Emulsion 1 Definition 2 Applications 3 Classification 4














- Slides: 28

Emulsion 1. Definition 2. Applications 3. Classification 4. Theory of emulsification 5. Stability of emulsion 6. Preservation of emulsion 7. Emulsion preparation 8. Nascent method 9. Dry gum 10. Wet gum 11. Incorporation of drugs into emulsion 12. Microemulsion

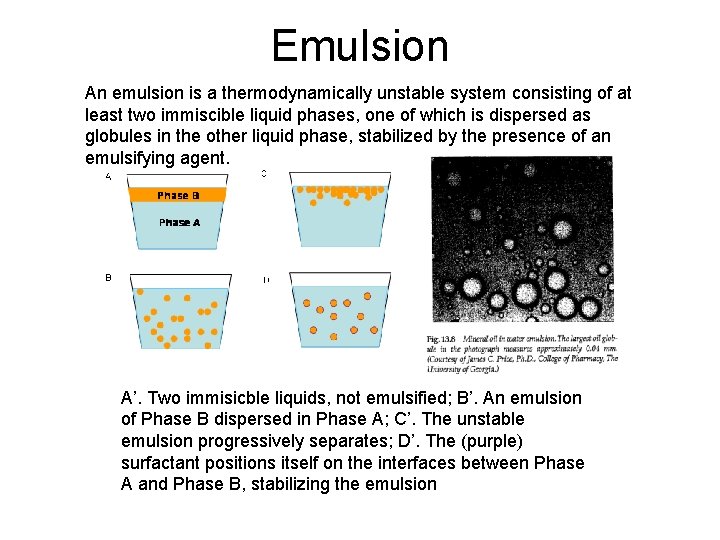
Emulsion An emulsion is a thermodynamically unstable system consisting of at least two immiscible liquid phases, one of which is dispersed as globules in the other liquid phase, stabilized by the presence of an emulsifying agent. A’. Two immisicble liquids, not emulsified; B’. An emulsion of Phase B dispersed in Phase A; C’. The unstable emulsion progressively separates; D’. The (purple) surfactant positions itself on the interfaces between Phase A and Phase B, stabilizing the emulsion

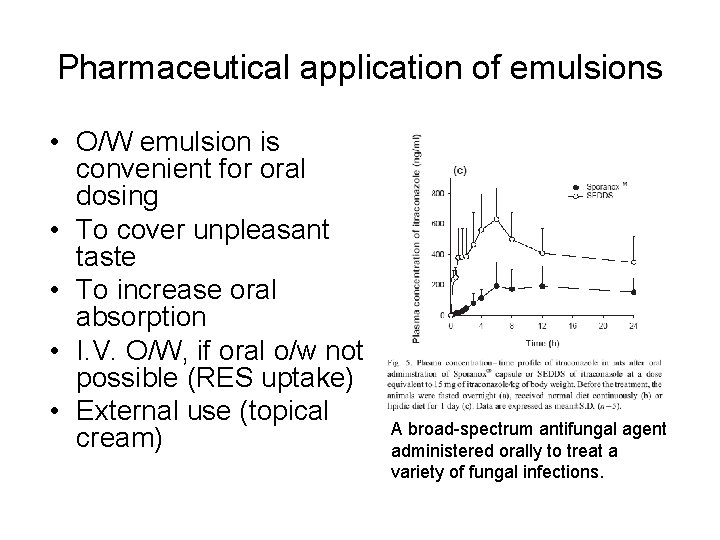
Pharmaceutical application of emulsions • O/W emulsion is convenient for oral dosing • To cover unpleasant taste • To increase oral absorption • I. V. O/W, if oral o/w not possible (RES uptake) • External use (topical cream) A broad-spectrum antifungal agent administered orally to treat a variety of fungal infections.

