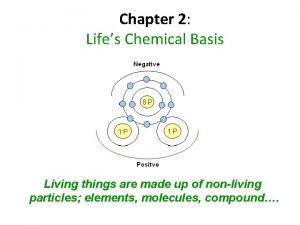
The Chemical Foundations of Life Chapter 2 pages







- Slides: 52

The Chemical Foundations of Life Chapter 2 (pages 22 -44)

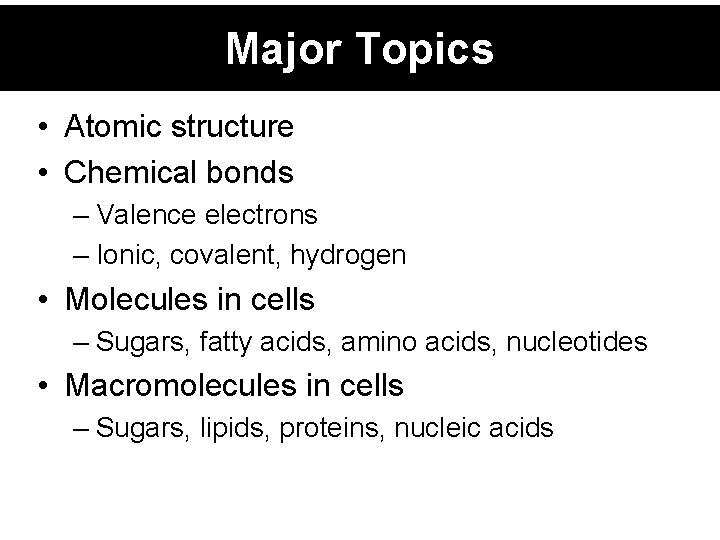
Major Topics • Atomic structure • Chemical bonds – Valence electrons – Ionic, covalent, hydrogen • Molecules in cells – Sugars, fatty acids, amino acids, nucleotides • Macromolecules in cells – Sugars, lipids, proteins, nucleic acids

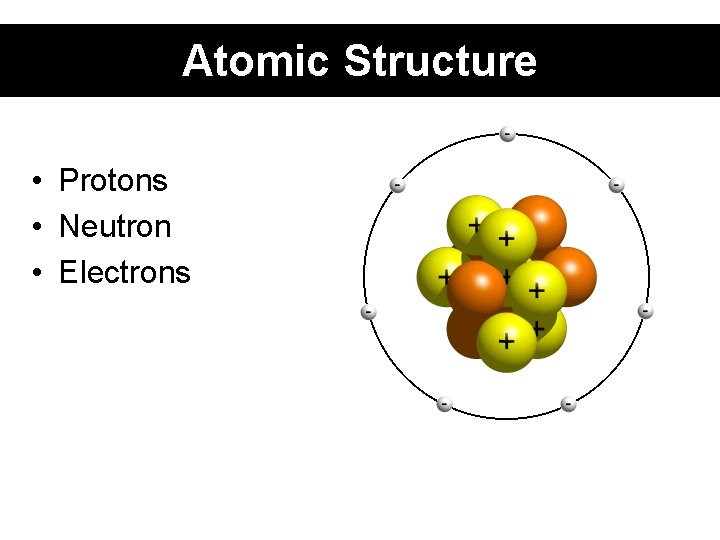
Atomic Structure • Protons • Neutron • Electrons

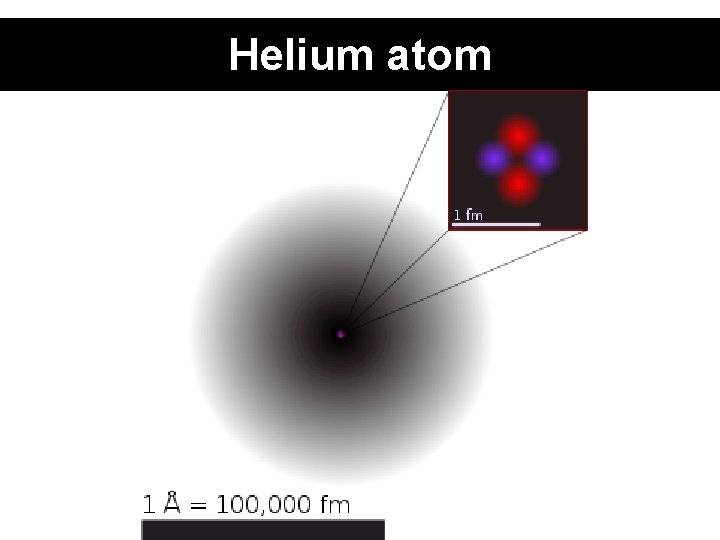
Helium atom

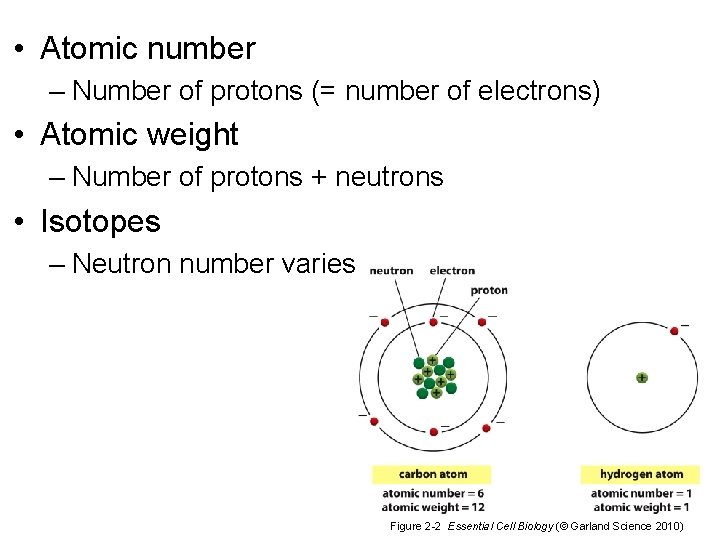
• Atomic number – Number of protons (= number of electrons) • Atomic weight – Number of protons + neutrons • Isotopes – Neutron number varies Figure 2 -2 Essential Cell Biology (© Garland Science 2010)

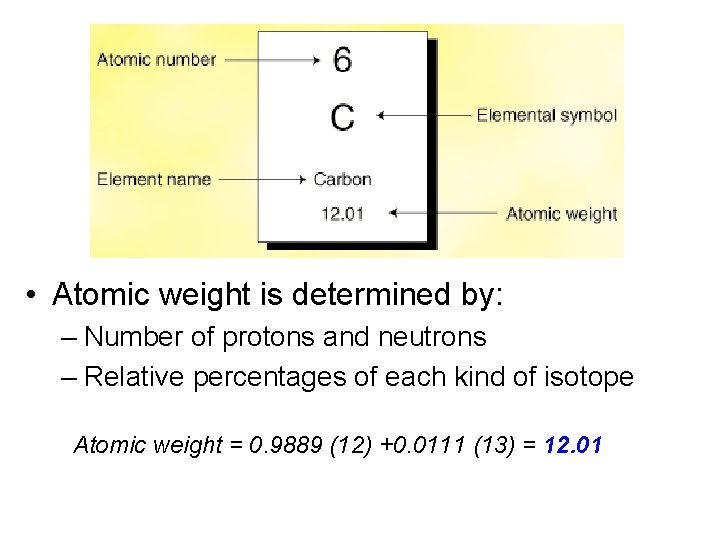
• Atomic weight is determined by: – Number of protons and neutrons – Relative percentages of each kind of isotope Atomic weight = 0. 9889 (12) +0. 0111 (13) = 12. 01

