Phases Phase Changes Chemical and Physical Changes Science
























- Slides: 56

Phases, Phase Changes, Chemical and Physical Changes Science Fifth Grade Mr. Pate

Standards • S 5 P 2. Students will explain the difference between a physical change and a chemical change. • a. Investigate physical changes by separating mixtures and manipulating (cutting, tearing, folding) paper to demonstrate examples of physical change. • b. Recognize that the changes in state of water (water vapor/steam, liquid, ice) are due to temperature differences and are examples of physical change.

Standards • c. Investigate the properties of a substance before, during, and after a chemical reaction to find evidence of change. • S 5 P 1. Students will verify that an object is the sum of its parts

Standards • a. Demonstrate that the mass of an object is equal to the sum of its parts by manipulating and measuring different objects made of various parts. • b. Investigate how common items have parts that are too small to be seen without magnification

Essential Questions • What is a chemical change? • What is a physical change? • How does an object equal the sum of its parts?

Task • The students will use the liquid mixture in the baggie to create a change in state. • The students will use a banana and break it into parts to see if the sum of the parts equal the whole.

Circumstance • The students will work in teacher assigned groups to perform the experiments. • They will use the lab sheets to record data and answer questions.

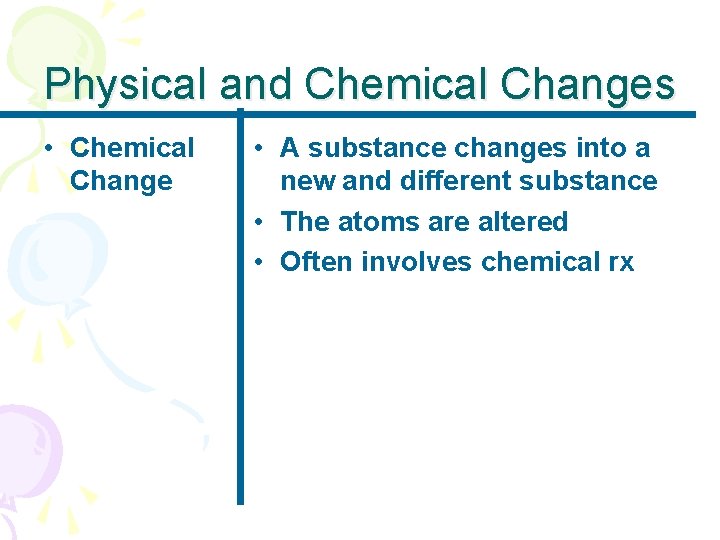
GOAL • To discuss the states of matter and the terms relative to changes in state • To distinguish between chemical and physical changes

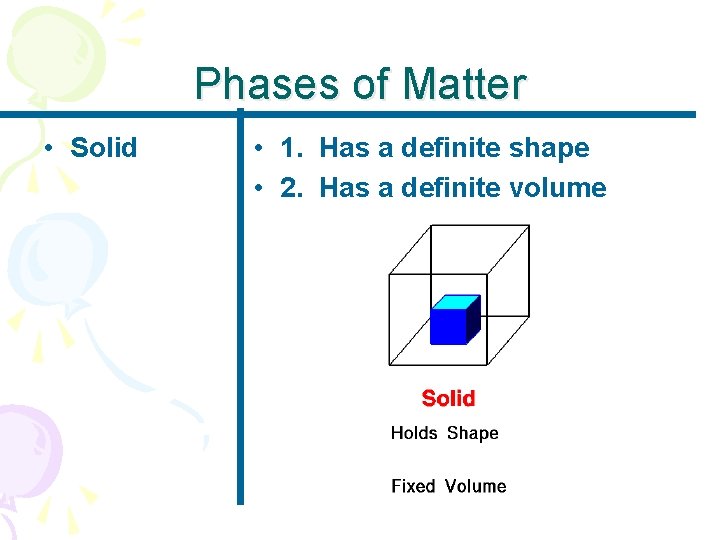
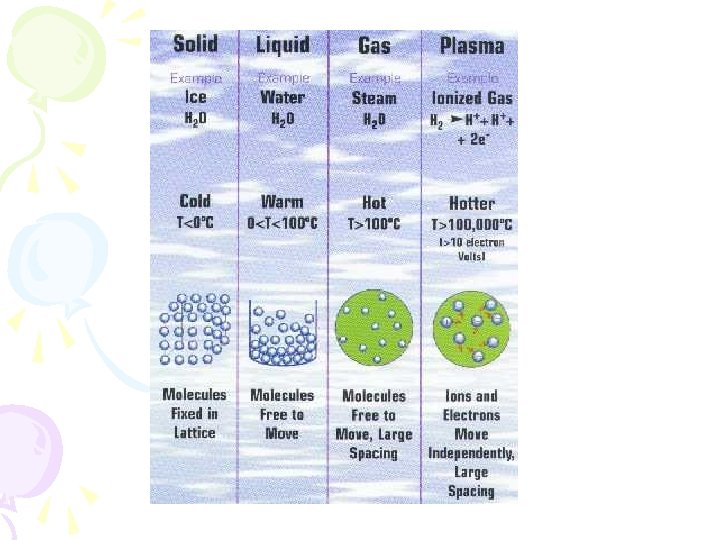
Phases of Matter • Solid • 1. Has a definite shape • 2. Has a definite volume

