Physical and Chemical Changes A Write On Activity



























- Slides: 50

Physical and Chemical Changes A Write On Activity

Objective: Chemical/Physical Change: Differentiate o The learner will be able to (ESSENTIAL) distinguish between a physical and chemical change.

Matter is everywhere. o Matter is anything that takes up space and has mass. o Matter is constantly experiencing both chemical and physical changes. o

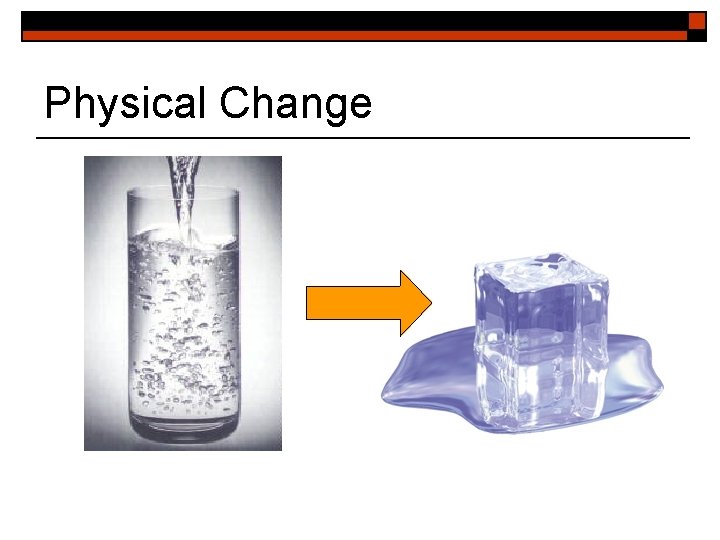
Physical Change o o Physical changes occur when matter changes its property but not its chemical nature. Physical property changes could include a change in: texture, shape, size, color, odor, volume, mass, weight, and density.

Physical Change

Chemical Change o o Chemical changes are changes matter undergoes when it becomes new or different matter. To identify a chemical change look for signs such as color change, bubbling and fizzing, light production, smoke, and presence of heat.

Chemical Change o A chemical change occurs when fireworks are used. Fireworks are made of metals such as magnesium and copper. These change chemically as they light up the sky.

Is it a chemical or physical change? o Sugar dissolving in tea • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Logs burning • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Breaking water up by separating it into hydrogen and oxygen • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Cutting paper • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Crushing an aspirin • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Metal rusting • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Lighter fluid burning • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o An egg rotting • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o An egg breaking • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Writing Activity o Write a paragraph about the difference between a chemical and physical change. Give examples of each.

Changing Matter Let’s Review Physical & Chemical Changes

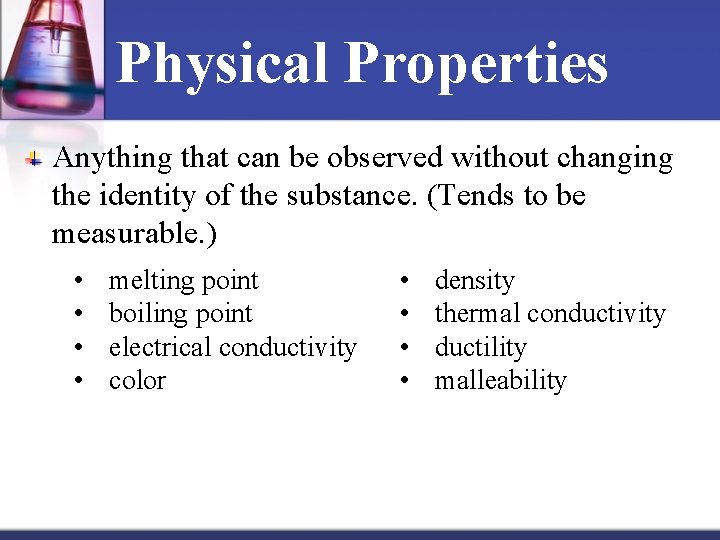
Physical Properties Anything that can be observed without changing the identity of the substance. (Tends to be measurable. ) Name 5 of the 10 …

Physical Properties Anything that can be observed without changing the identity of the substance. (Tends to be measurable. ) • • melting point boiling point electrical conductivity color • • density thermal conductivity ductility malleability

Chemical Properties The way a substance may change or react to form other substances Name 3 of the 6

Chemical Properties The way a substance may change or react to form other substances • • • heat of combustion reactivity with water p. H Oxidation Flammability • Reactivity to other chemicals

Physical Changes Do NOT CHANGE THE TYPE OF MATTER Nothing new or different is formed Could be a change in: ? ? ?

Physical Changes Do NOT CHANGE THE TYPE OF MATTER Nothing new or different is formed Could be a change in: Mass Size Volume Density Change in state Color Shape

Examples of Physical Changes Boiling Freezing Dissolving Breaking Making a mixture 2 or more types of matter (substances) mixed together Not in specific amounts Can be separated physically

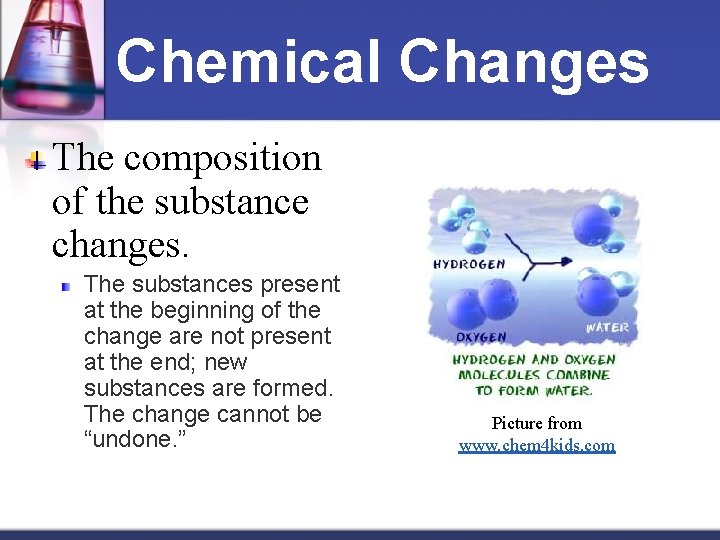
Chemical Changes The composition of the substance changes. The substances present at the beginning of the change are not present at the end; new substances are formed. The change cannot be “undone. ” Picture from www. chem 4 kids. com