The proton shrinks in size! • 2010 paper in Nature • Proton 0. 00000003 mm smaller than thought! – 4% smaller! • Fired “muons” at hydrogen atoms and measured energy

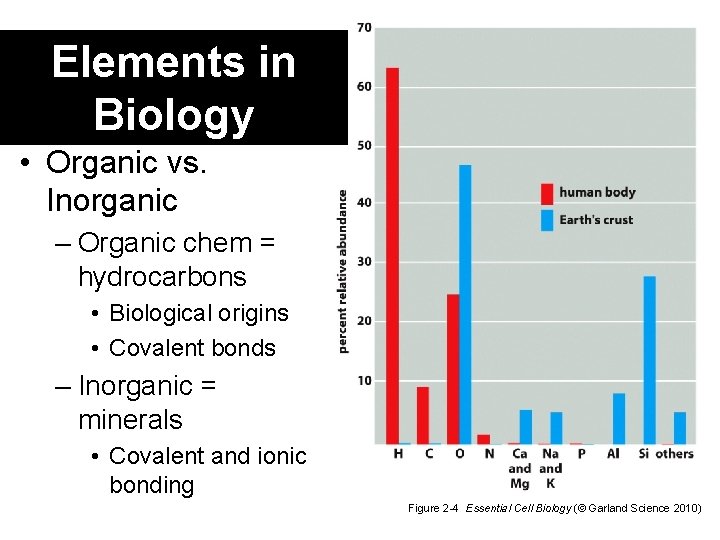
Elements in Biology • Organic vs. Inorganic – Organic chem = hydrocarbons • Biological origins • Covalent bonds – Inorganic = minerals • Covalent and ionic bonding Figure 2 -4 Essential Cell Biology (© Garland Science 2010)

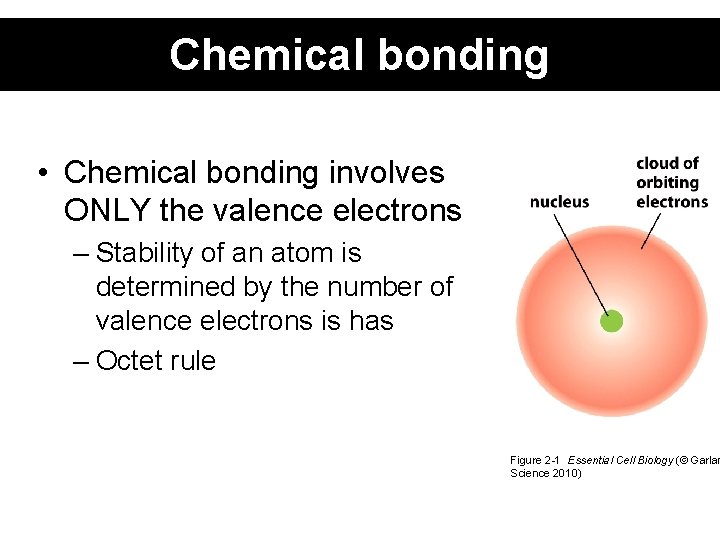
Chemical bonding • Chemical bonding involves ONLY the valence electrons – Stability of an atom is determined by the number of valence electrons is has – Octet rule Figure 2 -1 Essential Cell Biology (© Garlan Science 2010)

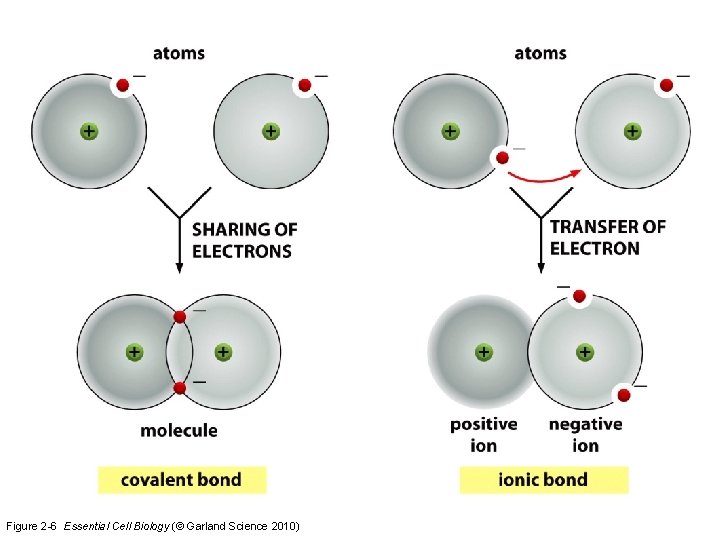
Chemical bonding in biology • Valence electron number determines if chemical bonding occurs – Octet rule: Atoms are happiest with a complete octet in their valence ring • Options to get that octet: – Share electrons – Give away electrons – Accept electrons Covalent bonding Ionic bonding

Figure 2 -6 Essential Cell Biology (© Garland Science 2010)

Covalent bonding • Predominant type of bonding in organic chemistry – Carbon can form four covalent bonds • Two types: – Polar – Nonpolar

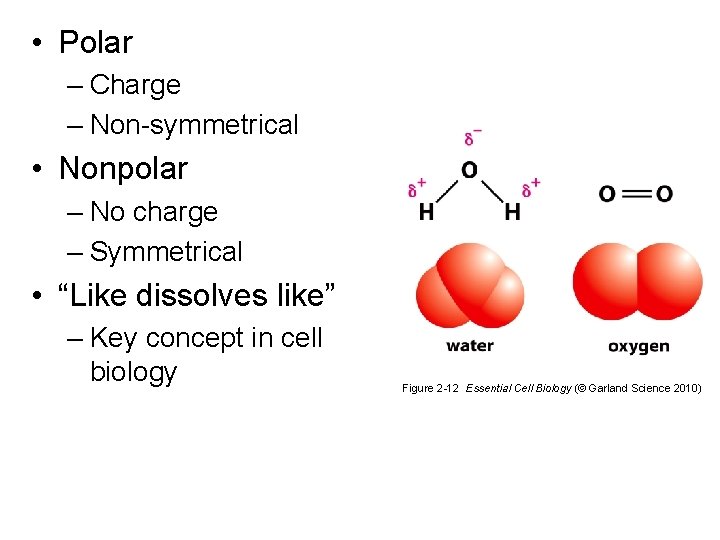
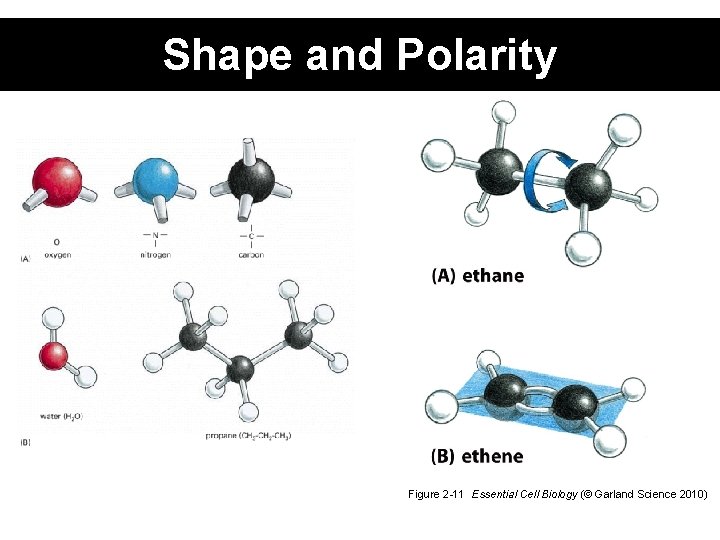
• Polar – Charge – Non-symmetrical • Nonpolar – No charge – Symmetrical • “Like dissolves like” – Key concept in cell biology Figure 2 -12 Essential Cell Biology (© Garland Science 2010)


