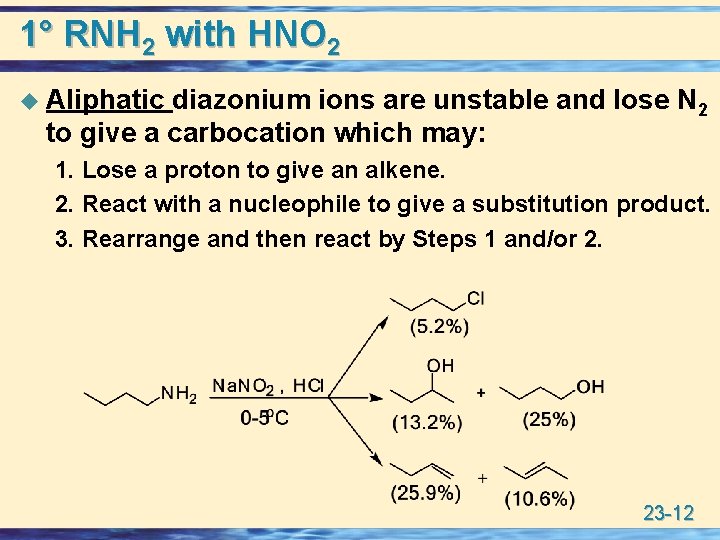
1° RNH 2 with HNO 2 u Aliphatic diazonium ions are unstable and lose N 2 to give a carbocation which may: 1. Lose a proton to give an alkene. 2. React with a nucleophile to give a substitution product. 3. Rearrange and then react by Steps 1 and/or 2. 23 -12

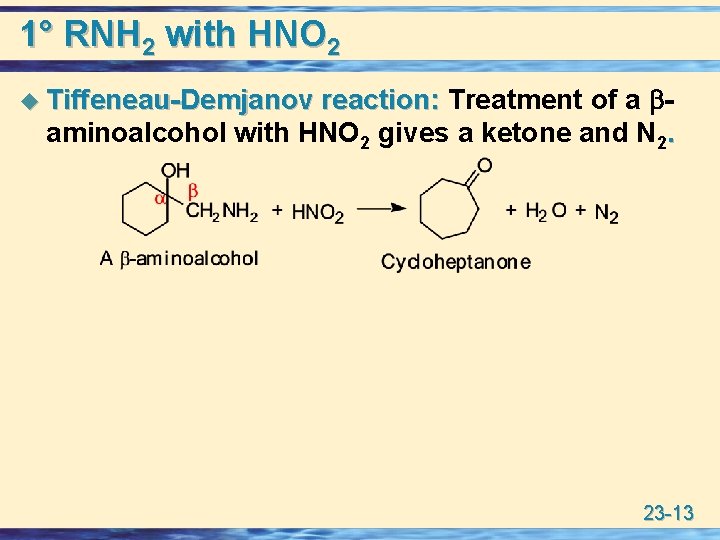
1° RNH 2 with HNO 2 reaction: Treatment of a aminoalcohol with HNO 2 gives a ketone and N 2. u Tiffeneau-Demjanov 23 -13

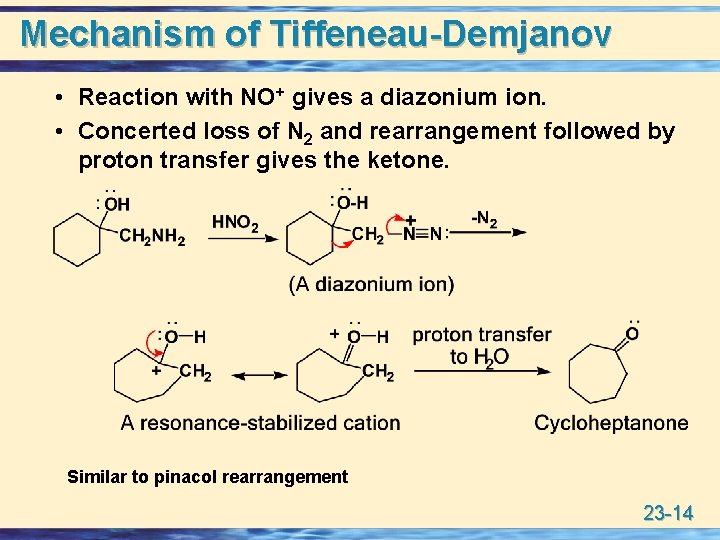
Mechanism of Tiffeneau-Demjanov • Reaction with NO+ gives a diazonium ion. • Concerted loss of N 2 and rearrangement followed by proton transfer gives the ketone. Similar to pinacol rearrangement 23 -14