Emulsion types Types • Oil-in-water (o/w) • Water-in-oil (w/o) • Oil-in-water-in-oil (o/w/o) • Water-in-oil-in-water (w/o/w) Determination of o/w or w/o • Water soluble dye (e. g. , methylene blue) • Dilution of emulsions • Conduction of current

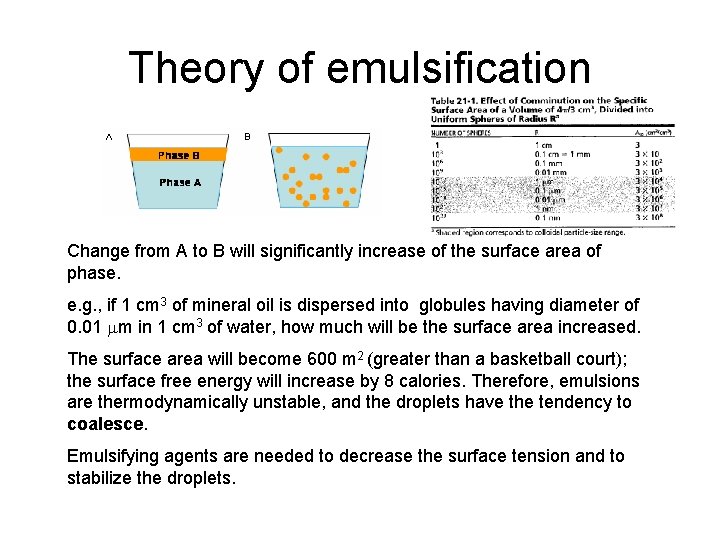
Theory of emulsification Change from A to B will significantly increase of the surface area of phase. e. g. , if 1 cm 3 of mineral oil is dispersed into globules having diameter of 0. 01 mm in 1 cm 3 of water, how much will be the surface area increased. The surface area will become 600 m 2 (greater than a basketball court); the surface free energy will increase by 8 calories. Therefore, emulsions are thermodynamically unstable, and the droplets have the tendency to coalesce. Emulsifying agents are needed to decrease the surface tension and to stabilize the droplets.

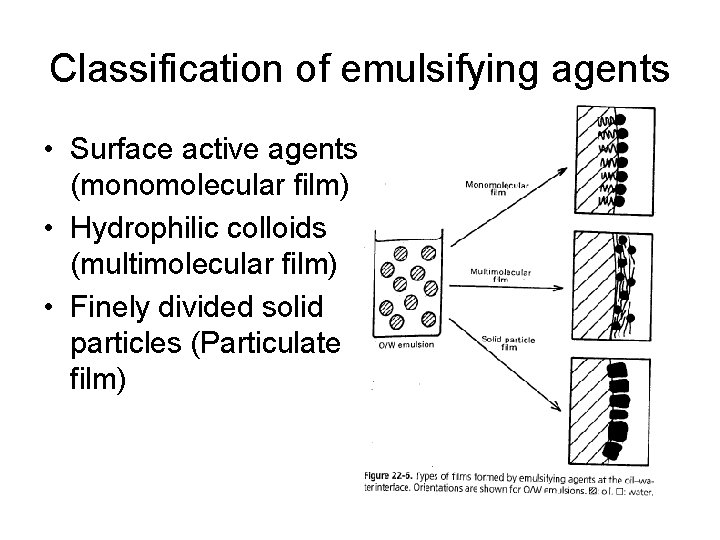
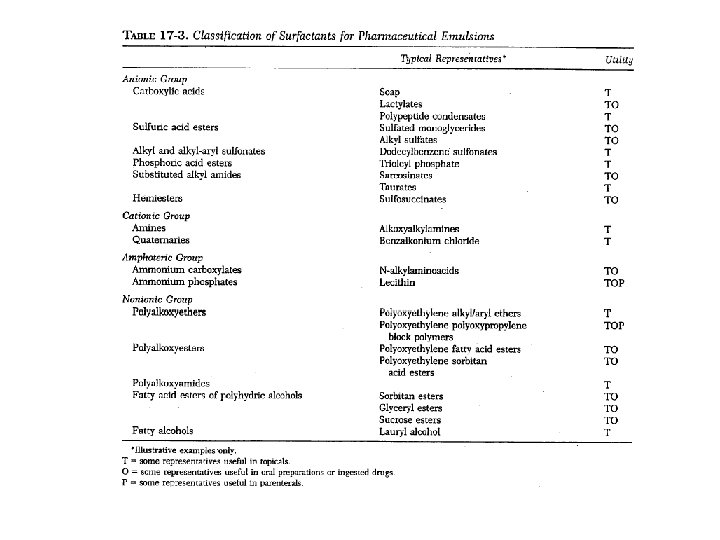
Classification of emulsifying agents • Surface active agents (monomolecular film) • Hydrophilic colloids (multimolecular film) • Finely divided solid particles (Particulate film)