Shape and Polarity Figure 2 -11 Essential Cell Biology (© Garland Science 2010)

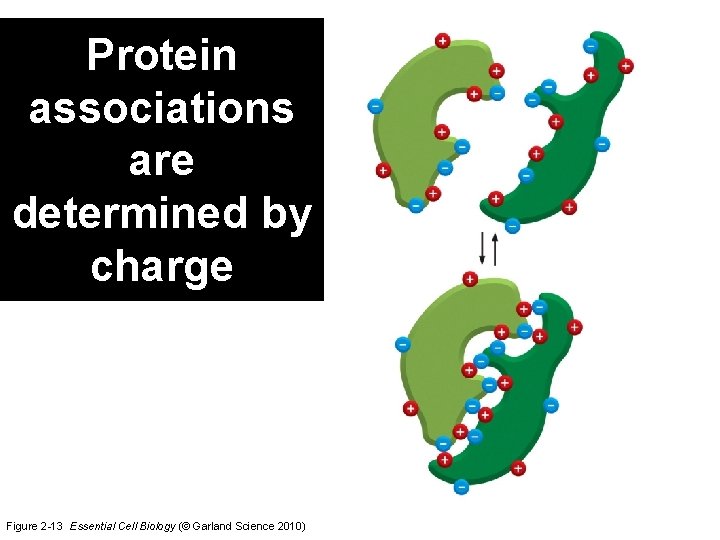
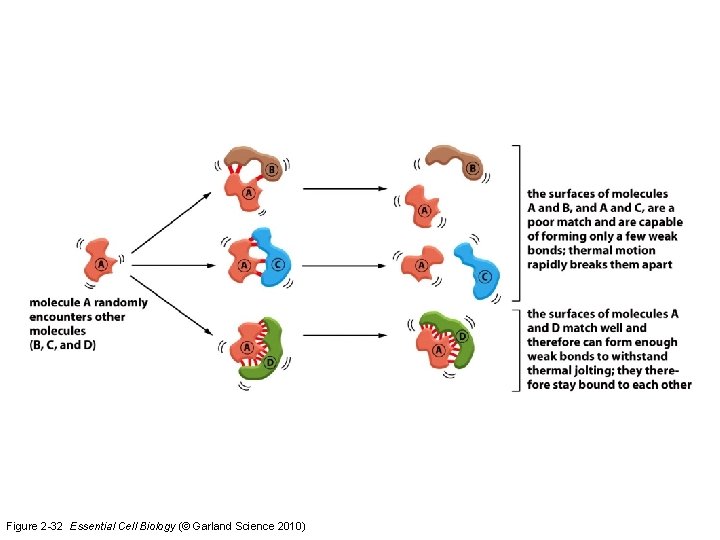
Protein associations are determined by charge Figure 2 -13 Essential Cell Biology (© Garland Science 2010)

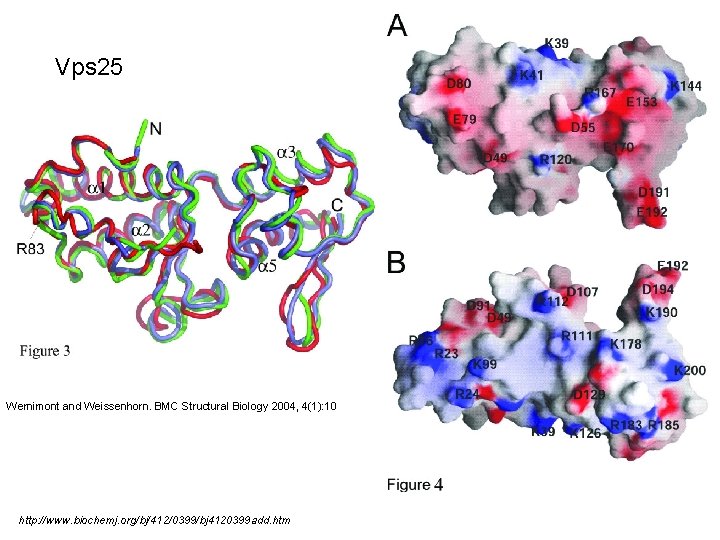
Vps 25 Wernimont and Weissenhorn. BMC Structural Biology 2004, 4(1): 10 http: //www. biochemj. org/bj/412/0399/bj 4120399 add. htm

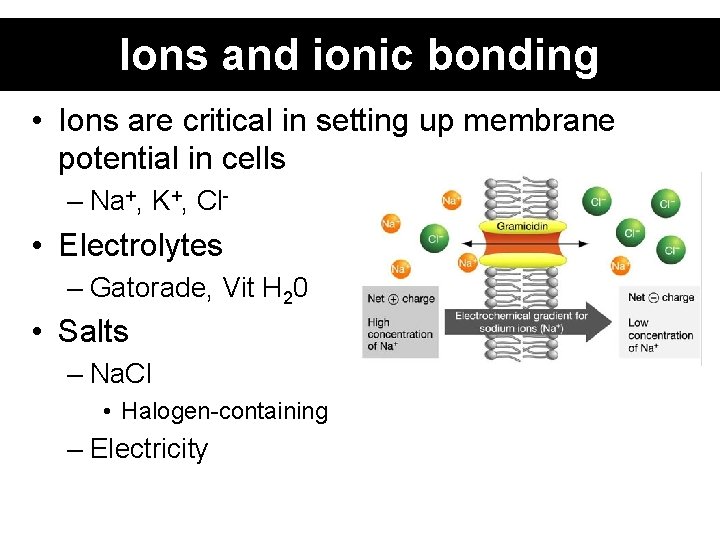
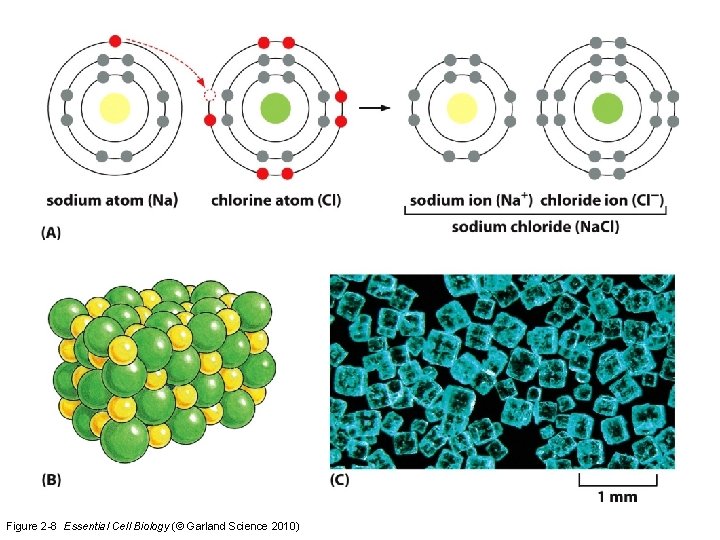
Ions and ionic bonding • Ions are critical in setting up membrane potential in cells – Na+, K+, Cl- • Electrolytes – Gatorade, Vit H 20 • Salts – Na. Cl • Halogen-containing – Electricity

