Chemical Reactions Life depends on chemical reactions Chemical



- Slides: 13

Chemical Reactions Life depends on chemical reactions

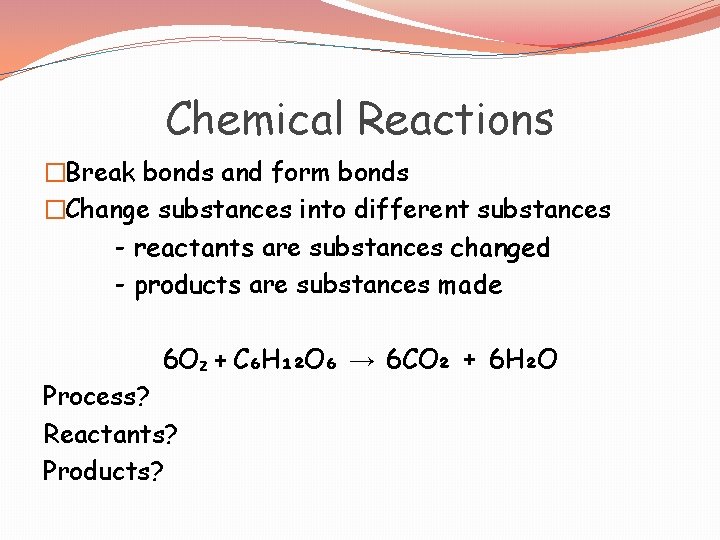
Chemical Reactions �Break bonds and form bonds �Change substances into different substances - reactants are substances changed - products are substances made 6 O₂ + C₆H₁₂O₆ → 6 CO₂ + 6 H₂O Process? Reactants? Products?

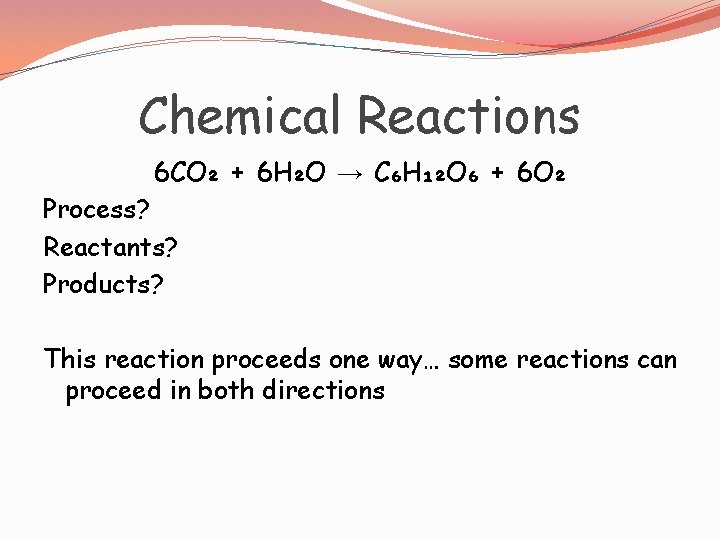
Chemical Reactions 6 CO₂ + 6 H₂O → C₆H₁₂O₆ + 6 O₂ Process? Reactants? Products? This reaction proceeds one way… some reactions can proceed in both directions