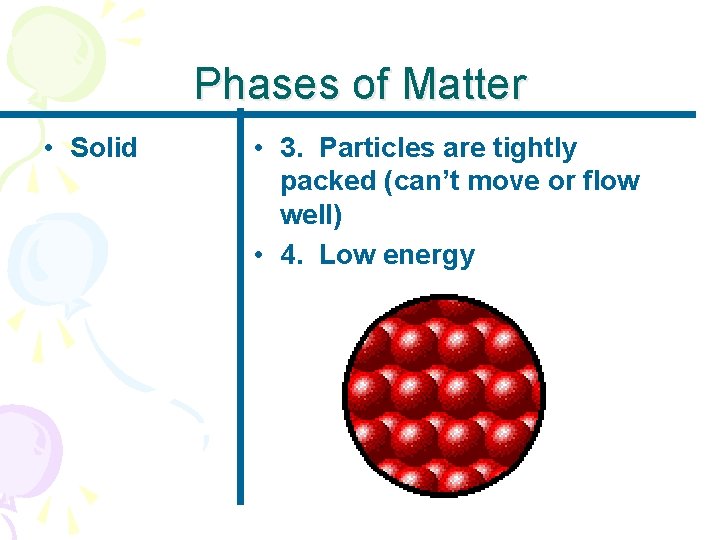
Phases of Matter • Solid • 3. Particles are tightly packed (can’t move or flow well) • 4. Low energy

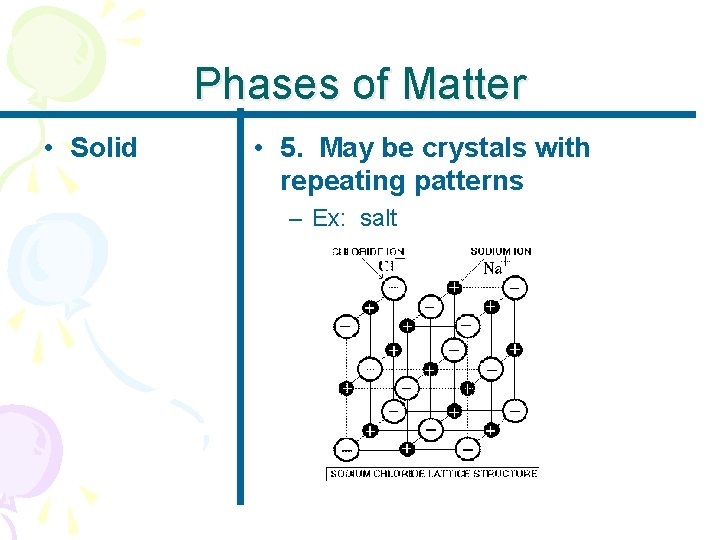
Phases of Matter • Solid • 5. May be crystals with repeating patterns – Ex: salt

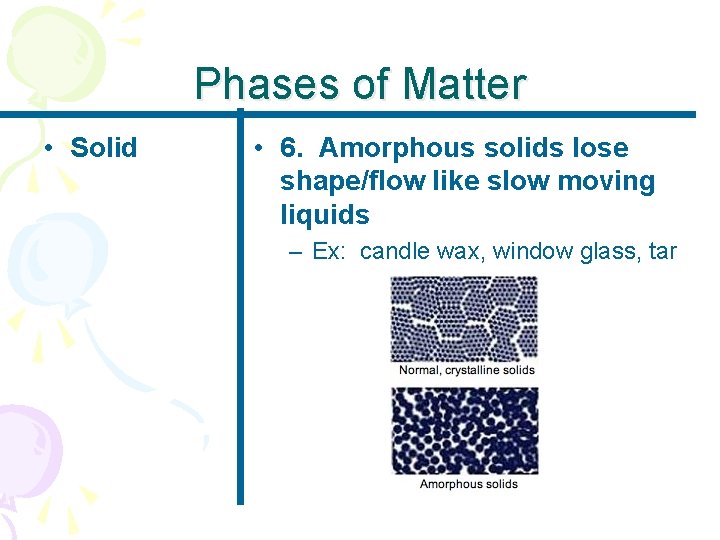
Phases of Matter • Solid • 6. Amorphous solids lose shape/flow like slow moving liquids – Ex: candle wax, window glass, tar