Figure 2 -8 Essential Cell Biology (© Garland Science 2010)

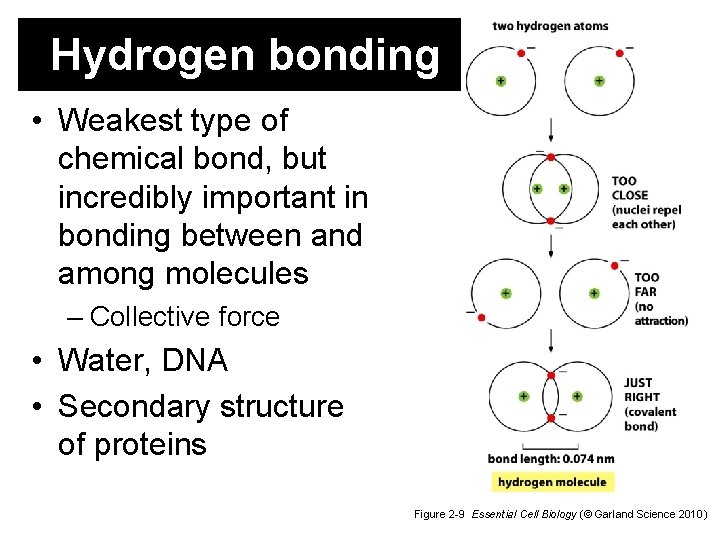
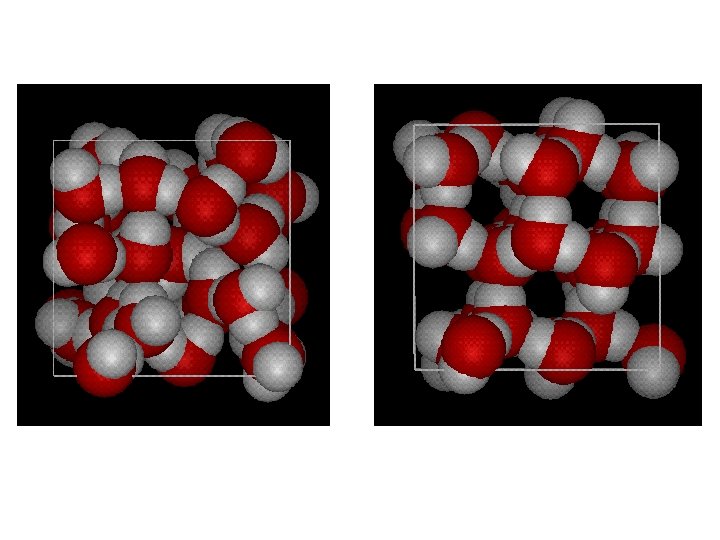
Hydrogen bonding • Weakest type of chemical bond, but incredibly important in bonding between and among molecules – Collective force • Water, DNA • Secondary structure of proteins Figure 2 -9 Essential Cell Biology (© Garland Science 2010)




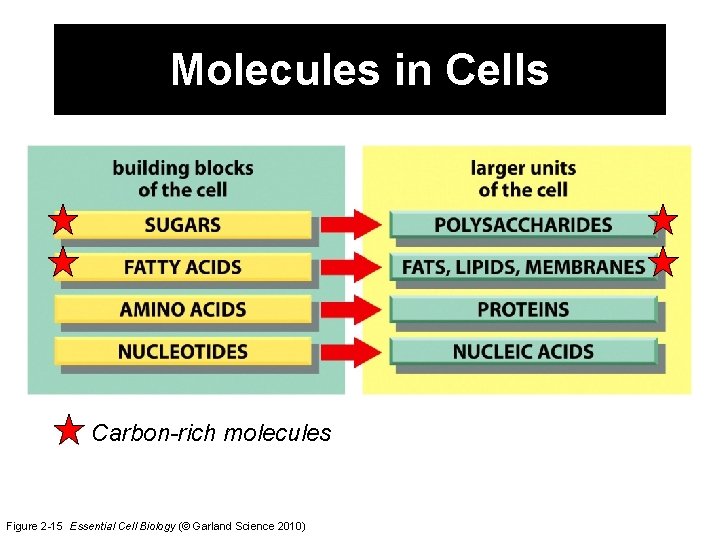
Molecules in Cells Carbon-rich molecules Figure 2 -15 Essential Cell Biology (© Garland Science 2010)

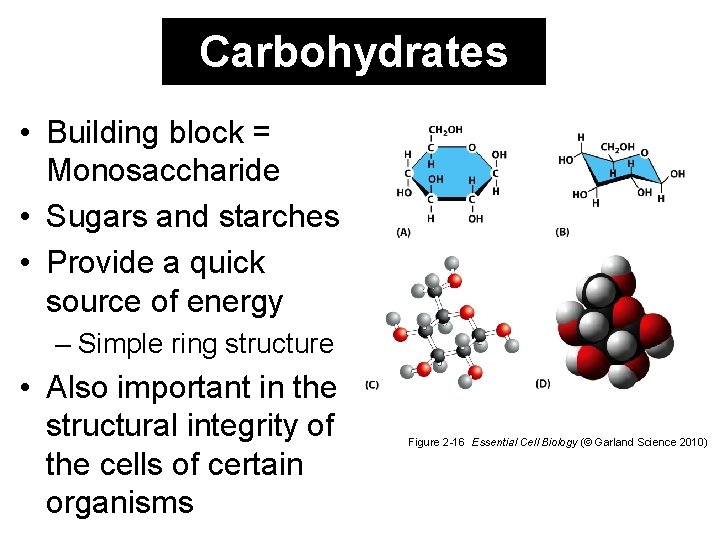
Carbohydrates • Building block = Monosaccharide • Sugars and starches • Provide a quick source of energy – Simple ring structure • Also important in the structural integrity of the cells of certain organisms Figure 2 -16 Essential Cell Biology (© Garland Science 2010)

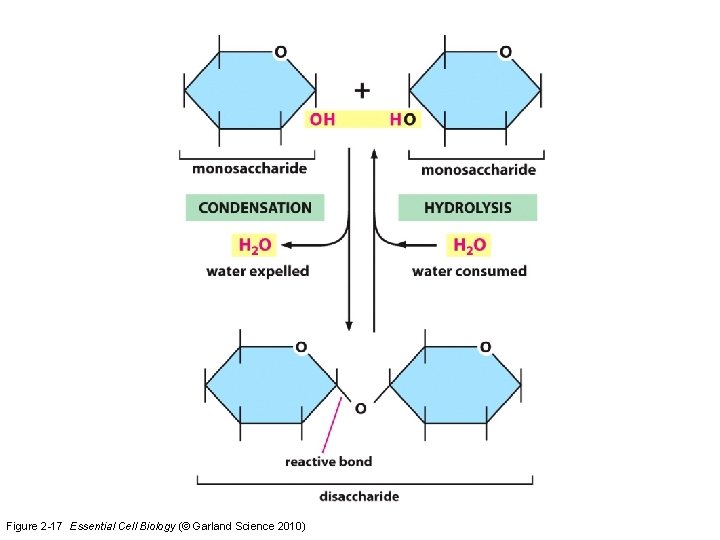
Figure 2 -17 Essential Cell Biology (© Garland Science 2010)