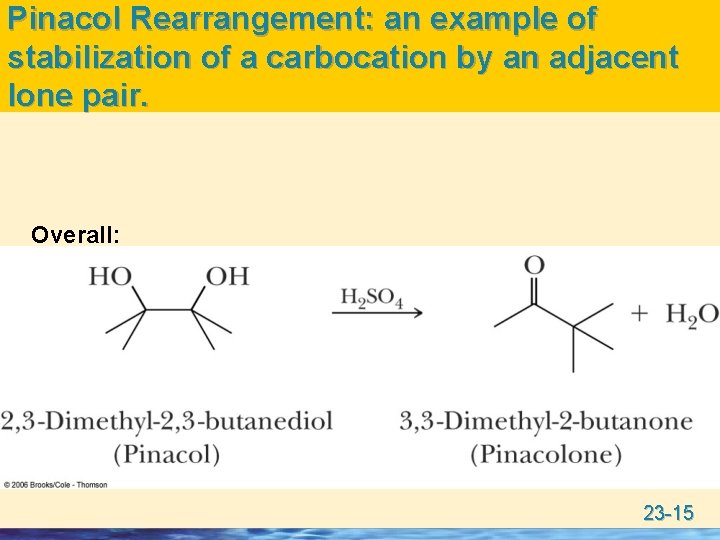
Pinacol Rearrangement: an example of stabilization of a carbocation by an adjacent lone pair. Overall: 23 -15

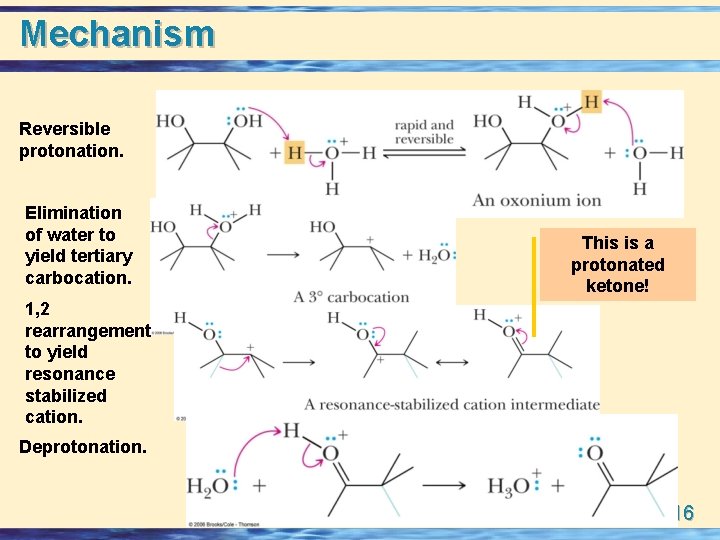
Mechanism Reversible protonation. Elimination of water to yield tertiary carbocation. This is a protonated ketone! 1, 2 rearrangement to yield resonance stabilized cation. Deprotonation. 23 -16

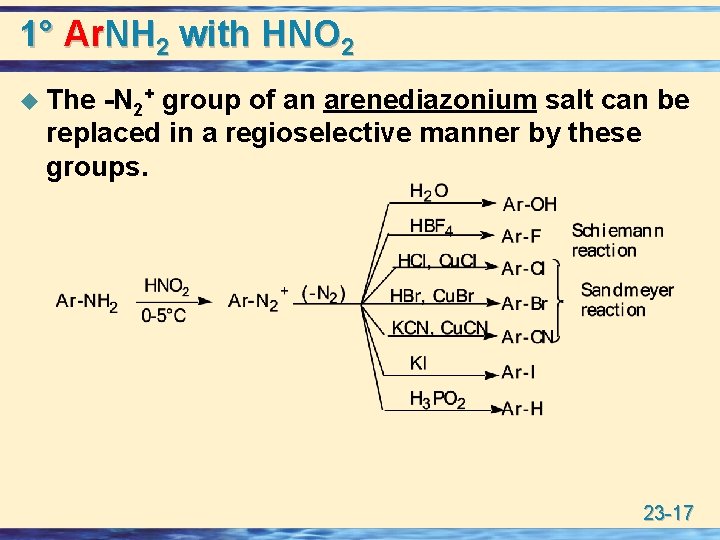
1° Ar. NH 2 with HNO 2 u The -N 2+ group of an arenediazonium salt can be replaced in a regioselective manner by these groups. 23 -17

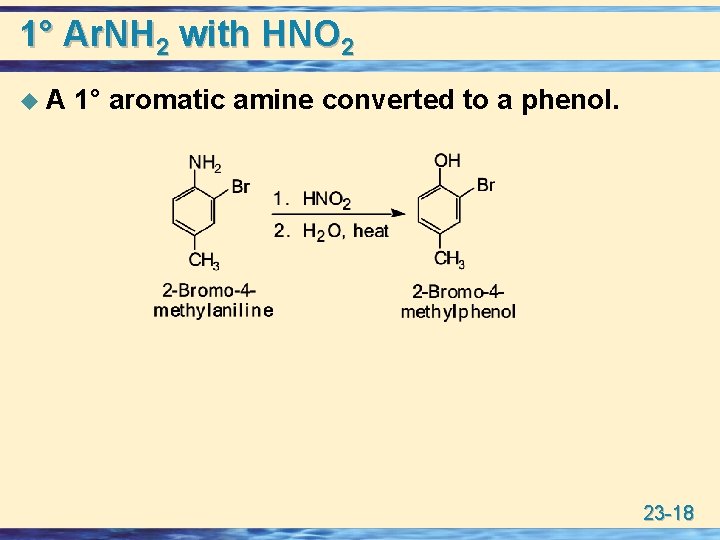
1° Ar. NH 2 with HNO 2 u. A 1° aromatic amine converted to a phenol. 23 -18

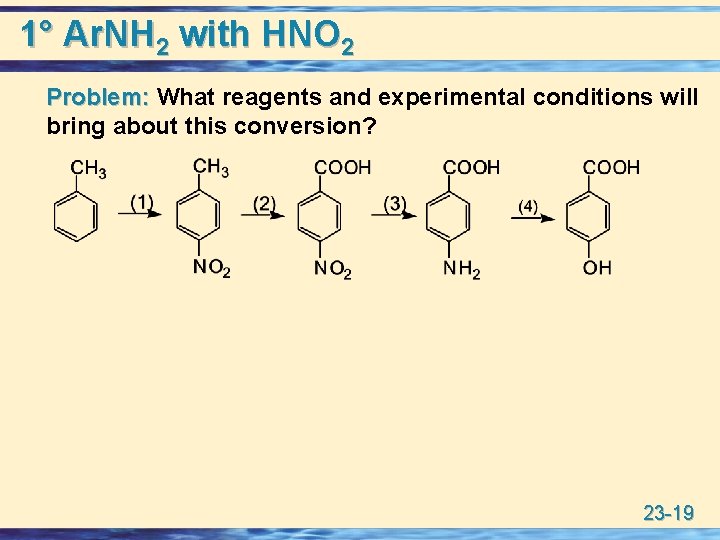
1° Ar. NH 2 with HNO 2 Problem: What reagents and experimental conditions will bring about this conversion? 23 -19

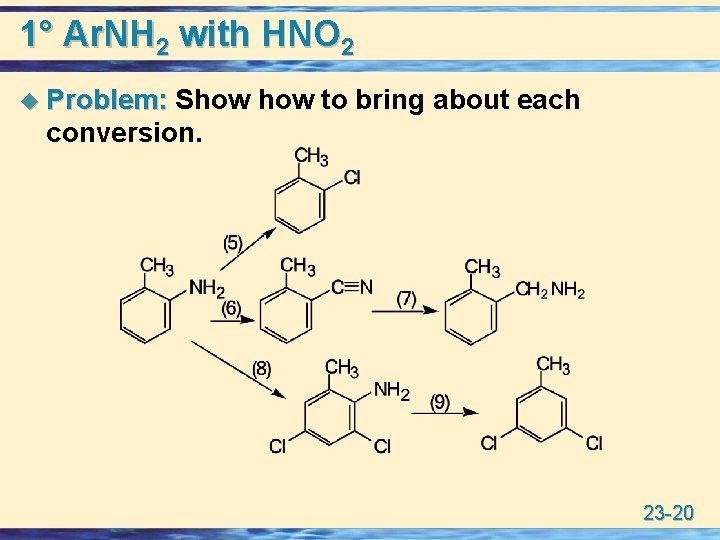
1° Ar. NH 2 with HNO 2 u Problem: Show to bring about each conversion. 23 -20