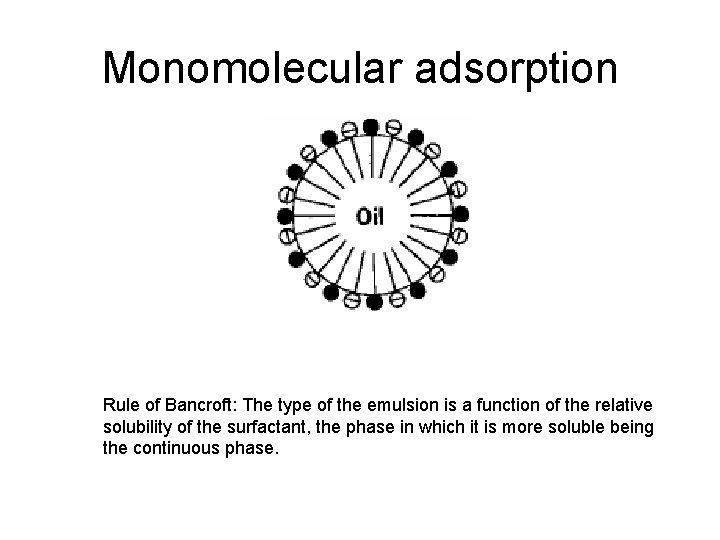
Monomolecular adsorption Rule of Bancroft: The type of the emulsion is a function of the relative solubility of the surfactant, the phase in which it is more soluble being the continuous phase.



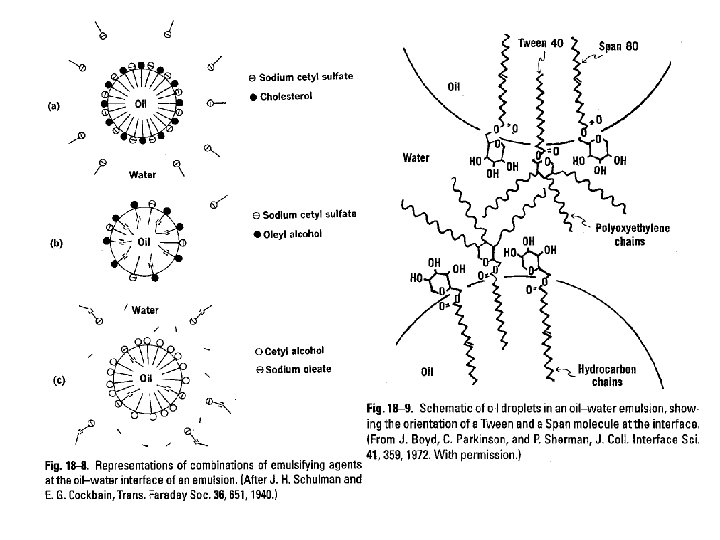
Multimolecular adsorption and film formation 1. Hydrated lyophilic colloids (hydrocolloids) • • • providing a protective sheath around the droplets imparting a charge to the dispersed droplets (so that they repel each other) swelling to increase the viscosity of the system (so that droplets are less likely to merge) 2. Classification of hydrocolloids • • vegetable derivatives, e. g. , acacia, tragacanth, agar, pectin, lecithin animal derivatives, e. g. , gelatin, lanolin, cholesterol Semi-synthetic agents, e. g. , methylcellulose, carboxymethylcellulose Synthetic agents, e. g. , carbomers (PEG and acrylic acid)