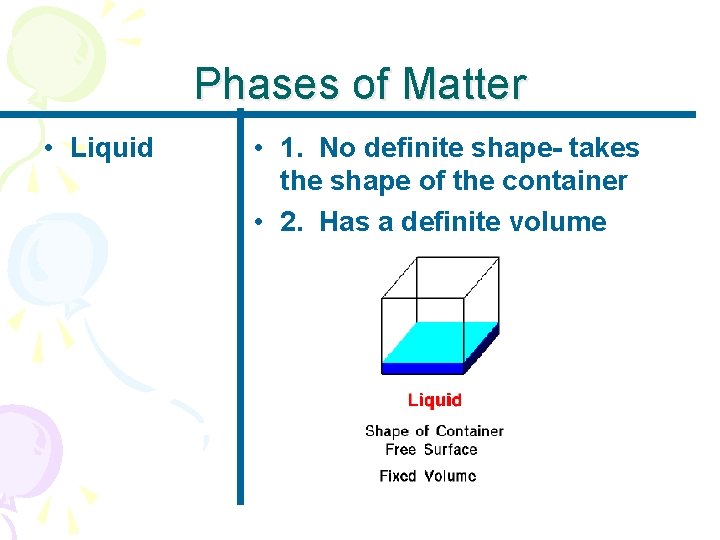
Phases of Matter • Liquid • 1. No definite shape- takes the shape of the container • 2. Has a definite volume

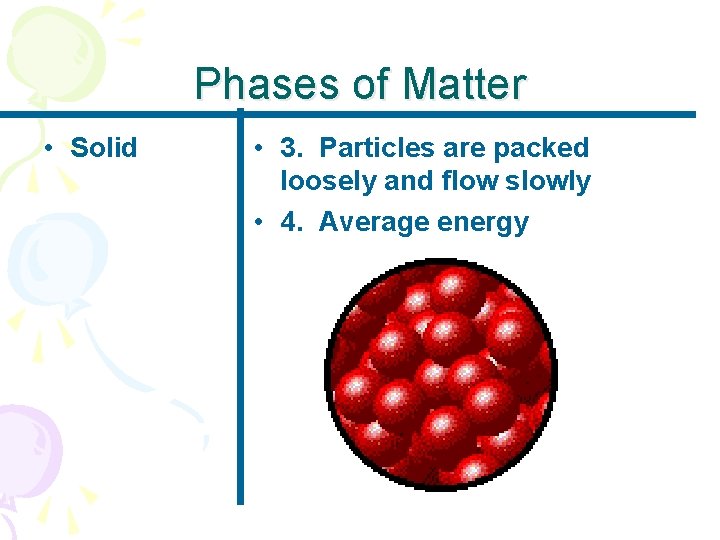
Phases of Matter • Solid • 3. Particles are packed loosely and flow slowly • 4. Average energy

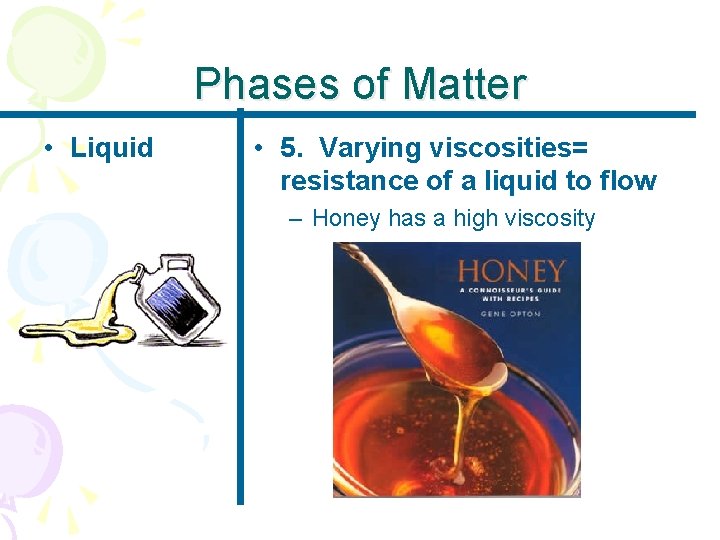
Phases of Matter • Liquid • 5. Varying viscosities= resistance of a liquid to flow – Honey has a high viscosity

