Chemical and Physical Changes Physical Changes that do












- Slides: 20

Chemical and Physical Changes

Physical Changes that do not affect the nature or the characteristic properties of a substance Ex. Breaking glass, phase changes

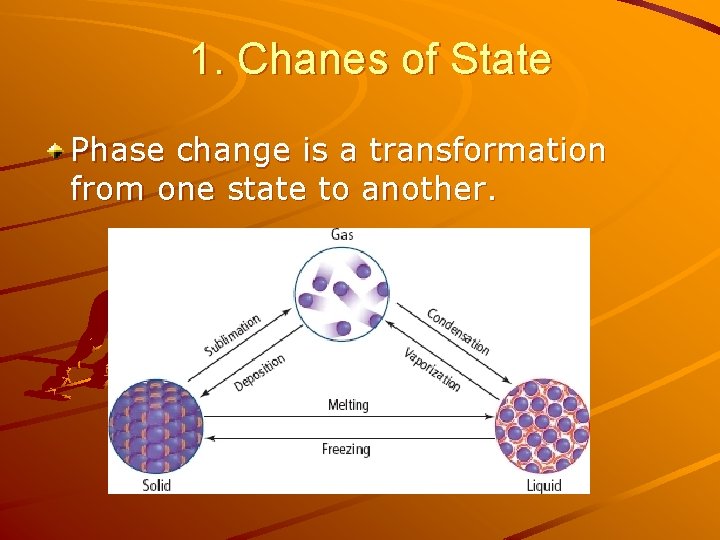
1. Chanes of State Phase change is a transformation from one state to another.

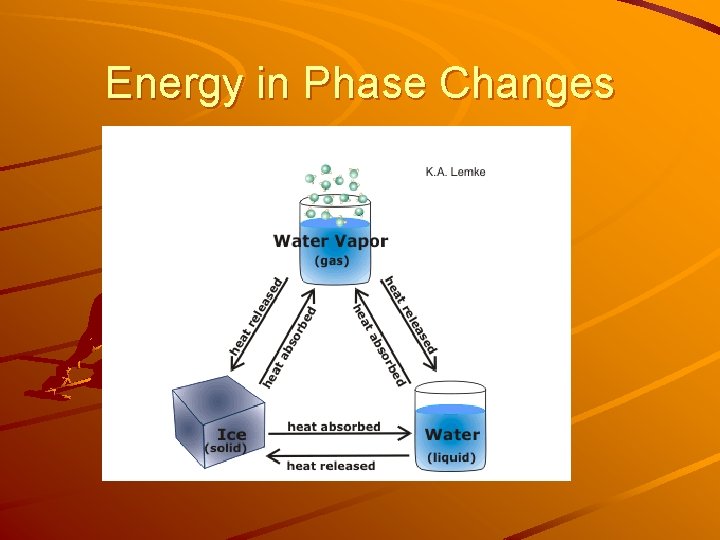
Energy in Phase Changes

Phase changes & Particle Model Phase change don’t affect characteristic properties Only the forces of attraction change Particles don’t change= “same”

Energy in phase changes Absorbs energy: liquid water + energy water vapour Releases energy liquid water ice + energy

2. Dissolution Creation of a solution by a solute dissolving in a solvent. Solutes may: Release energy -temp. increases

Absorb Energy: - temp. decreases

3. Deformation Means changing the shape of a material.

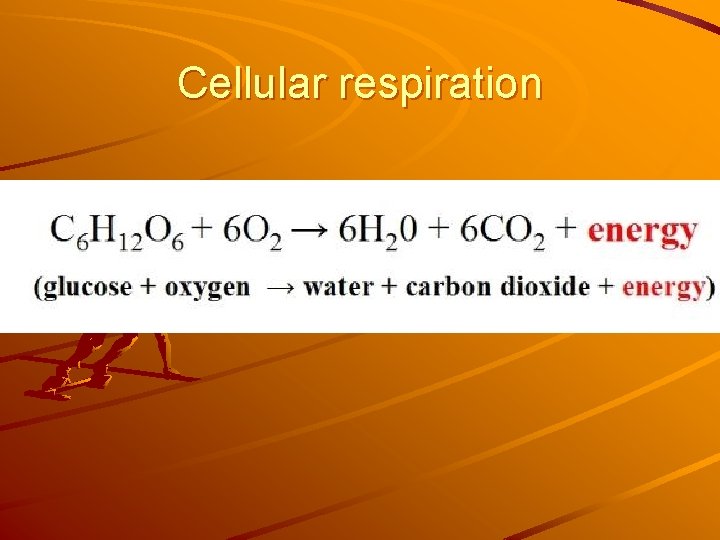
Chemical Changes that transform the nature and characteristic properties of a substance Ex. Burning wood, cooking food, Human body: - cellular respiration - digestion of food