Using bacteria to fight cancer • Clostridium sporogenes • Harmless bacteria found in soil • Anaerobic – Tumor cells = no O 2 • Enzyme + cancer drug = activated drug

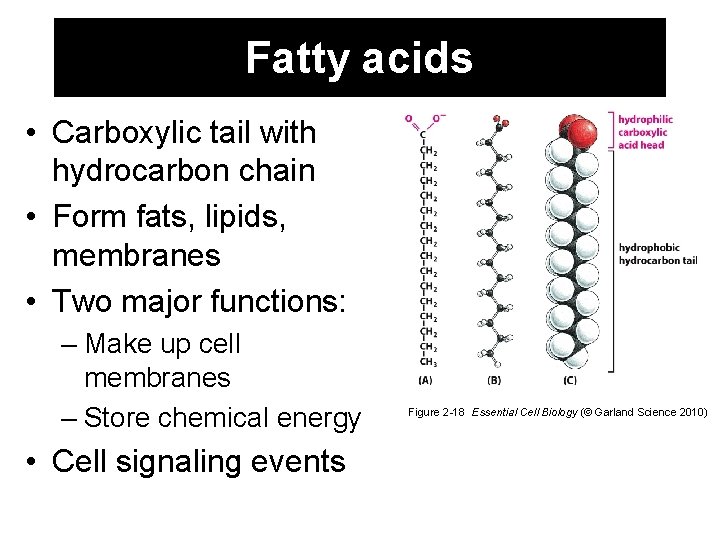
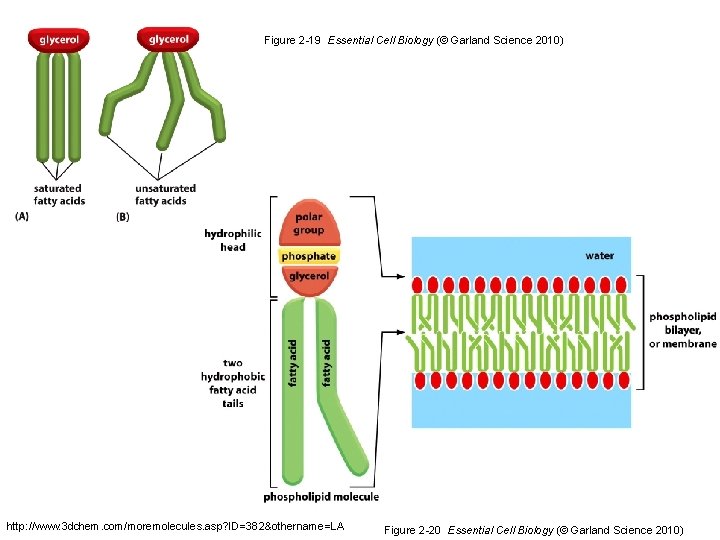
Fatty acids • Carboxylic tail with hydrocarbon chain • Form fats, lipids, membranes • Two major functions: – Make up cell membranes – Store chemical energy • Cell signaling events Figure 2 -18 Essential Cell Biology (© Garland Science 2010)

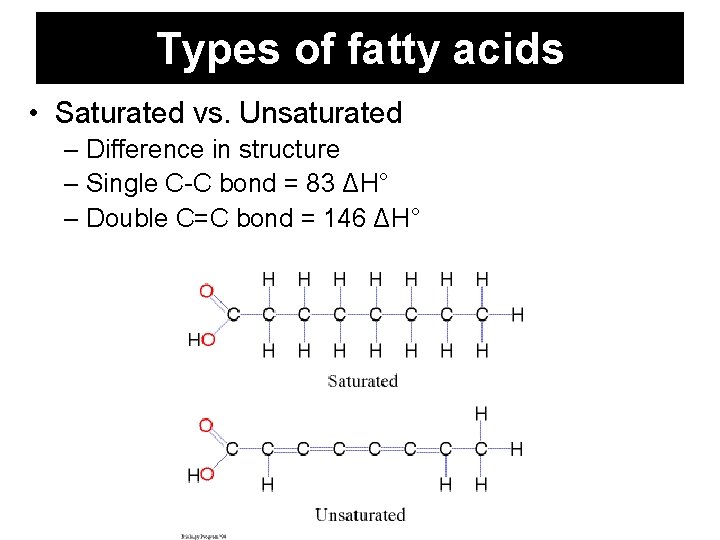
Types of fatty acids • Saturated vs. Unsaturated – Difference in structure – Single C-C bond = 83 ΔH° – Double C=C bond = 146 ΔH°

Saturated vs. Unsaturated • Saturated fats – Animal products – Processed foods – Solid at room temperature • Unsaturated fats – Nuts – Olive oil – Avocados – Liquid at room temperature

Fats in neural development • Axons covered by myelin sheath • Fat cells • Fatty acids are delivered to infants through breast milk

Figure 2 -19 Essential Cell Biology (© Garland Science 2010) http: //www. 3 dchem. com/moremolecules. asp? ID=382&othername=LA Figure 2 -20 Essential Cell Biology (© Garland Science 2010)

Membrane fluidity varies among species • Bigger mammals=less unsaturated fatty acids than smaller mammals – Less membrane fluidity – Lower Na+-K+-ATPase activity • Smaller animals= more unsaturated fatty acids than bigger mammals – More membrane fluidity – Higher Na+-K+-ATPase activity – Increased permeability of the membrane?