Solid particle adsorption • Description: Finely divided solid particles that are wetted to some degree by both oil and water can act as emulsifying agents. This results from their being concentrated at the interface, where they produce a particulate film around the dispersed droplets to prevent coalescence. • Example of agents: bentonite (Al 2 O 3. 4 Si. O 2. H 2 O), veegum (Magnesium Aluminum Silicate), hectorite, magnesium hydroxide, aluminum hydroxide and magnesium trisilicate • Auxiliary Emulsifying Agents A variety of fatty acids (e. g. , stearic acid), fatty alcohols (e. g. , stearyl or cetyl alcohol), and fatty esters (e. g. , glyceryl monostearate) serve to stabilize emulsions through their ability to thicken the emulsion. Because these agents have only weak emulsifying properties, they are always use in combination with other emulsifiers.

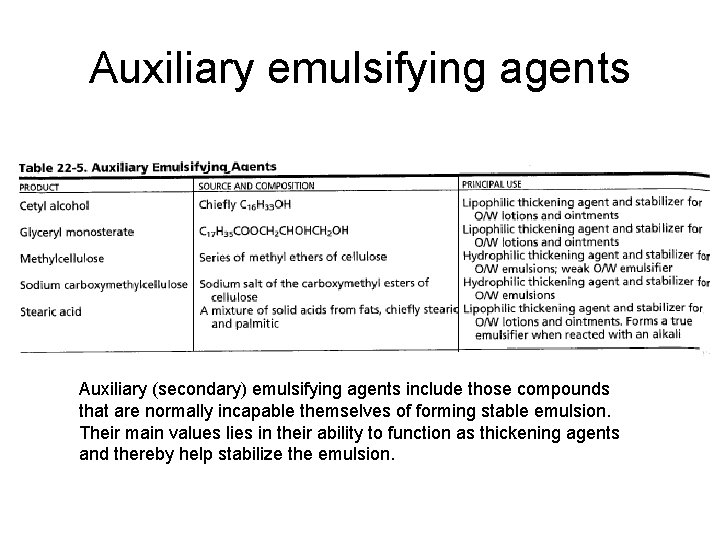
Auxiliary emulsifying agents Auxiliary (secondary) emulsifying agents include those compounds that are normally incapable themselves of forming stable emulsion. Their main values lies in their ability to function as thickening agents and thereby help stabilize the emulsion.

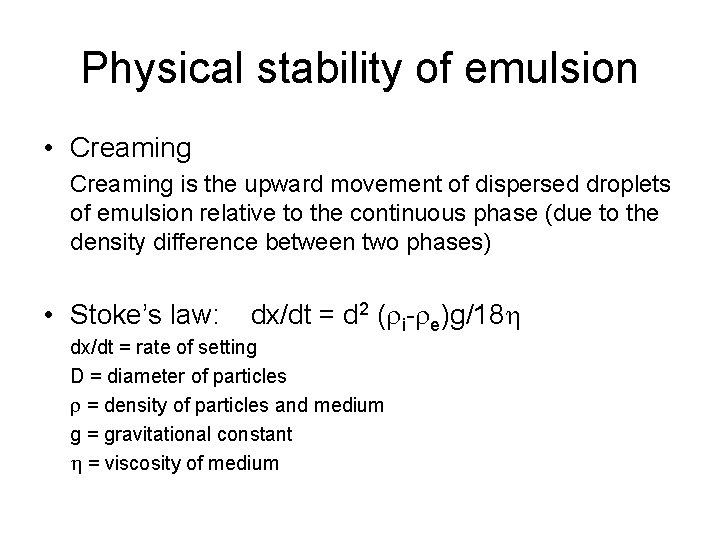
Physical stability of emulsion • Creaming is the upward movement of dispersed droplets of emulsion relative to the continuous phase (due to the density difference between two phases) • Stoke’s law: dx/dt = d 2 ( i- e)g/18 h dx/dt = rate of setting D = diameter of particles = density of particles and medium g = gravitational constant h = viscosity of medium

Physical stability of emulsion • Breaking, coalescence, aggregation • Breaking is the destroying of the film surrounding the particles. • Coalescence is the process by which emulsified particles merge with each to form large particles. • Aggregation: dispersed particles come together but do not fuse. • The major fact preventing coalescence is the mechanical strength of the interfacial film.

