Chemistry Atoms smallest unit of matter Atoms contain
- Slides: 5

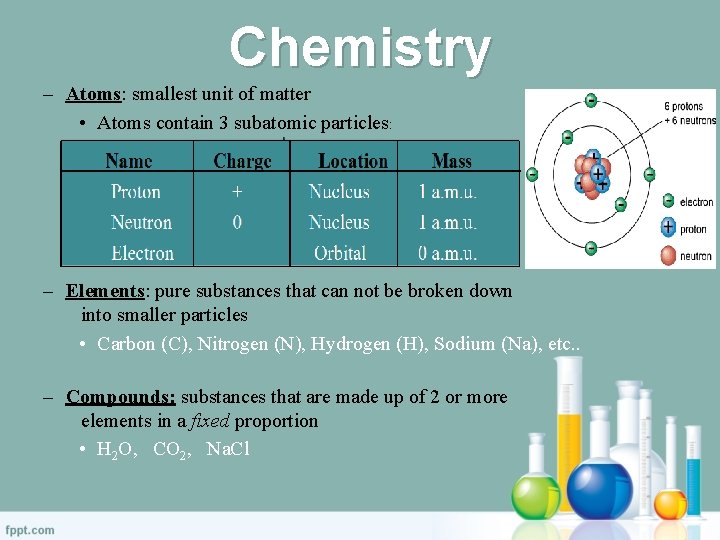
Chemistry – Atoms: smallest unit of matter • Atoms contain 3 subatomic particles: – Elements: pure substances that can not be broken down into smaller particles • Carbon (C), Nitrogen (N), Hydrogen (H), Sodium (Na), etc. . – Compounds: substances that are made up of 2 or more elements in a fixed proportion • H 2 O, CO 2, Na. Cl

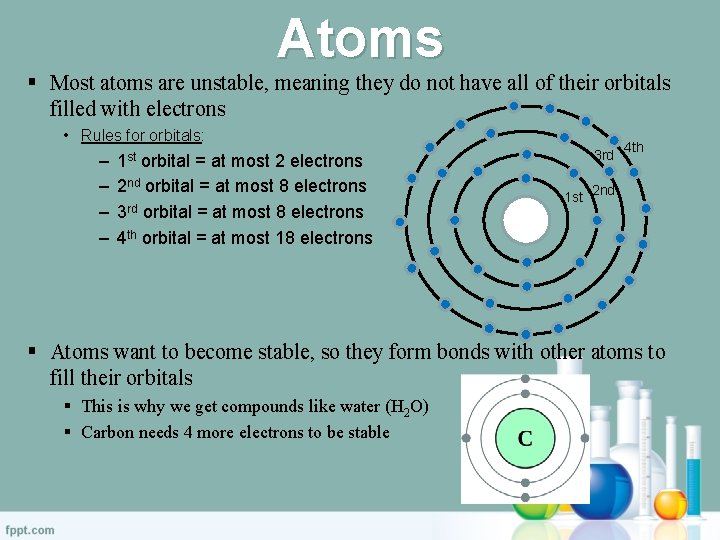
Atoms § Most atoms are unstable, meaning they do not have all of their orbitals filled with electrons • Rules for orbitals: – – 1 st orbital = at most 2 electrons 2 nd orbital = at most 8 electrons 3 rd orbital = at most 8 electrons 4 th orbital = at most 18 electrons 3 rd 1 st 4 th 2 nd § Atoms want to become stable, so they form bonds with other atoms to fill their orbitals § This is why we get compounds like water (H 2 O) § Carbon needs 4 more electrons to be stable

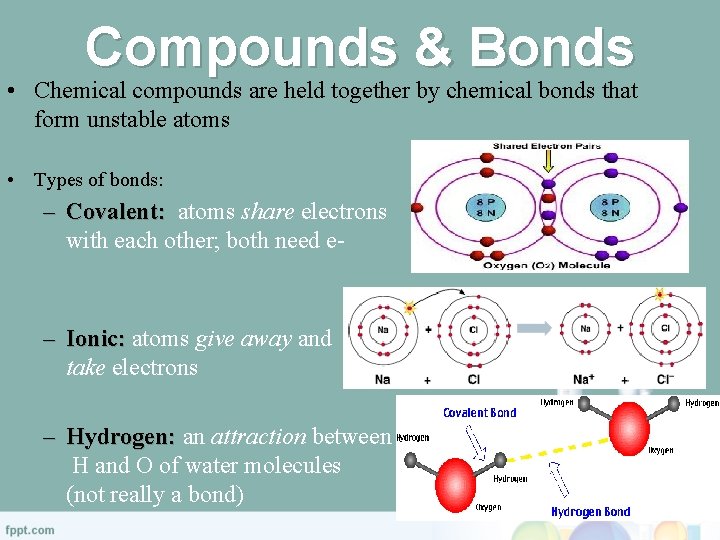
Compounds & Bonds • Chemical compounds are held together by chemical bonds that form unstable atoms • Types of bonds: – Covalent: atoms share electrons with each other; both need e- – Ionic: atoms give away and take electrons – Hydrogen: an attraction between H and O of water molecules (not really a bond)

Isotopes • Isotopes are naturally occurring elements with the same # of protons & electrons, but a different # of neutrons • Has a different atomic mass Ex: 1 H – mass 1 a. m. u. (Protium) 2 H – mass 2 a. m. u. (Deuterium) 3 H – mass 3 a. m. u. (Tritium) Normal hydrogen Isotopes of hydrogen • Usually radioactive (unstable) – Power plants – Uranium – Medicine – MRI, diagnostic tracers, cancer treatments – Radioactive (carbon) Dating – determining the age of objects

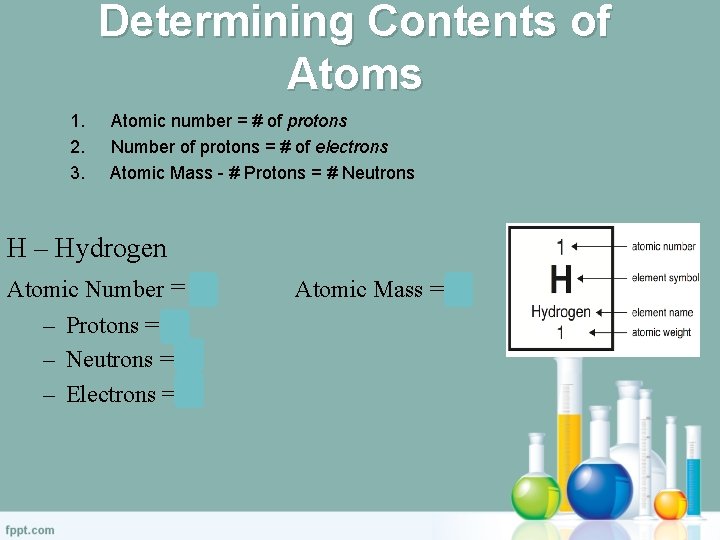
Determining Contents of Atoms 1. 2. 3. Atomic number = # of protons Number of protons = # of electrons Atomic Mass - # Protons = # Neutrons H – Hydrogen Atomic Number = 1 – Protons = 1 – Neutrons = 0 – Electrons = 1 Atomic Mass = 1