Chapter 7 Chemical Formulas and Chemical Compounds Chemical






































- Slides: 41

Chapter 7 Chemical Formulas and Chemical Compounds

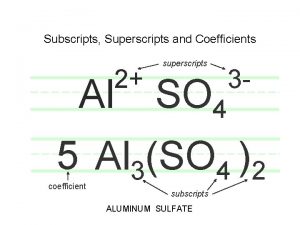
Chemical Formulas • Number of atoms of each kind • Examples – C 8 H 18 – Al 2(SO 4)3

Monatomic Ions • Formed by gaining or losing electrons • S-block – +1 or +2 charge • P-Block – Groups 15, 16, 17 • 1 -, 2 -, or 3 - charge – Group 14 • 2+ charge

Monatomic Ions • D-block – 2+, 3+ charge • Sometimes 1+ and 4+ • Nomenclature – Cations • Element’s name – Anion • Ending of name is dropped • Add -ide

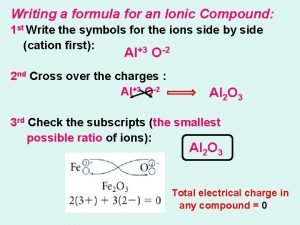
Binary Ionic Compounds • • • Composed of 2 atoms Neutral compounds Cation written first Cross over to balance charge Name of cation given first Name of anion given second

Problem • Write the formulas for the binary ionic compounds formed between the following elements – Zinc and iodine – Zinc and sulfur – Potassium and iodine – Magnesium and chlorine – Sodium and sulfur – Aluminum and nitrogen

Problem • Name the binary ionic compounds indicated by the following formulas – Ag. Cl – Zn. O – Ca. Br 2 – Sr. F 2 – Ba. O – Ca. Cl 2

Binary Ionic Compounds • Elements that form 2 or more cations • Stock System – Use Roman numerals to indicate ion’s charge • Examples – Fe 2+ →Iron(II) – Fe 3+ →Iron(III) • Cu. Cl 2 →copper(II) chloride

Problem • Write the formula and give the name for the compound formed by the ions – Cr 3+ and F– Cu 2+ and Br– Fe 2+ and O 2– Pb 2+ and Cl– Sn 2+ and F– Hg 2+ and S 2 -

Problem • Give the names for the following compounds – Cu. O – Co. F 3 – Sn. I 4 – Fe. S

Polyatomic Ions • Oxyanions – One less oxygen →-ite – One less oxygen than -ite →hypo– One more oxygen than -ate →per- • • Cl. O- →hypochlorite Cl. O 2 - →chlorite Cl. O 3 - →chlorate Cl. O 4 - →perchlorate

Problem • Write the formula for – tin(IV) sulfate – Lithium nitrate – Copper(II) sulfate – Sodium carbonate – Calcium nitrite – Potassium perchlorate

Problem • Give the names for the following compounds – Ca(OH)2 – KCl. O 3 – NH 4 OH – Fe. Cr. O 4 – KCl. O

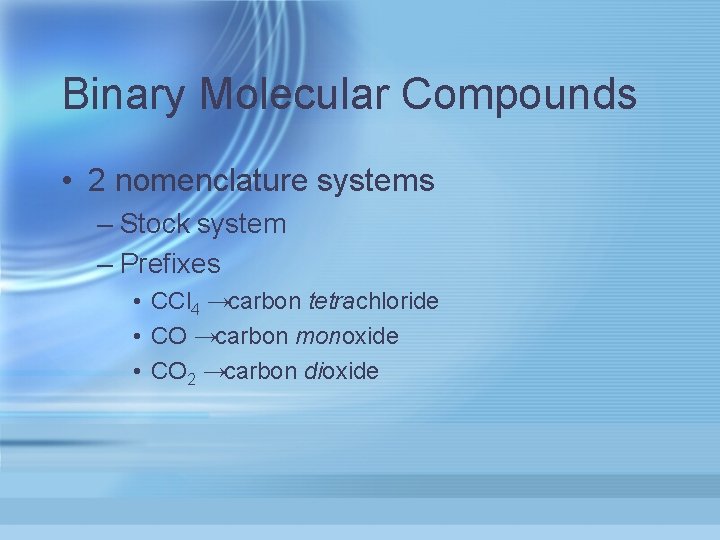
Binary Molecular Compounds • 2 nomenclature systems – Stock system – Prefixes • CCl 4 →carbon tetrachloride • CO →carbon monoxide • CO 2 →carbon dioxide

Prefixes • • • 1 →mono 2 →di 3 →tri 4 →tetra 5 →penta 6 →hexa 7 →hepta 8 →octa 9 →nona 10 →deca

Rules 1. Less electronegative element 1 st 2. 2 nd element →combine prefix, root, and ending -ide 3. O or a of prefix is dropped when root starts with vowel • P 4 O 10 →tetraphosphorus decoxide