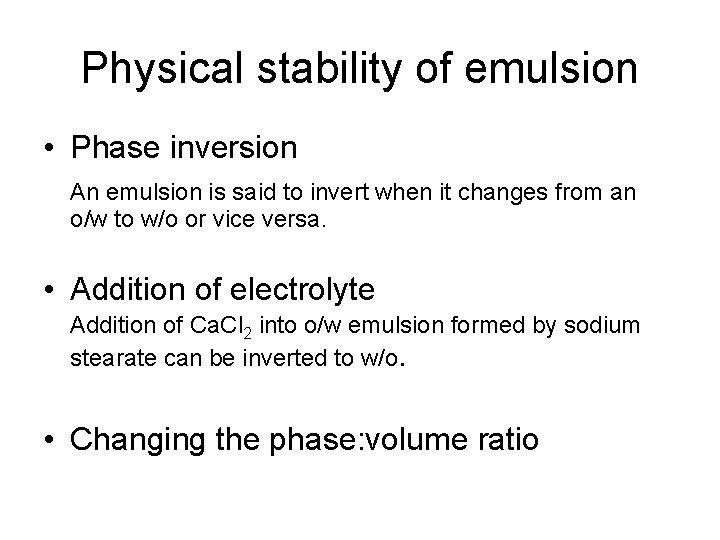
Physical stability of emulsion • Phase inversion An emulsion is said to invert when it changes from an o/w to w/o or vice versa. • Addition of electrolyte Addition of Ca. Cl 2 into o/w emulsion formed by sodium stearate can be inverted to w/o. • Changing the phase: volume ratio

Preservation of emulsions • Growth of microorganisms in emulsions • Preservatives should be in aqueous phase. • Preservatives should be in unionized state to penetrate the bacteria • Preservatives must not bind to other components of the emulsion

Methods of emulsion preparation • • • Continental or dry gum method English of wet gum method Bottle or Forbes bottle method Auxiliary method In situ soap method Calcium soaps: w/o emulsions contain oils such as oleic acid, in combination with lime water (calcium hydroxide solution, USP). Prepared by mixing equal volumes of oil and lime water.

Nascent soap • Oil phase: olive oil/oleic acid; olive oil may be replaced by other oils, but oleic acid must be added • • Lime water: Ca(OH)2 should be freshly prepared. Equal volume of oil and lime water The emulsion formed is w/o or o/w? Method of preparation: Bottle method: Mortar method: when the formulation contains solid insoluble such as zinc oxide and calamine.

Dry gum method (4: 2: 1 method) • • • The continental method is used to prepare the initial or primary emulsion from oil, water, and a hydrocolloid or "gum" type emulsifier (usually acacia). The primary emulsion, or emulsion nucleus, is formed from 4 parts oil, 2 parts water, and 1 part emulsifier. The 4 parts oil and 1 part emulsifier represent their total amounts for the final emulsion. In a mortar, the 1 part gum (e. g. , acacia) is levigated with the 4 parts oil until the powder is thoroughly wetted; then the 2 parts water are added all at once, and the mixture is vigorously and continually triturated until the primary emulsion formed is creamy white. Additional water or aqueous solutions may be incorporated after the primary emulsion is formed. Solid substances (e. g. , active ingredients, preservatives, color, flavors) are generally dissolved and added as a solution to the primary emulsion. Oil soluble substance, in small amounts, may be incorporated directly into the primary emulsion. Any substance which might reduce the physical stability of the emulsion, such as alcohol (which may precipitate the gum) should be added as near to the end of the process as possible to avoid breaking the emulsion. When all agents have been incorporated, the emulsion should be transferred to a calibrated vessel, brought to final volume with water, then homogenized or blended to ensure uniform distribution of ingredients.