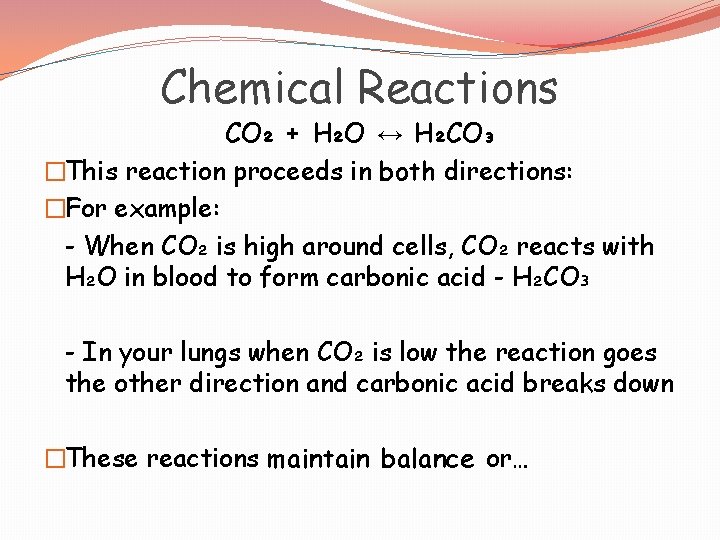
Chemical Reactions CO₂ + H₂O ↔ H₂CO₃ �This reaction proceeds in both directions: �For example: - When CO₂ is high around cells, CO₂ reacts with H₂O in blood to form carbonic acid - H₂CO₃ - In your lungs when CO₂ is low the reaction goes the other direction and carbonic acid breaks down �These reactions maintain balance or…

Chemical Reactions �Equilibrium - equal rate in both directions - the reactant and product concentrations stay the same � All chemical reactions involve changes in energy - Energy is added to reactants to break bonds - When new bonds are formed energy is released

Chemical Reactions 6 O₂ + C₆H₁₂O₆ → 6 CO₂ + 6 H₂O �How do we know energy is released during chemical reactions in our body? - Body temperature �How does our body maintain homeostasis to keep from overheating? - High specific heat of water �How do chemical reactions get started?

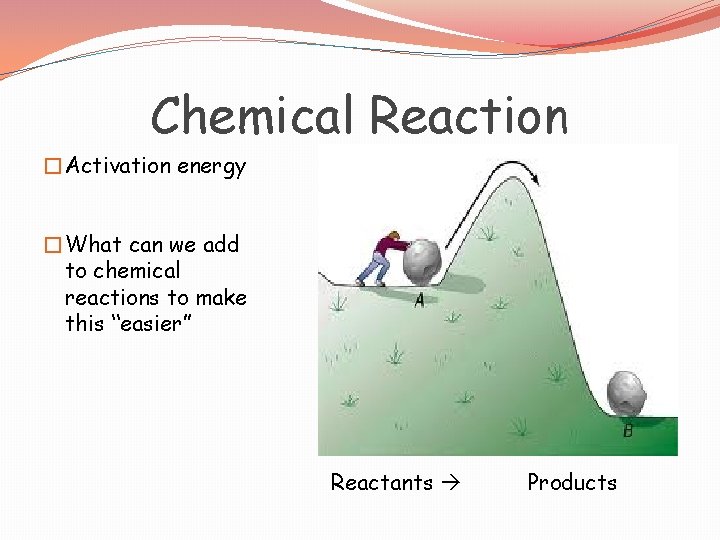
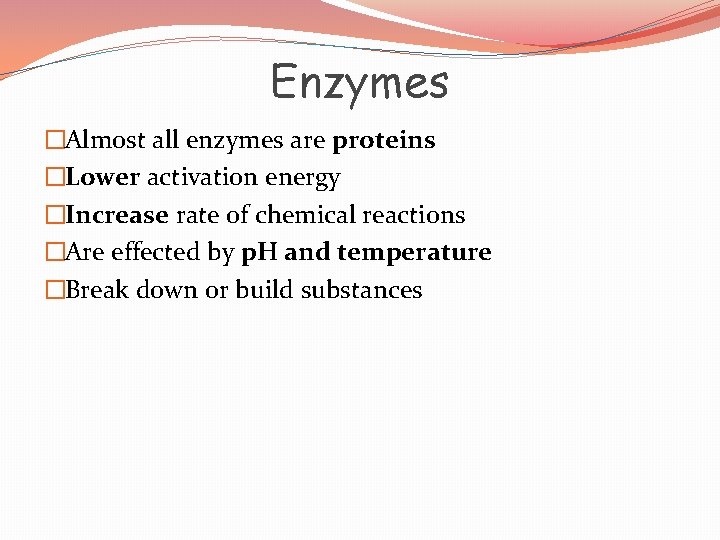
Chemical Reactions � Energy is added to reactants to break bonds �Energy is released when new bonds are formed � Activation energy is the amount of energy that must be absorbed by the reactants for a chemical reaction to start