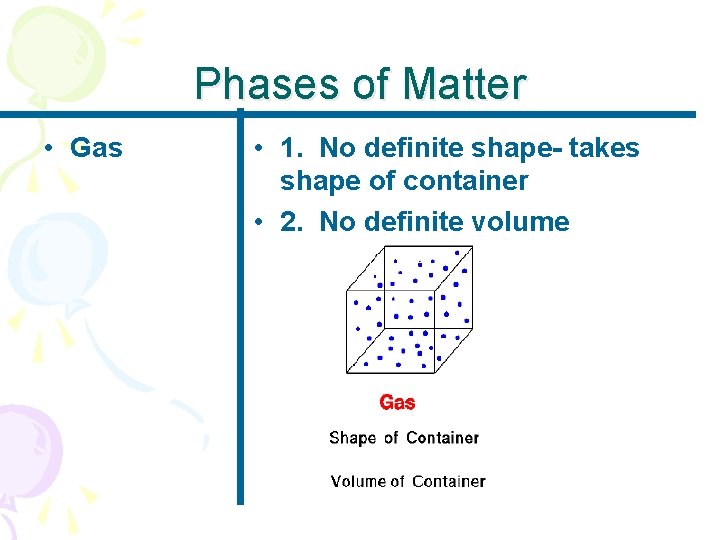
Phases of Matter • Gas • 1. No definite shape- takes shape of container • 2. No definite volume

Phases of Matter • Gas • 3. Particles are spread far apart- fill all spaces • 4. Contantly moving and bumping into eachother • 5. High energy

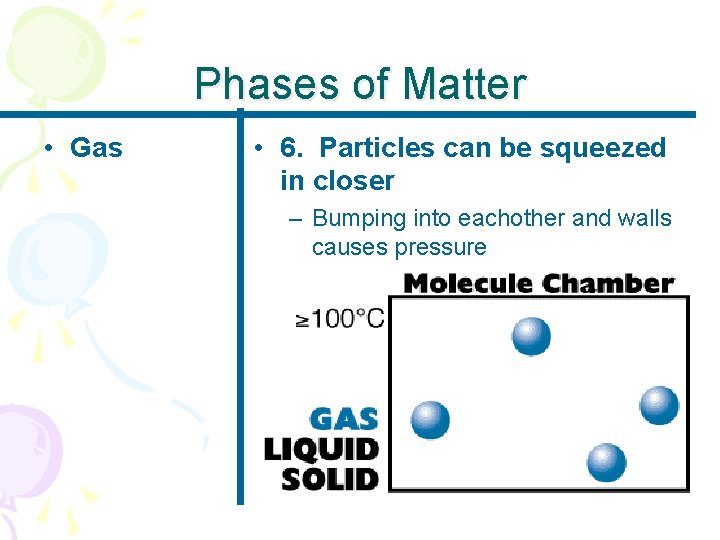
Phases of Matter • Gas • 6. Particles can be squeezed in closer – Bumping into eachother and walls causes pressure

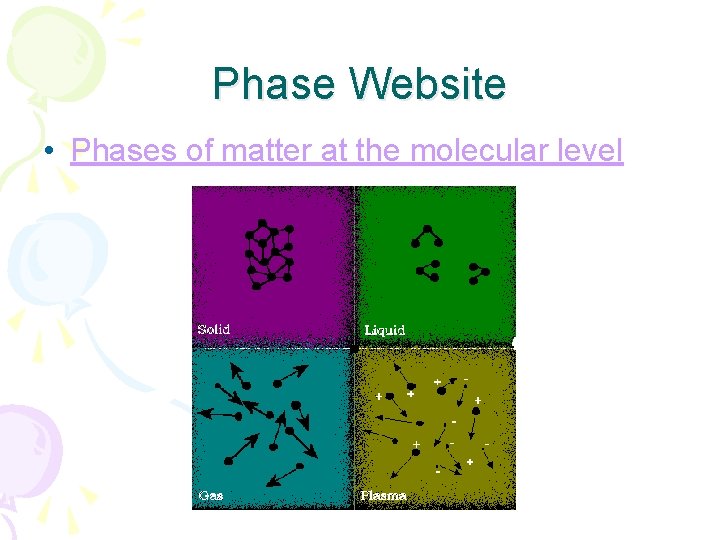
Phase Website • Phases of matter at the molecular level

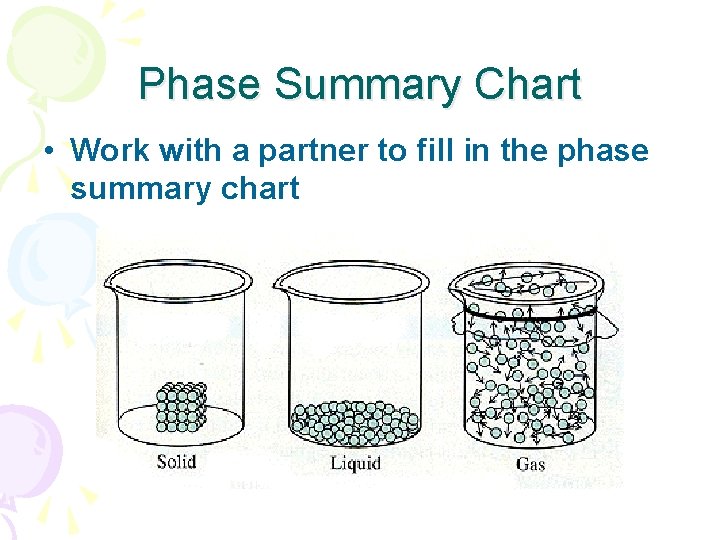
Phase Summary Chart • Work with a partner to fill in the phase summary chart