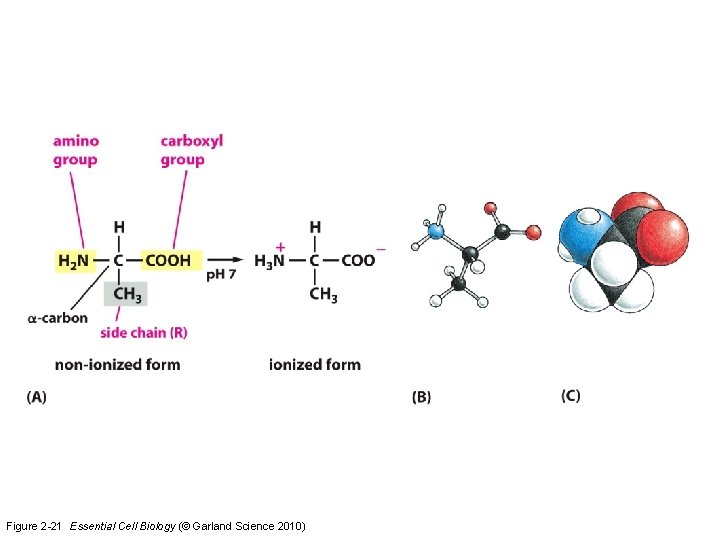
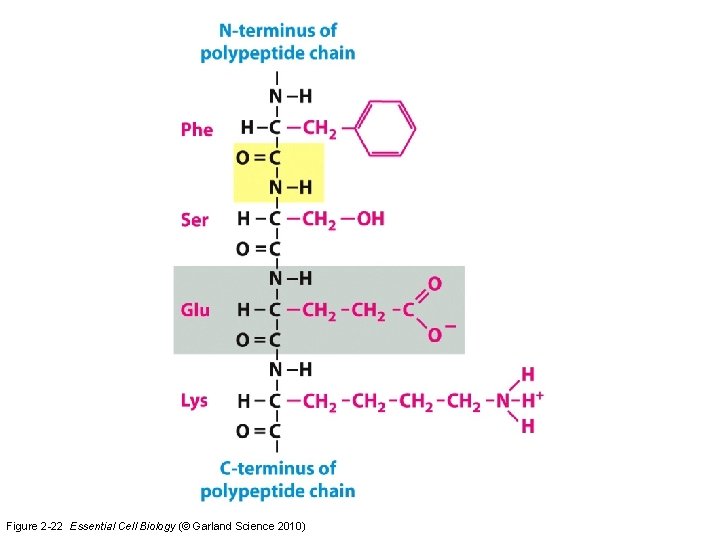
Amino acids • Link together to form proteins • Defining property: all possess a carboxylic acid group and an amino group – COOH – NH 2 • Proteins are polymers of amino acids

Figure 2 -21 Essential Cell Biology (© Garland Science 2010)

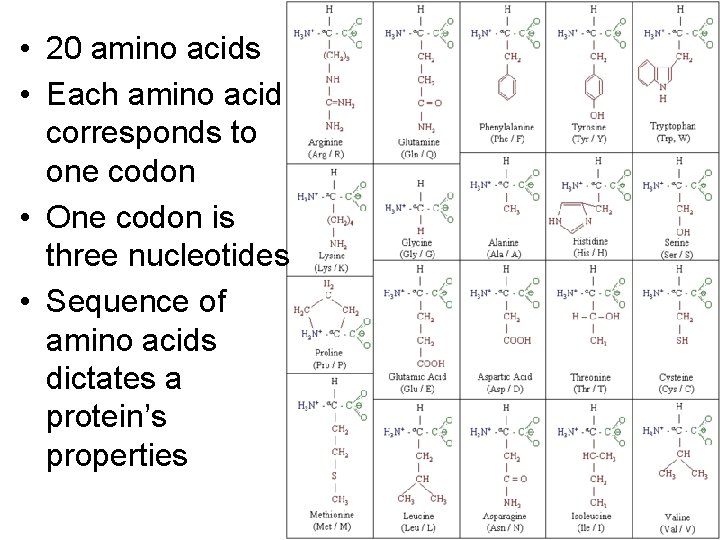
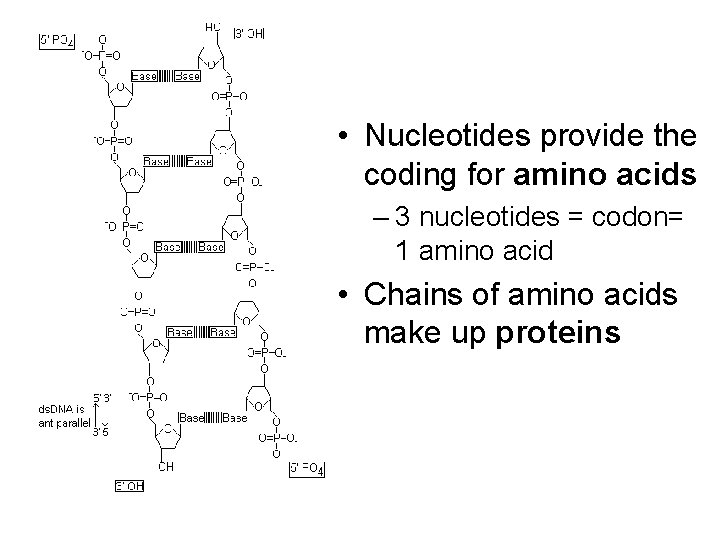
• 20 amino acids • Each amino acid corresponds to one codon • One codon is three nucleotides • Sequence of amino acids dictates a protein’s properties

Figure 2 -22 Essential Cell Biology (© Garland Science 2010)

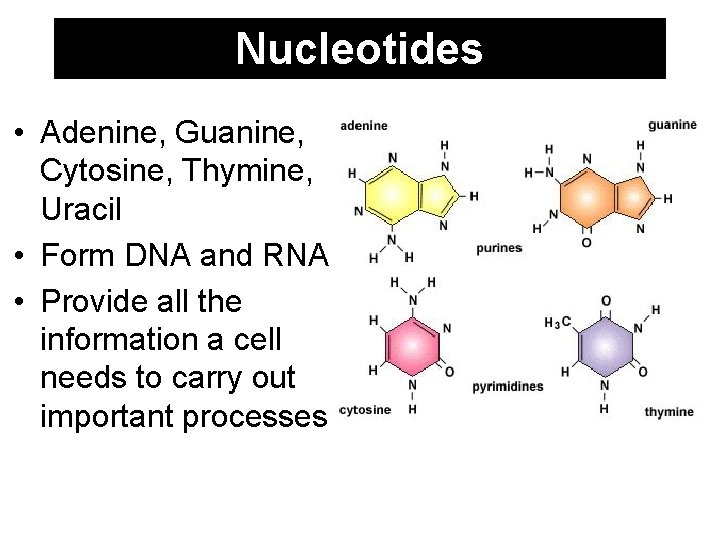
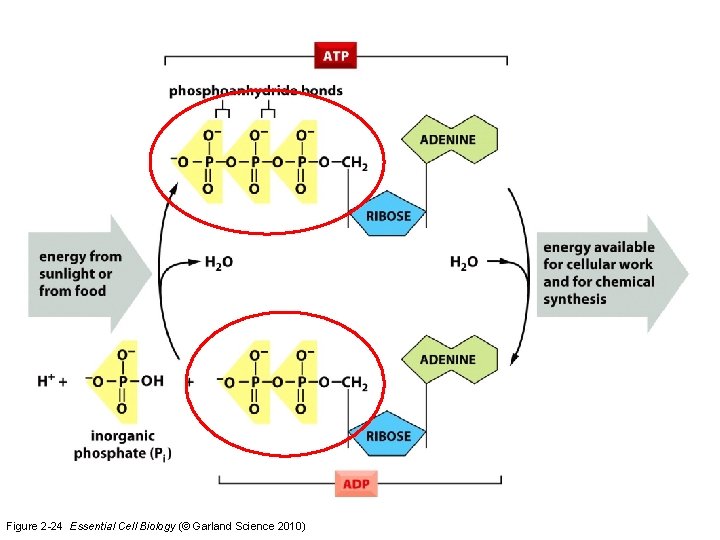
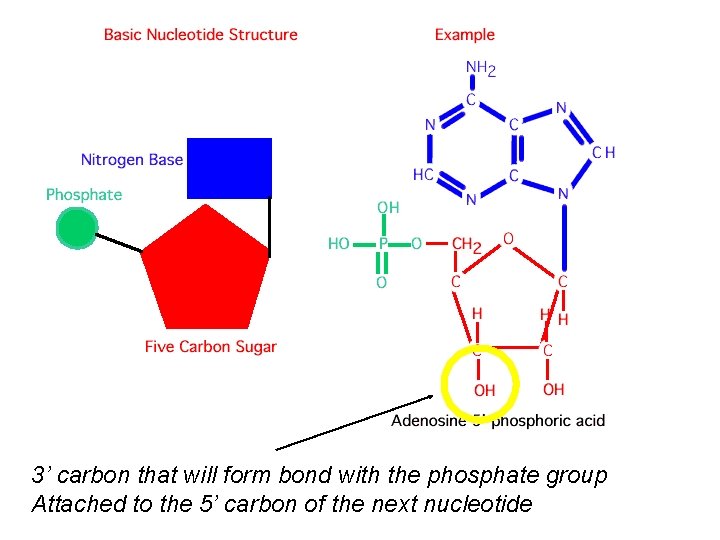
Nucleotides • Adenine, Guanine, Cytosine, Thymine, Uracil • Form DNA and RNA • Provide all the information a cell needs to carry out important processes