Signs of a chemical change Change of colour Greater changes of heat Generation of Light Release of a Gas Formation of a Precipitate (a solid material forms in a liquid)

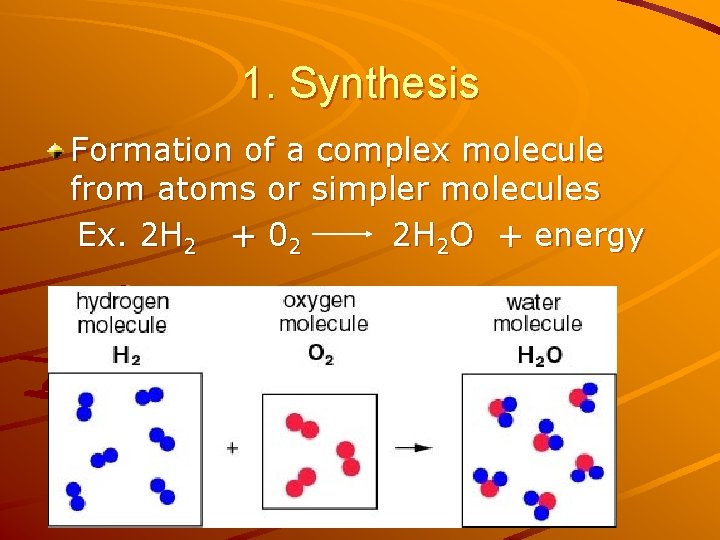
1. Synthesis Formation of a complex molecule from atoms or simpler molecules Ex. 2 H 2 + 02 2 H 2 O + energy

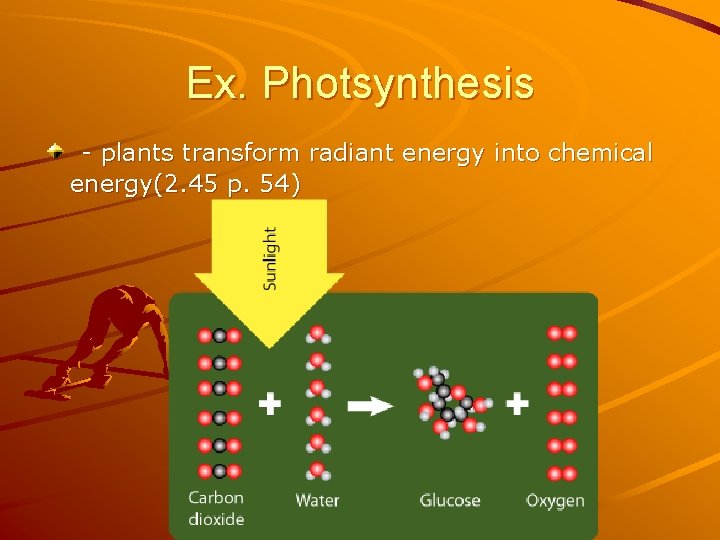
Ex. Photsynthesis - plants transform radiant energy into chemical energy(2. 45 p. 54)