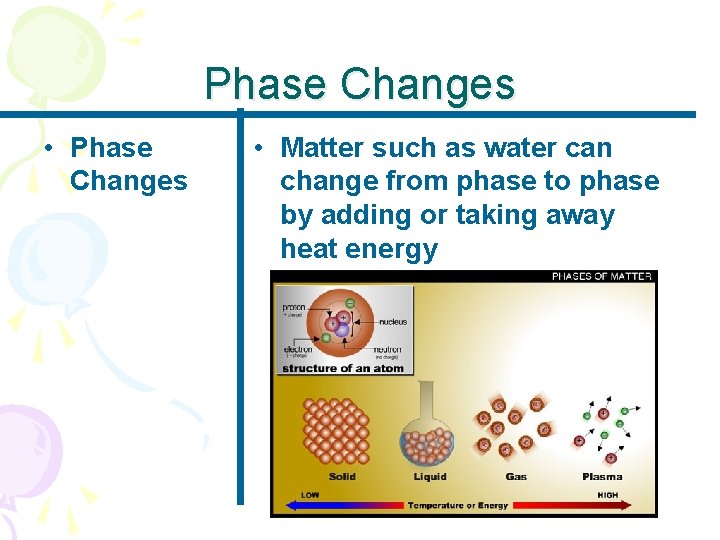
Phase Changes • Phase Changes • Matter such as water can change from phase to phase by adding or taking away heat energy

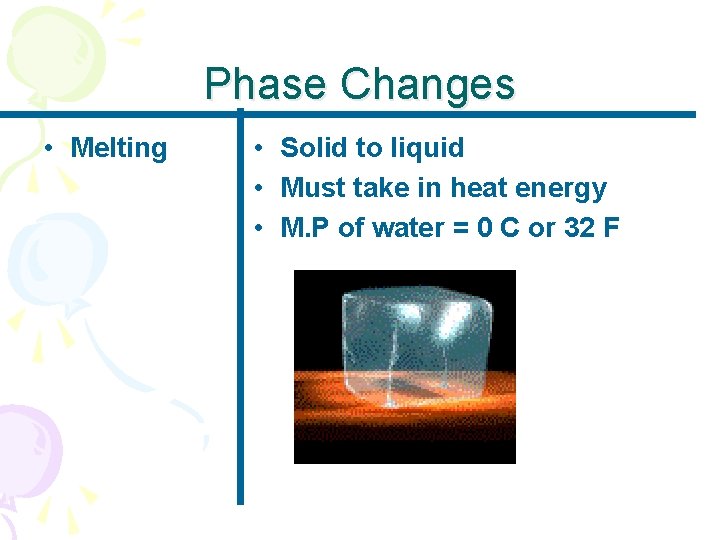
Phase Changes • Melting • Solid to liquid • Must take in heat energy • M. P of water = 0 C or 32 F

Examples of Melting

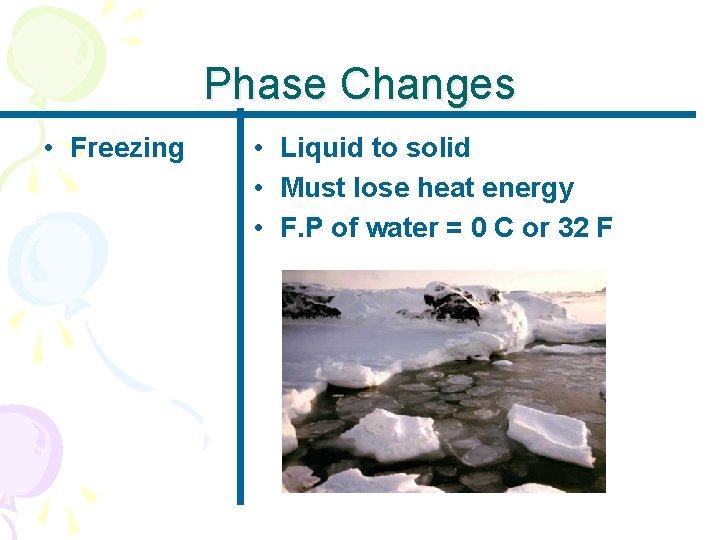
Phase Changes • Freezing • Liquid to solid • Must lose heat energy • F. P of water = 0 C or 32 F

Examples of Freezing

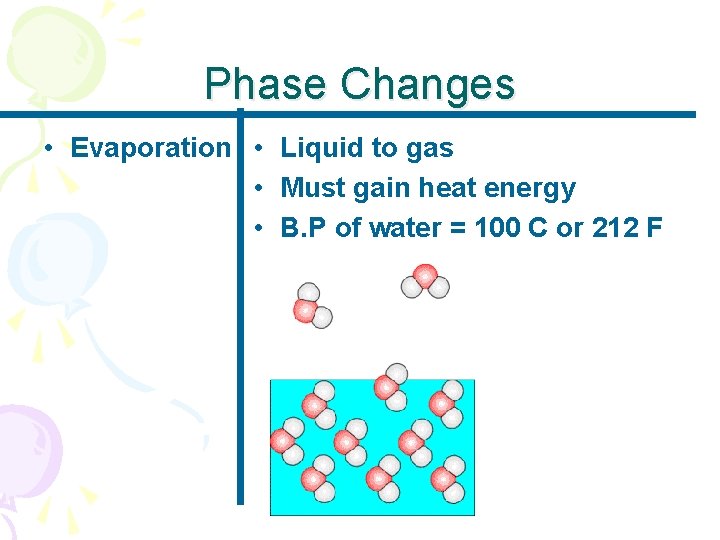
Phase Changes • Evaporation • Liquid to gas • Must gain heat energy • B. P of water = 100 C or 212 F

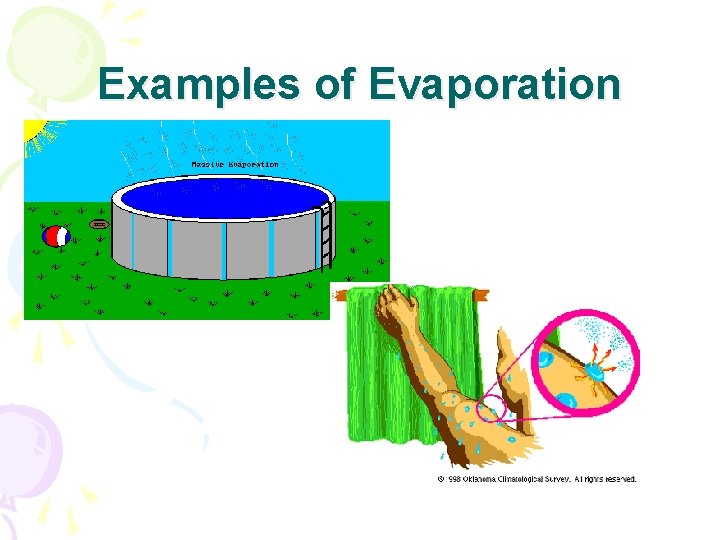
Examples of Evaporation

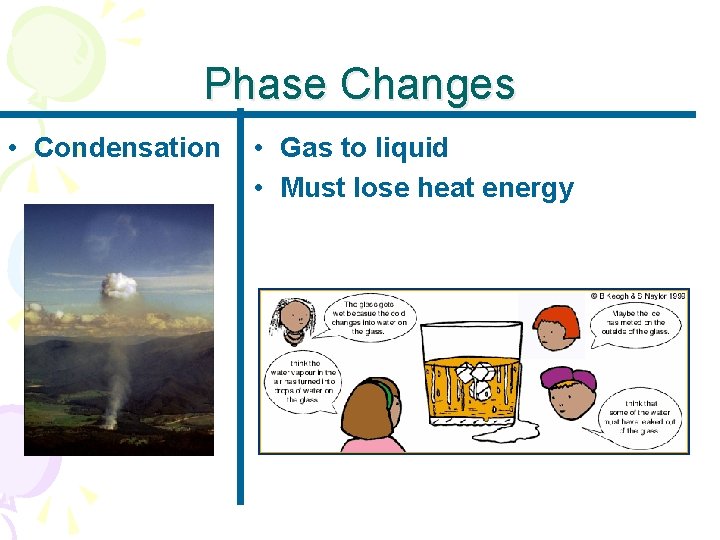
Phase Changes • Condensation • Gas to liquid • Must lose heat energy