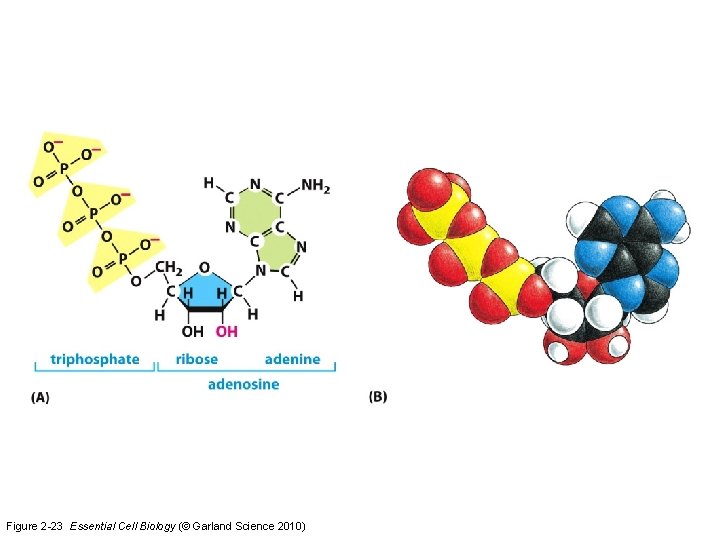
Figure 2 -23 Essential Cell Biology (© Garland Science 2010)

Figure 2 -24 Essential Cell Biology (© Garland Science 2010)

3’ carbon that will form bond with the phosphate group Attached to the 5’ carbon of the next nucleotide

• Nucleotides provide the coding for amino acids – 3 nucleotides = codon= 1 amino acid • Chains of amino acids make up proteins

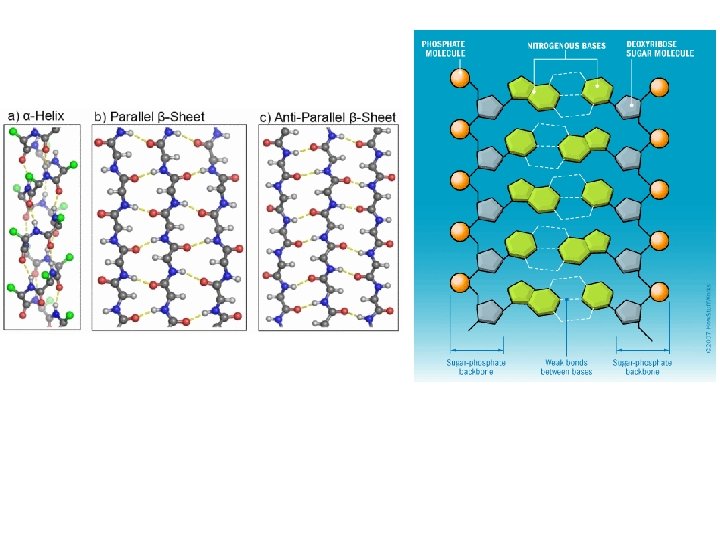
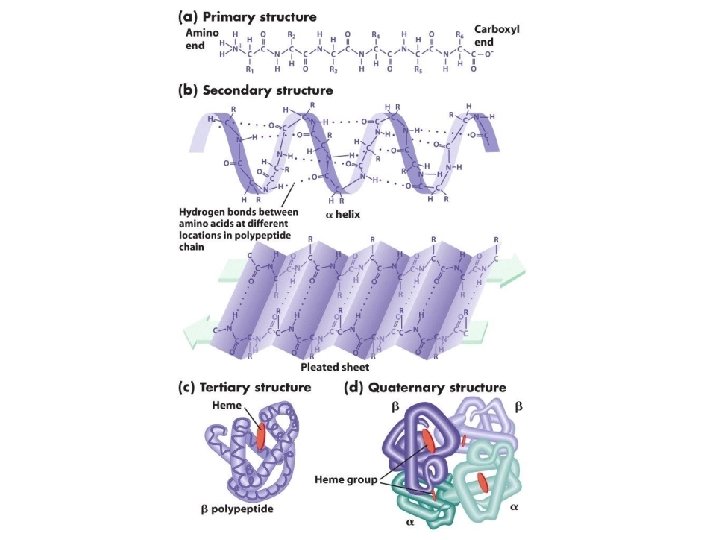
Proteins • Sequence of nucleotides • Levels of protein organization – Primary: sequence – Secondary: alpha helix and beta sheets – Tertiary: final specific geometry that a protein assumes – Quaternary: clustering of several protein chains into a final specific structure


Figure 2 -32 Essential Cell Biology (© Garland Science 2010)

Diseases attributable to defects in protein function • Sickle Cell Anemia • Huntington’s disease • Achondroplasia

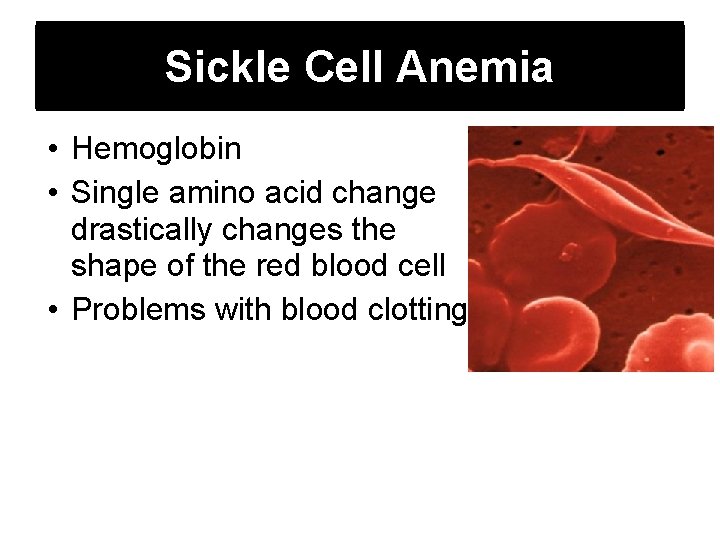
Sickle Cell Anemia • Hemoglobin • Single amino acid change drastically changes the shape of the red blood cell • Problems with blood clotting