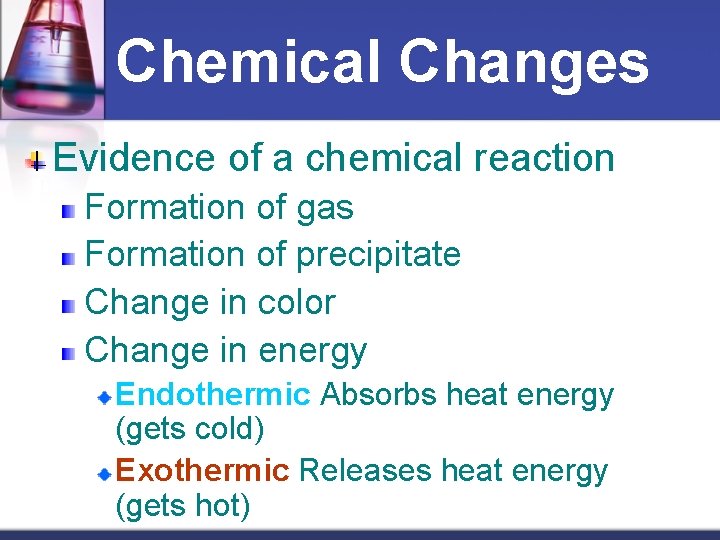
Chemical Changes Evidence of a chemical reaction Formation of g? ? ? Formation of p? ? ? Change in c? ? ? Change in e? ? ?

Chemical Changes Evidence of a chemical reaction Formation of gas Formation of precipitate Change in color Change in energy Endothermic Absorbs heat energy (gets cold) Exothermic Releases heat energy (gets hot)

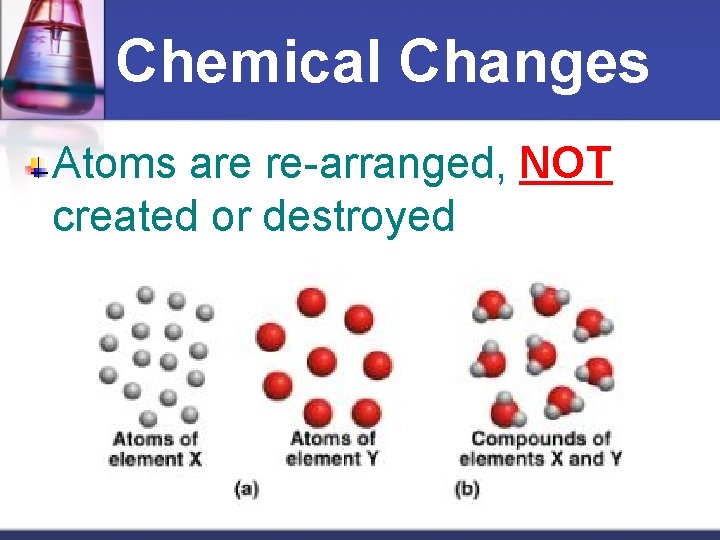
Chemical Changes Atoms are re-arranged, NOT created or destroyed

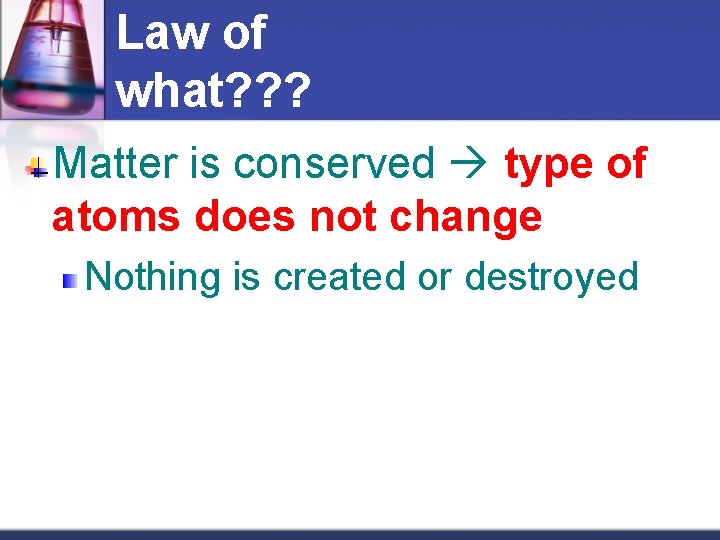
Law of what? ? ? Matter is conserved type of atoms does not change Nothing is created or destroyed

Law of Conservation of Mass is conserved amount of atoms cannot change Nothing is created or destroyed

Computer Lab Tasks Find an example of physical and chemical changes- safe demo Go test your skills at physical vs. chemical changes http: //vital. cs. ohiou. edu/steamwebsite/downloads/Change. Lab. swf http: //www. glencoe. com/sites/common_assets/science/virtual_labs/E 03. html Try out the periodic table sites http: //www. rsc. org/periodic-table http: //www. ptable. com/ http: //www. abpischools. org. uk/page/modules/interactiveperiodictable/activity. cfm ? co. Site. Navigation_all. Topic=1