Problem • Name the following binary molecular compounds – As 2 O 5 – SO 3 – ICl 3 – PBr 5

Problem • Write the formula for – Oxygen difluoride – Carbon tetraiodide – Phosphorus trichloride – Dinitrogen trioxide

Oxidation Numbers 1. Atoms in pure element → 0 2. More electronegative element → negative charge as an anion 3. Fluorine →-1 4. Oxygen →-2 1. Exceptions: peroxides →-1 fluoride →+2 5. Hydrogen →+1 1. Exception: w/metals →-1

Oxidation Numbers 6. Sum of neutral compound equals 0 7. Sum of atoms in polyatomic ion equals charge of ion

Problems • Assign oxidation numbers to each atom in the following compounds or ions: – HF – H 2 O – UF 6 – H 2 SO 4 – Cl. O 3 -

Problems • Assign oxidation numbers to each atom in the following compounds or ions: – CF 4 – SO 2 – P 4 O 10 – HCl. O 3

Oxidation Numbers, Formulas, & Names • Oxidation numbers can be used like ionic charges to determine formulas • Use stock system – Roman numerals show oxidation number • SO 2 - sulfur(IV) oxide • SO 3 - sulfur(VI) oxide

Problem • Give the name according to the stock system of the following compounds: – PCl 3 – N 2 O – Pb. O 2 – CI 4 – As 2 S 3 – NCl 3

Problem • Give the formula for the following compounds: – Sodium(I) oxide – Sulfur(II) chloride – Nitrogen(V) oxide – Carbon(IV) sulfide – Phosphorus(III) iodide

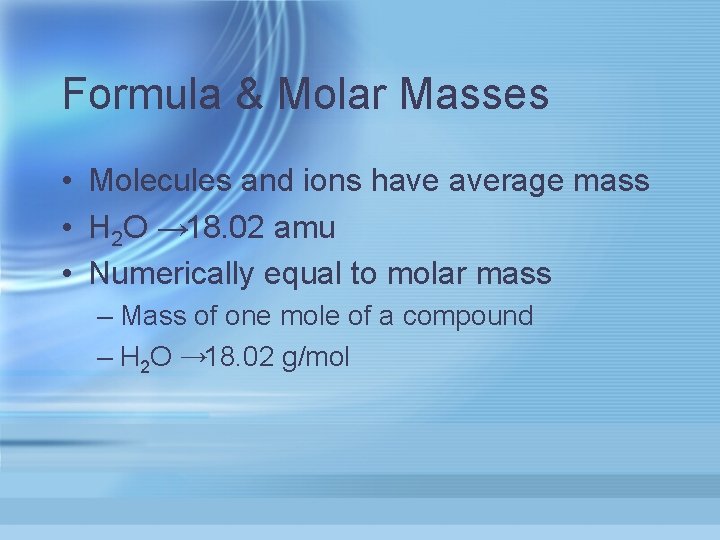
Formula & Molar Masses • Molecules and ions have average mass • H 2 O → 18. 02 amu • Numerically equal to molar mass – Mass of one mole of a compound – H 2 O → 18. 02 g/mol

Problems • What is the molar mass of: – KCl. O 3 – H 2 SO 4 – Ca(NO 3)2 – Ba(OH)2 – Na. NO 3 – Al 2 S 3

Conversions • Molar mass is a conversion factor – g/mol – mol/g

Problems • What is the mass in grams of 2. 50 mol of oxygen gas? • What is the mass in grams of 0. 257 mol of calcium nitrate? • How many moles are in 3. 82 g SO 2? How many molecules are in this sample of SO 2?

Problem • Ibuprofen, C 13 H 18 O 2, is the active ingredient in many nonprescription pain relievers. Its molar mass is 206. 29 g/mol. – If the tablets in a bottle contain a total of 33 g of ibuprofen, how many moles of ibuprofen are in the bottle? – How many molecules of ibuprofen are in the bottle? – What is the total mass in grams of carbon in 33 g of ibuprofen?

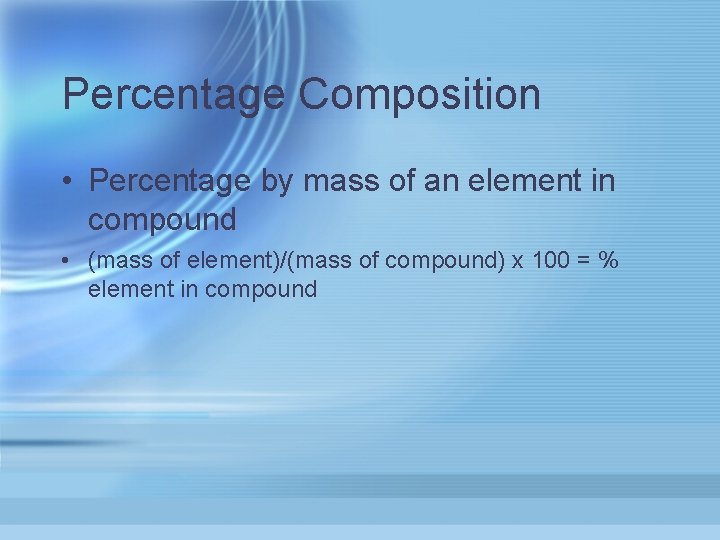
Percentage Composition • Percentage by mass of an element in compound • (mass of element)/(mass of compound) x 100 = % element in compound

Problem • Find the percentage composition of copper(I) sulfide, Cu 2 S. • Calculate the percentage composition of sodium nitrate.