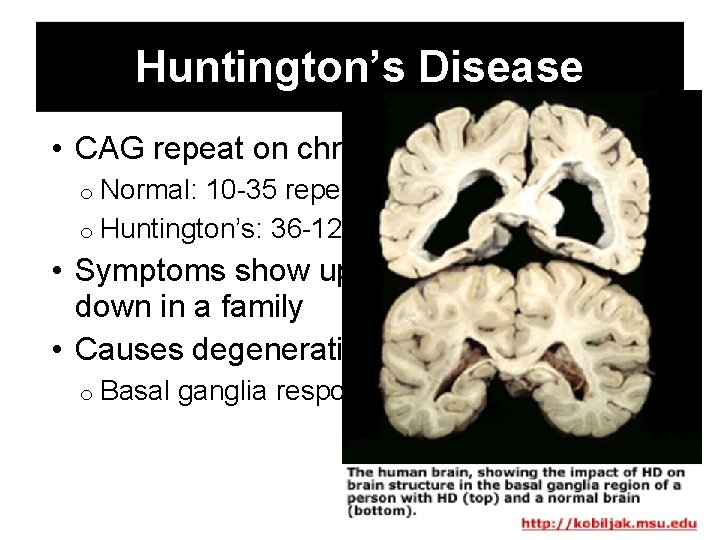
Huntington’s Disease • CAG repeat on chromosome 4 o Normal: 10 -35 repeats o Huntington’s: 36 -120 • Symptoms show up earlier as it is passed down in a family • Causes degeneration of nervous tissue o Basal ganglia responsible for movement

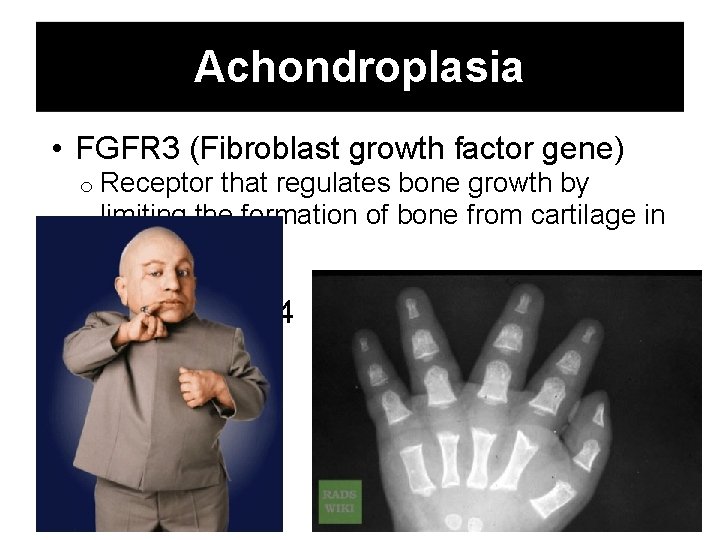
Achondroplasia • FGFR 3 (Fibroblast growth factor gene) o Receptor that regulates bone growth by limiting the formation of bone from cartilage in the long bones o Hyperactive • Chromosome 4

Mad Cow Disease • How is Mad Cow Disease transmitted? • How is Alzheimer’s “transmitted? ” • How could these two possibly be related? – Protein vs. Prion Molecular Biology of prion diseases. Prusiner, SB, Science 1991; 252; 1515 -1522

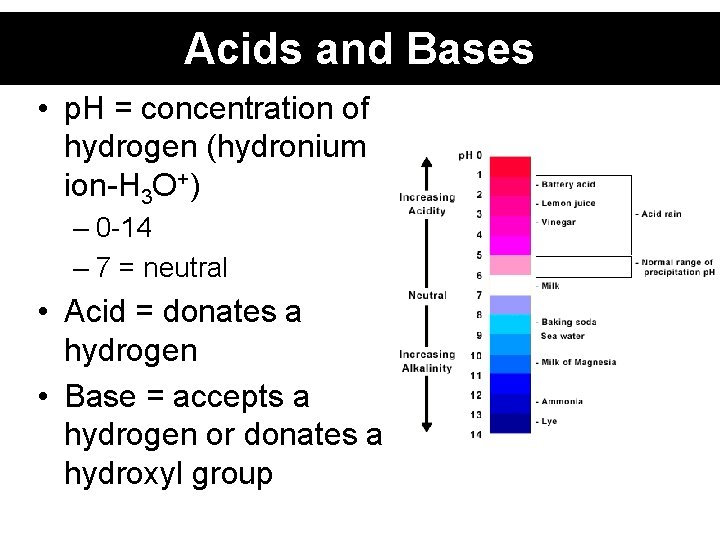
Acids and Bases • p. H = concentration of hydrogen (hydronium ion-H 3 O+) – 0 -14 – 7 = neutral • Acid = donates a hydrogen • Base = accepts a hydrogen or donates a hydroxyl group

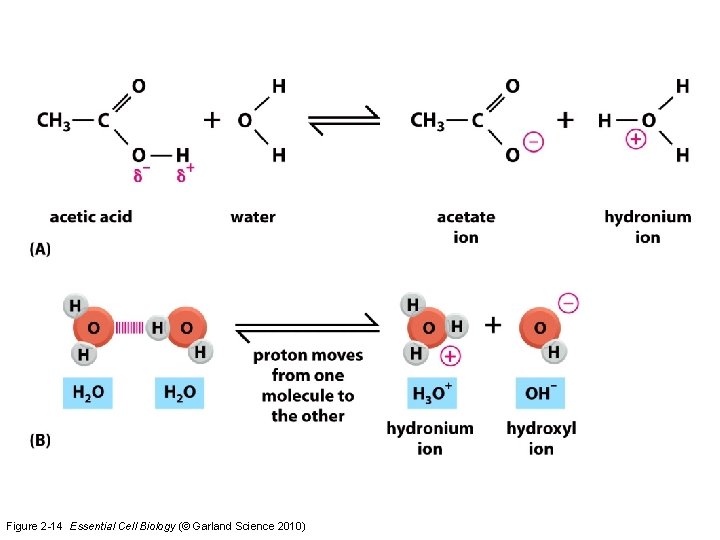
Figure 2 -14 Essential Cell Biology (© Garland Science 2010)
 Printed pages vs web pages
Printed pages vs web pages Jsf sample
Jsf sample Empirical formula pogil
Empirical formula pogil Chemical formulas and chemical compounds chapter 7 review
Chemical formulas and chemical compounds chapter 7 review Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations The chemical context of life chapter 2
The chemical context of life chapter 2 Chapter 2 life's chemical basis
Chapter 2 life's chemical basis Chapter 2 chemical basis of life
Chapter 2 chemical basis of life What are the three c's of healthy relationships
What are the three c's of healthy relationships Chapter 6 skills for healthy relationships
Chapter 6 skills for healthy relationships Chapter 2 foundations of resident care
Chapter 2 foundations of resident care Chapter 2 foundations of individual behavior
Chapter 2 foundations of individual behavior Foundations of government guided reading activity section 1
Foundations of government guided reading activity section 1 Chapter 4 foundations background to american history
Chapter 4 foundations background to american history Foundations in personal finance chapter 1 test
Foundations in personal finance chapter 1 test Foundations of government vocabulary
Foundations of government vocabulary Chapter 1: foundations of government pdf
Chapter 1: foundations of government pdf Foundations of government (chapter 1 test form a)
Foundations of government (chapter 1 test form a) Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc
V cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot