Physical and Chemical Changes Concept of Change l








- Slides: 9

Physical and Chemical Changes

Concept of Change l Change - the act of altering a substance u Changes can be physical or chemical u When considering what TYPE of change has occurred, ask yourself… “Did the identity change? ” “Can I separate it? ”

Physical Change l Physical change - a change that occurs that does not change the identity of the substance and can be separated. u Examples: u Tearing paper u Phase Changes like Melting, Freezing, Boiling

Chemical Changes l Chemical change - a change that occurs causing the identity of the substance to change and cannot be separated. u Examples: u Burning paper u Digesting food l A chemical change is also called a chemical reaction

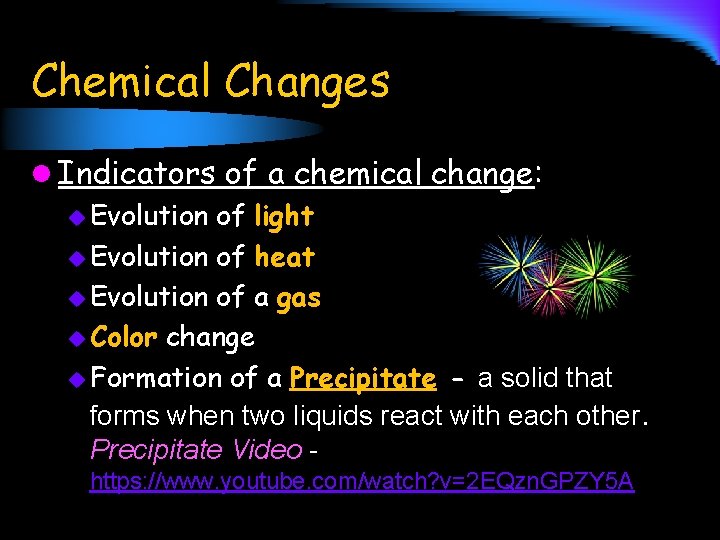
Chemical Changes l Indicators of a chemical change: u Evolution of light u Evolution of heat u Evolution of a gas u Color change u Formation of a Precipitate - a solid that forms when two liquids react with each other. Precipitate Video https: //www. youtube. com/watch? v=2 EQzn. GPZY 5 A

Possible Exceptions l Not all color changes are chemical reactions… l When you mix water and Kool-Aid the color changes. But is it a chemical change? l Why? How do you know? l What are some other exceptions?

Is it Physical or Chemical? Change Melting cheese Burning wood Baking cookies Wadding up paper Bicycle rusting Physical Chemical

Think-Pair-Share l Look at the real-world examples on the back of the notes. l Think (1 min) – Answer individually l Pair (2 min) – Get with a partner and discuss your answers l Share (3 min) – Go over whole class

Snowball Fight l On the paper and write an example of a change from the chart. l Crumple and wad up the paper into a “snow”ball. l When I say so, throw the snowball at someone else in the room. l Open up a snowball near you and decide if the example is Physical or Chemical and write your name on it! l Crumple it up again and throw it one more time. Write Agree or Disagree and your name again. l Put it in the Apple Tray.