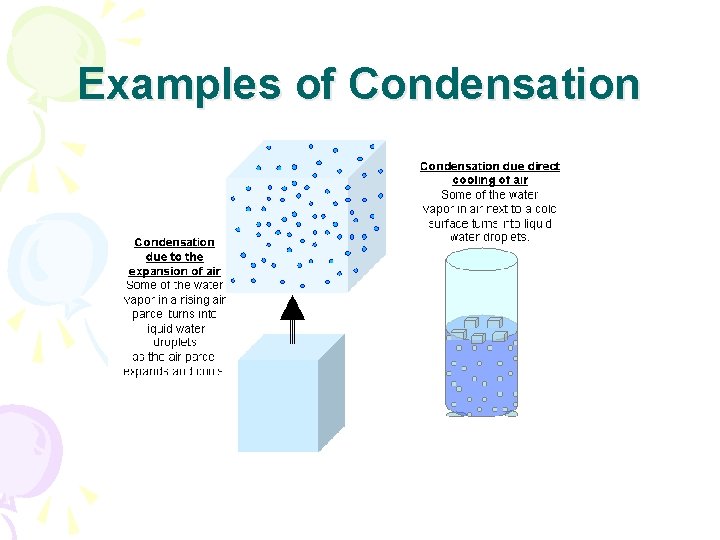
Examples of Condensation

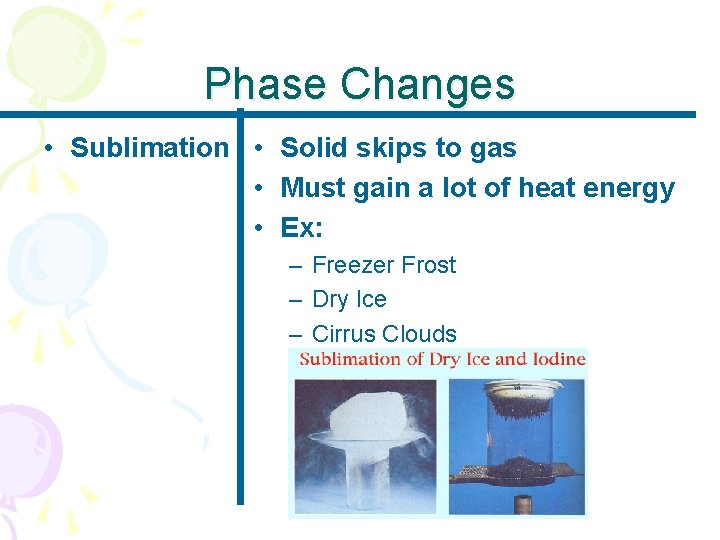
Phase Changes • Sublimation • Solid skips to gas • Must gain a lot of heat energy • Ex: – Freezer Frost – Dry Ice – Cirrus Clouds

Phase Change Website • Click here to view a phase change

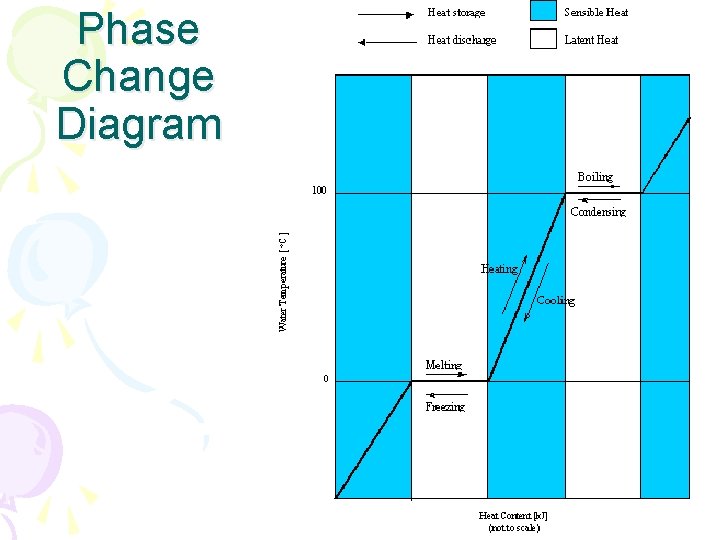
Phase Change Diagram

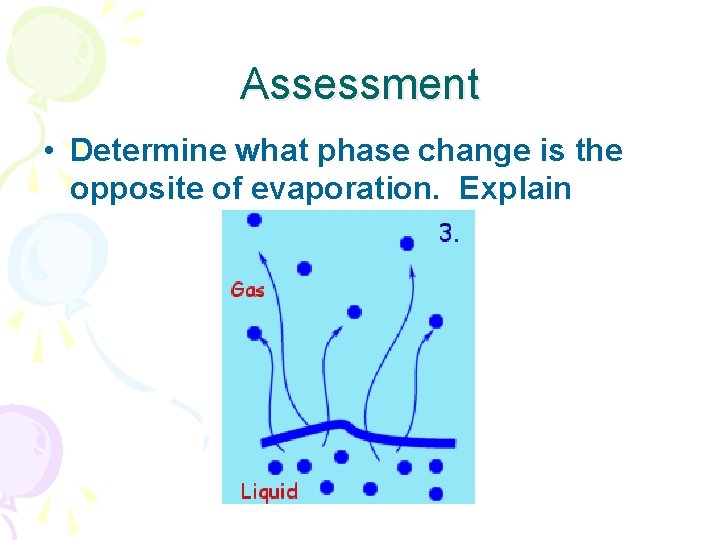
Assessment • Determine what phase change is the opposite of evaporation. Explain

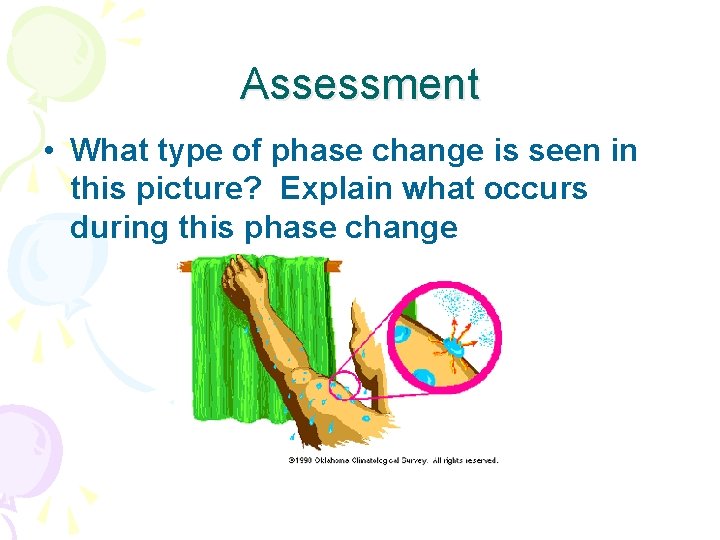
Assessment • What type of phase change is seen in this picture? Explain what occurs during this phase change

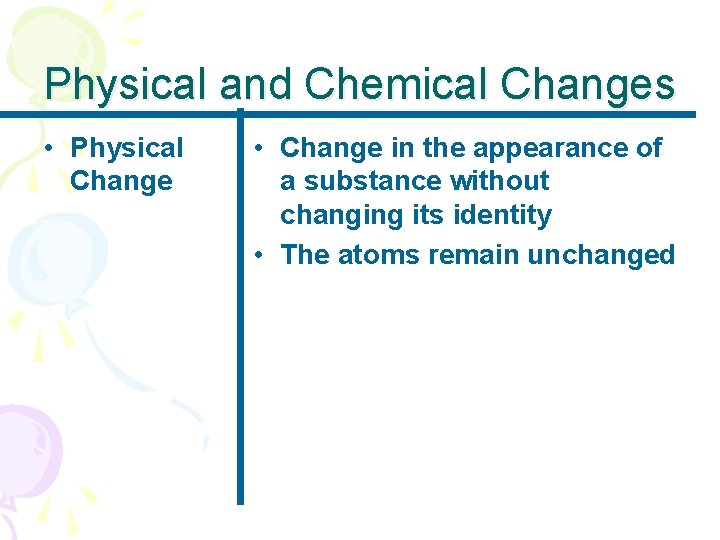
Physical and Chemical Changes • Physical Change • Change in the appearance of a substance without changing its identity • The atoms remain unchanged












Physical and Chemical Changes • Chemical Change • A substance changes into a new and different substance • The atoms are altered • Often involves chemical rx







Ice Cream Baggie Procedure: 1. Get a 1 gallon bag from Mr. Pate 2. Put two cups of ice into the bag. 3. Put ½ cup of rock salt on the ice and mix. 4. Place the liquid mixture baggie inside the gallon bag and seal the gallon bag. 5. Begin to gently shake and roll the bag in your hands. Pass to another group member if your hands get too cold. 6. Make sure that you are mixing the baggie with the liquid thoroughly. 7. You will need to mix thoroughly for at least 15 minutes. Check the clock to make sure. 8. If you have questions, raise your hand talk to Mr. Pate.

Banana Procedure: 1. Using the balance and the gram stackers, find the mass of the banana. To do this, find the mass of the paper plate (Measure 1). Next, place the banana on the plate and find a new measure (Measure 2). 2. Subtract measure 1 from measure 2 to find the mass of the banana. 3. Carefully peel the banana into its edible and inedible parts. 4. Place the edible part of the banana on the plate and find its mass(Measure 3). 5. Subtract Measure 1 from Measure 3 to find the mass of the edible part of the banana. 6. Place the inedible part of the banana on the plate and find the mass (Measure 4). 7. Subtract Measure 1 from Measure 4 to find the inedible mass.