Chemical Reaction �Activation energy �What can we add to chemical reactions to make this “easier” Reactants Products

Enzymes �Catalysts: - decrease activation energy - increase reaction rates - in living things are called enzymes

Enzymes �Almost all enzymes are proteins �Lower activation energy �Increase rate of chemical reactions �Are effected by p. H and temperature �Break down or build substances

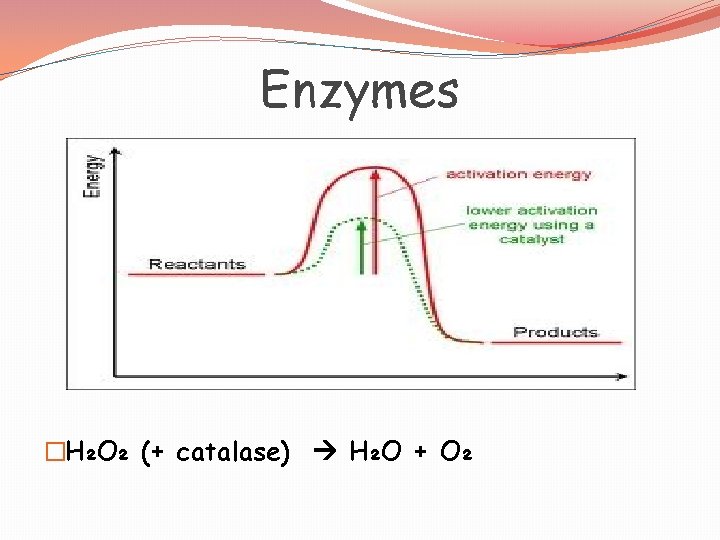
Enzymes �H₂O₂ (+ catalase) H₂O + O₂

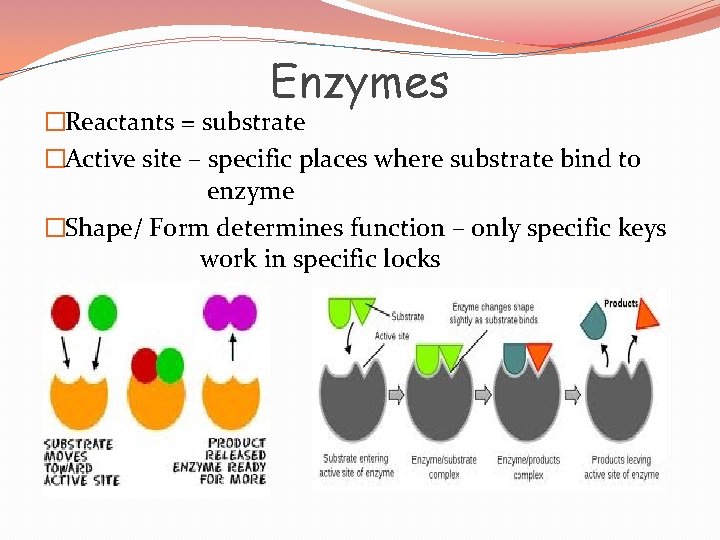
Enzymes �Reactants = substrate �Active site – specific places where substrate bind to enzyme �Shape/ Form determines function – only specific keys work in specific locks

Enzymes �Enzyme video �Key words: - Enzyme - Activation energy - Substrate (reactants) - Active site - Products
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Life depends on the sun
Life depends on the sun Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations An example of redox reaction
An example of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers The rate of weathering depends upon the area's ____.
The rate of weathering depends upon the area's ____. The method used for transferring a patient depends on
The method used for transferring a patient depends on Thermal energy depends on
Thermal energy depends on Reflection of sound experiment
Reflection of sound experiment Earthquake intensity depends primarily on the height of
Earthquake intensity depends primarily on the height of