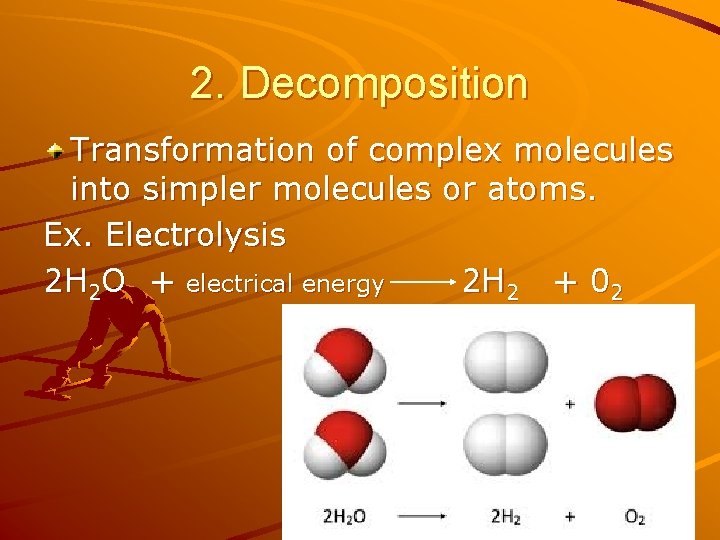
2. Decomposition Transformation of complex molecules into simpler molecules or atoms. Ex. Electrolysis 2 H 2 O + electrical energy 2 H 2 + 02

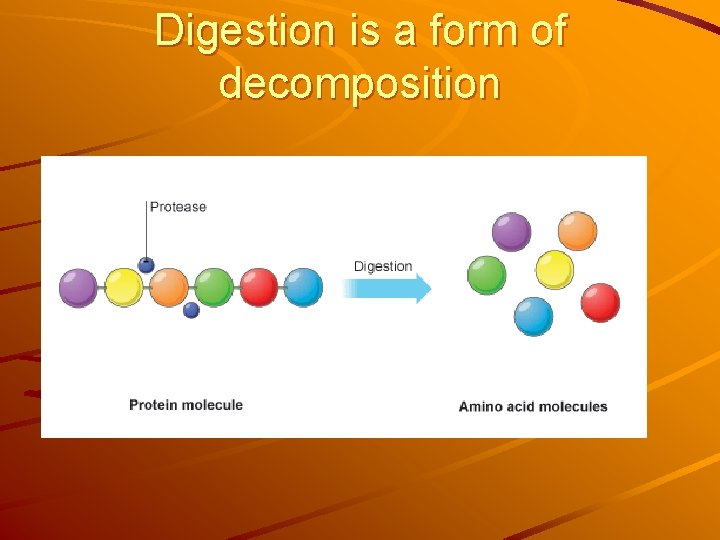
Digestion is a form of decomposition

3. Oxidation Chemical reaction involving oxygen or a substance that has similar properties to oxygen. Slow- rusting of metals release energy Rapid- combustion of fuels, very fast

Ex. Oxidation of Copper 2 Cu + O 2 2 Cu. O

Cellular respiration

4. Precipitation Formation of a solid that is less soluble, or not soluble, following the mixture of two solutions. Ex. Enzymes produce Precipitation in milk to Produce cheese

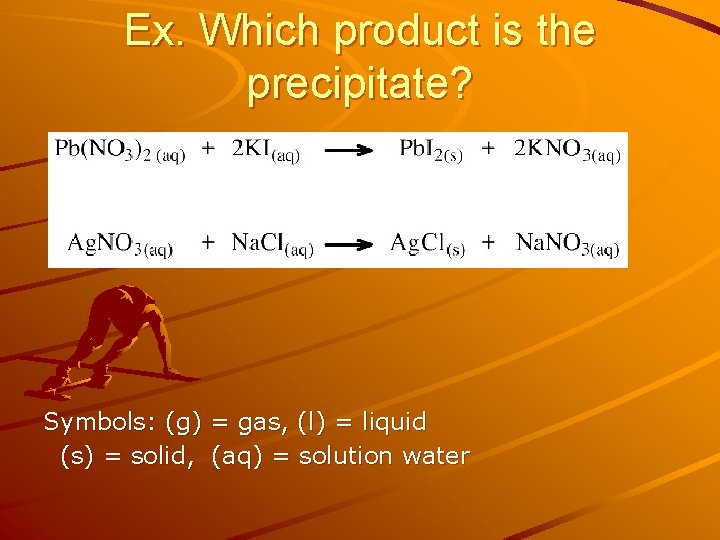
Ex. Which product is the precipitate? Symbols: (g) = gas, (l) = liquid (s) = solid, (aq) = solution water