Preparing emulsion by dry gum method • • • Cod liver oil Acacia Syrup Flavor oil Purified water, qs ad 50 m. L 12. 5 g 10 m. L 0. 4 m. L 100 m. L 1. Accurately weigh or measure each ingredient 2. Place cod liver oil in dry mortar 3. Add acacia and give it a very quick mix 4. Add 25 m. L of water and immediately triturate to form the thick, white, homogenous primary emulsion 5. Add the flavor and mix 6. Add syrup and mix 7. Add sufficient water to total 100 m. L

Wet gum method • In this method, the proportions of oil, water, and emulsifier are the same (4: 2: 1), but the order and techniques of mixing are different. The 1 part gum is triturated with 2 parts water to form a mucilage; then the 4 parts oil is added slowly, in portions, while triturating. After all the oil is added, the mixture is triturated for several minutes to form the primary emulsion. Then other ingredients may be added as in the continental method. Generally speaking, the English method is more difficult to perform successfully, especially with more viscous oils, but may result in a more stable emulsion.

Bottle method • This method may be used to prepare emulsions of volatile oils, or oleaginous substances of very low viscosities. This method is a variation of the dry gum method. One part powdered acacia (or other gum) is placed in a dry bottle and four parts oil are added. The bottle is capped and thoroughly shaken. To this, the required volume of water is added all at once, and the mixture is shaken thoroughly until the primary emulsion forms. It is important to minimize the initial amount of time the gum and oil are mixed. The gum will tend to imbibe the oil, and will become more waterproof.

Auxiliary method • An emulsion prepared by other methods can also usually be improved by passing it through a hand homogenizer, which forces the emulsion through a very small orifice, reducing the dispersed droplet size to about 5 microns or less.

Incorporation of medicinal agents • Addition of drug during emulsion formation • Addition of drugs to a preformed emulsion 1. Addition of oleaginous materials into a w/o emulsion 2. Addition of oleaginous materials to an o/w emulsion 3. Addition of water soluble materials to a w/o emulsion 4. Addition of water soluble materials to an o/w emulsion

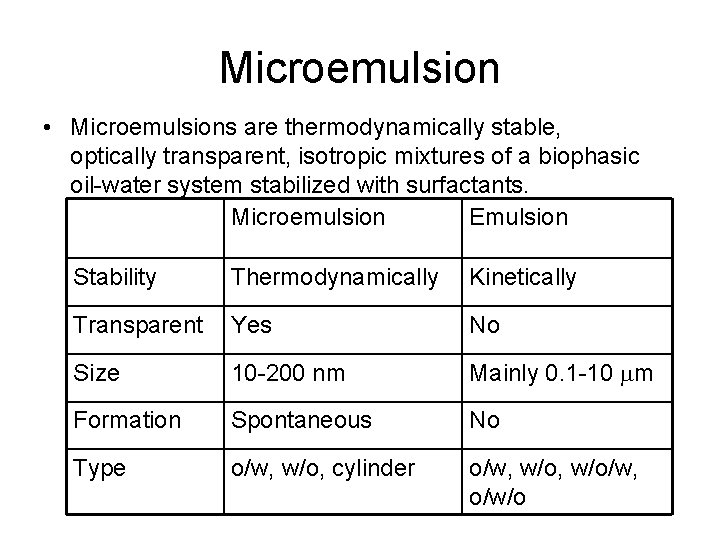
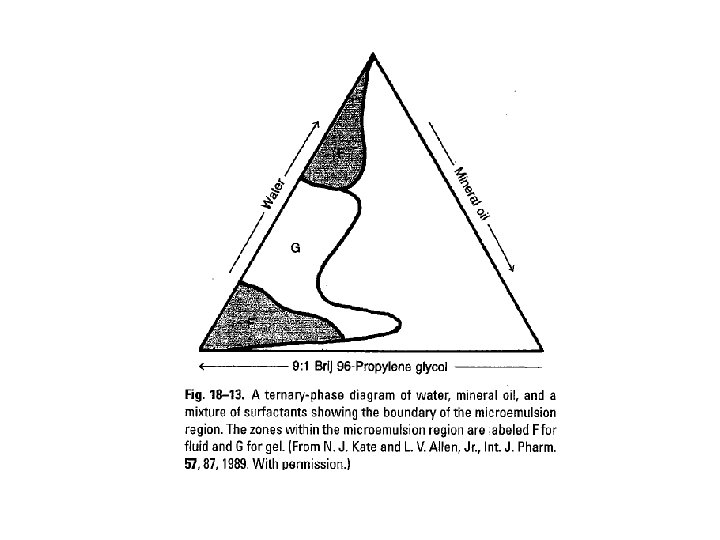
Microemulsion • Microemulsions are thermodynamically stable, optically transparent, isotropic mixtures of a biophasic oil-water system stabilized with surfactants. Microemulsion Emulsion Stability Thermodynamically Kinetically Transparent Yes No Size 10 -200 nm Mainly 0. 1 -10 mm Formation Spontaneous No Type o/w, w/o, cylinder o/w, w/o/w, o/w/o