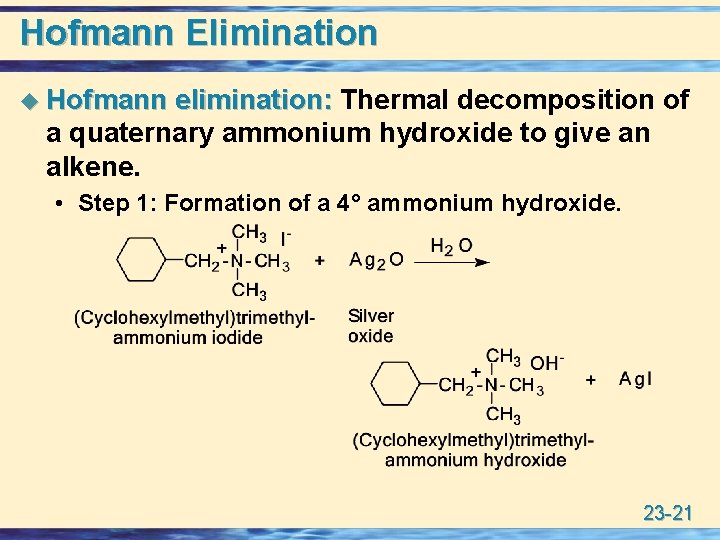
Hofmann Elimination u Hofmann elimination: Thermal decomposition of a quaternary ammonium hydroxide to give an alkene. • Step 1: Formation of a 4° ammonium hydroxide. 23 -21

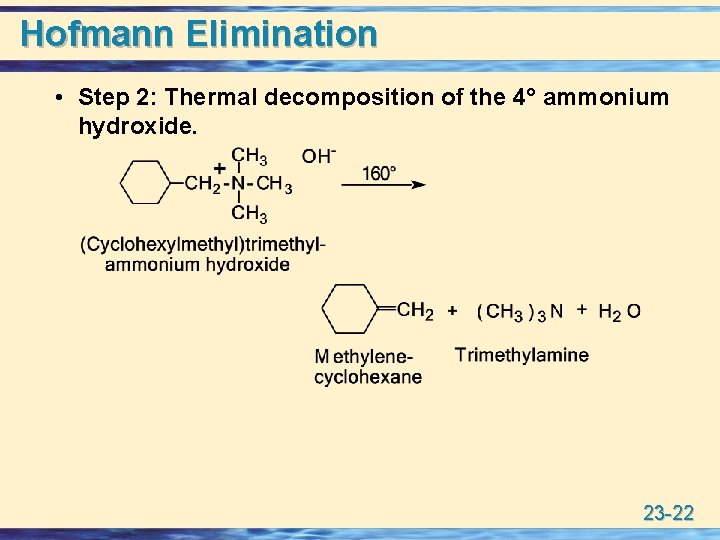
Hofmann Elimination • Step 2: Thermal decomposition of the 4° ammonium hydroxide. 23 -22

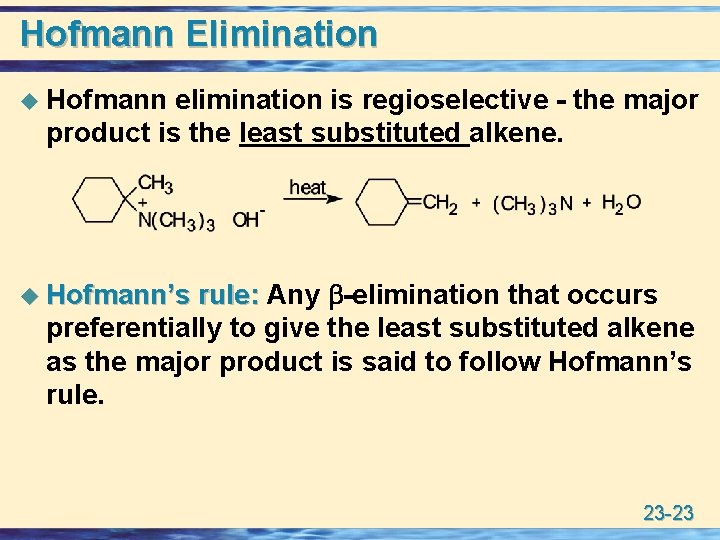
Hofmann Elimination u Hofmann elimination is regioselective - the major product is the least substituted alkene. rule: Any -elimination that occurs preferentially to give the least substituted alkene as the major product is said to follow Hofmann’s rule. u Hofmann’s 23 -23