Problem • As some salts crystallize from a water solution, they bind water molecules in their crystal structure. Sodium carbonate forms such a hydrate, in which 10 water molecules are present for every formula unit of sodium carbonate. Find the mass percentage of water in sodium carbonate decahydrate, Na 2 CO 3· 10 H 2 O, which has a molar mass of 286. 14 g/mol.

Empirical Formulas • Compound with subscripts showing smallest whole-number mole ratio of atoms – BH 3 vs. B 2 H 6 • • Convert percent composition to mass composition Convert mass composition to composition in moles

Empirical Formulas • • Divide each number of moles by smallest number of moles Round to nearest whole number

Problem • Quantitative analysis shows that a compound contains 32. 38% sodium, 22. 65% sulfur, and 44. 99% oxygen. Find the empirical formula of this compound.

Problem • Analysis of a 10. 150 g sample of a compound known to contain only phosphorus and oxygen indicates a phosphorus content of 4. 433 g. What is the empirical formula of this compound?

Molecular Formula • x(empirical formula) = molecular formula • Must know formula mass • (Experimental formula mass)/(Empirical formula mass)

Problem • The empirical formula of a compound of phosphorus and oxygen was found to be P 2 O 5. Experimentation shows that the molar mass of this compound is 283. 89 g/mol. What is the compound’s molecular formula?

Problem • Determine the molecular formula of the compound with a empirical formula of CH and a formula mass of 78. 110 amu • A sample of a compound with a formula mass of 34. 00 amu is found to consist of 0. 44 g H and 6. 92 g O. Find its molecular formula.

Chapter Review • Pg. 251 – 3 abcd, 4 bcd, 5 de, 6 ace, 7 bd, 8 ab, 10 ace, 11 cd, 16 cd, 23 bce, 24 bce, 25 bde, 28 ac, 30 bd, 31, 32, 36, 38
 Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Modern chemistry chapter 7
Modern chemistry chapter 7 Chemical formulas and names
Chemical formulas and names Tetraphosphorus octoxide formula
Tetraphosphorus octoxide formula Venn diagram covalent and ionic bonds
Venn diagram covalent and ionic bonds Naming compounds and writing formulas
Naming compounds and writing formulas Prefixes for hydrates
Prefixes for hydrates Monatomic ion definition chemistry
Monatomic ion definition chemistry Tetraiodine nonaoxide formula
Tetraiodine nonaoxide formula Section 3 writing formulas and naming compounds
Section 3 writing formulas and naming compounds Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Chemical names and formulas worksheet chapter 9
Chemical names and formulas worksheet chapter 9 Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key A chemical formula shows the
A chemical formula shows the Bunary compound
Bunary compound Which two formulas represent compounds
Which two formulas represent compounds Lesson 17 technicolor atoms flame test worksheet answers
Lesson 17 technicolor atoms flame test worksheet answers Nhmno4
Nhmno4 Properties of ionic compounds
Properties of ionic compounds Chapter 7 chapter assessment ionic compounds and metals
Chapter 7 chapter assessment ionic compounds and metals In an ionic compound the chemical formula represents
In an ionic compound the chemical formula represents Counting atoms worksheet
Counting atoms worksheet # of moles formula
# of moles formula Covalent compound formula
Covalent compound formula Writing formulas criss-cross method worksheet
Writing formulas criss-cross method worksheet Ionic compounds review
Ionic compounds review Unit chemical bonding forming ionic compounds ws 2
Unit chemical bonding forming ionic compounds ws 2 Chemical formula covalent compounds
Chemical formula covalent compounds Difference between molecular and covalent
Difference between molecular and covalent Systematic name
Systematic name Naming chemical compounds flowchart
Naming chemical compounds flowchart Chemical compounds
Chemical compounds Chapter 7 ionic compounds and metals
Chapter 7 ionic compounds and metals Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Chapter 7 ionic compounds and metals assessment answer key
Chapter 7 ionic compounds and metals assessment answer key Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Subscript in chemical formula
Subscript in chemical formula How to write an ionic compound formula
How to write an ionic compound formula Chemical formulas
Chemical formulas Criss cross method chemistry
Criss cross method chemistry Chemical formulas list
Chemical formulas list