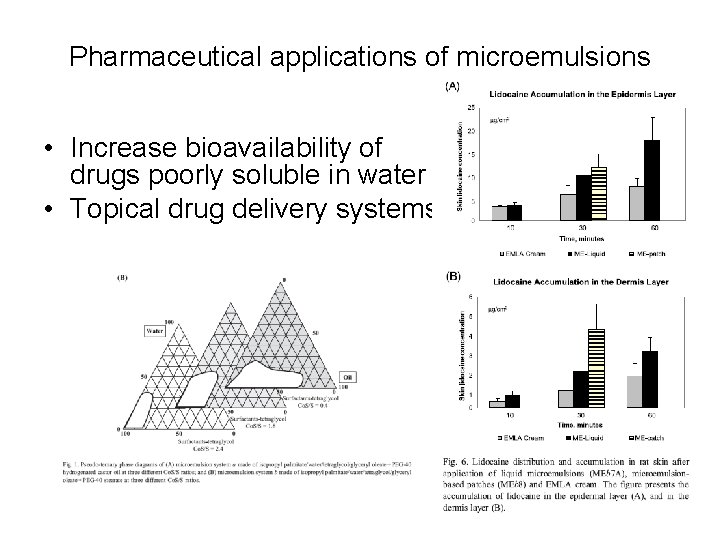
Pharmaceutical applications of microemulsions • Increase bioavailability of drugs poorly soluble in water • Topical drug delivery systems

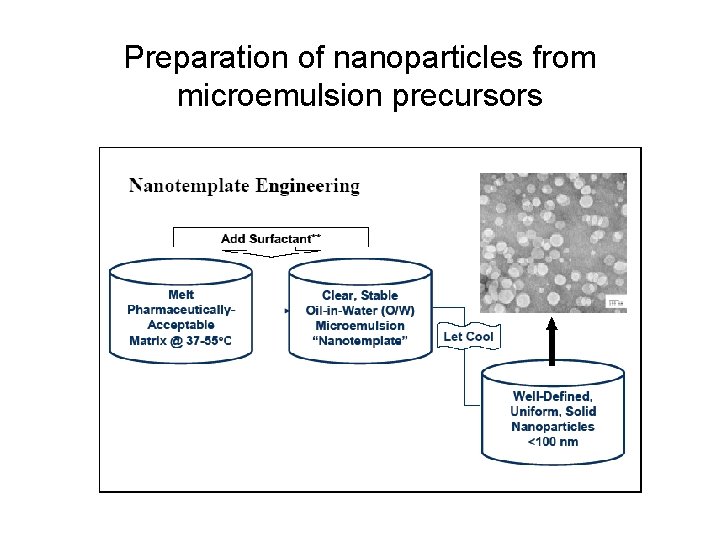
Preparation of nanoparticles from microemulsion precursors