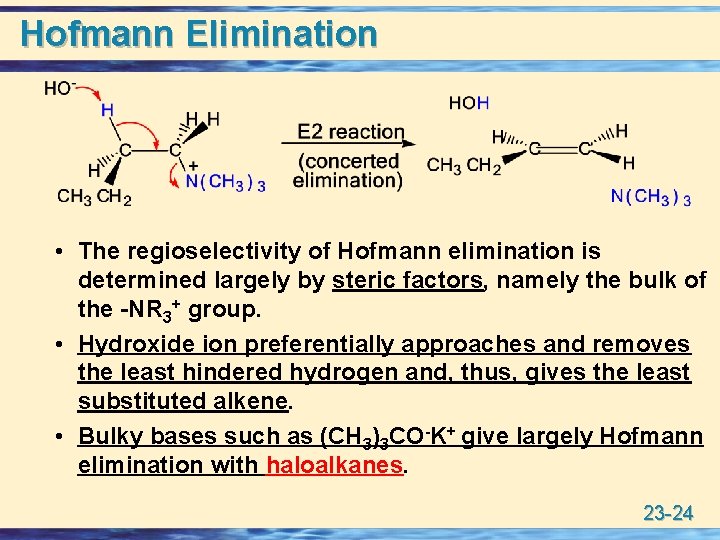
Hofmann Elimination • The regioselectivity of Hofmann elimination is determined largely by steric factors, namely the bulk of the -NR 3+ group. • Hydroxide ion preferentially approaches and removes the least hindered hydrogen and, thus, gives the least substituted alkene. • Bulky bases such as (CH 3)3 CO-K+ give largely Hofmann elimination with haloalkanes. 23 -24

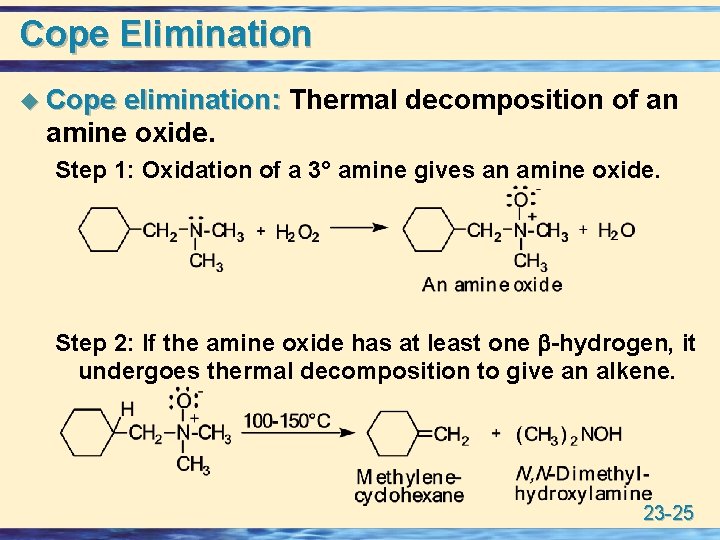
Cope Elimination u Cope elimination: Thermal decomposition of an amine oxide. Step 1: Oxidation of a 3° amine gives an amine oxide. Step 2: If the amine oxide has at least one -hydrogen, it undergoes thermal decomposition to give an alkene. 23 -25

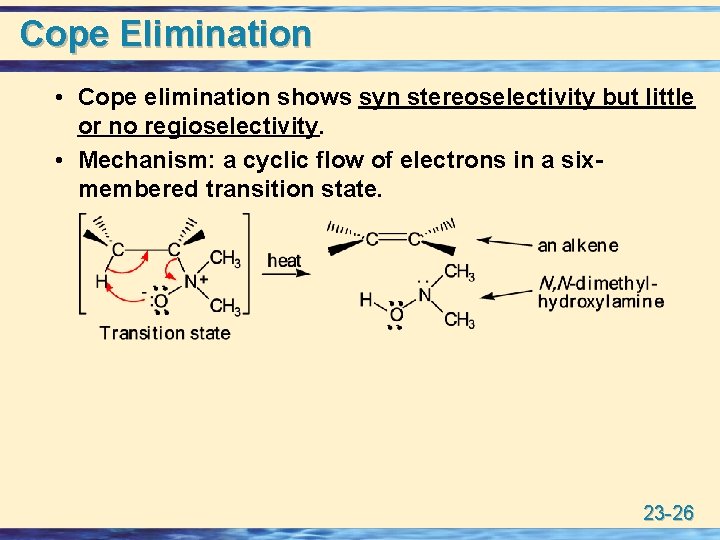
Cope Elimination • Cope elimination shows syn stereoselectivity but little or no regioselectivity. • Mechanism: a cyclic flow of electrons in a sixmembered transition state. 23 -26