Chapter 7 Chemical Formulas and Chemical Compounds Yes





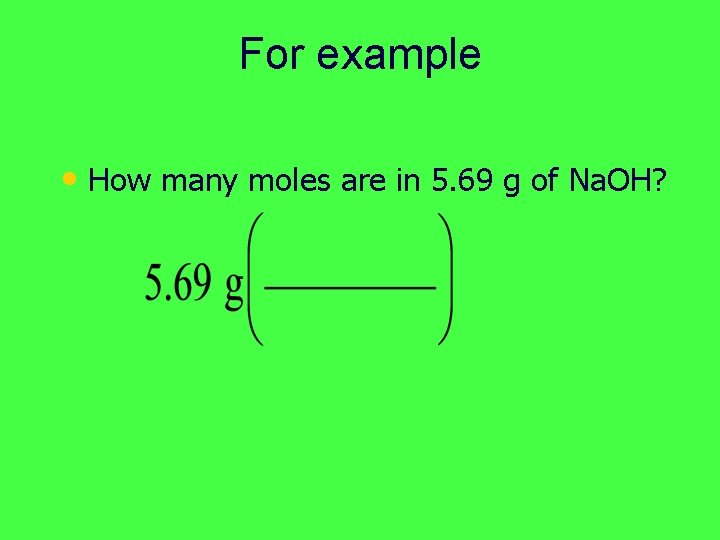








- Slides: 34

Chapter 7 “Chemical Formulas and Chemical Compounds” Yes, you will need a calculator for this chapter!

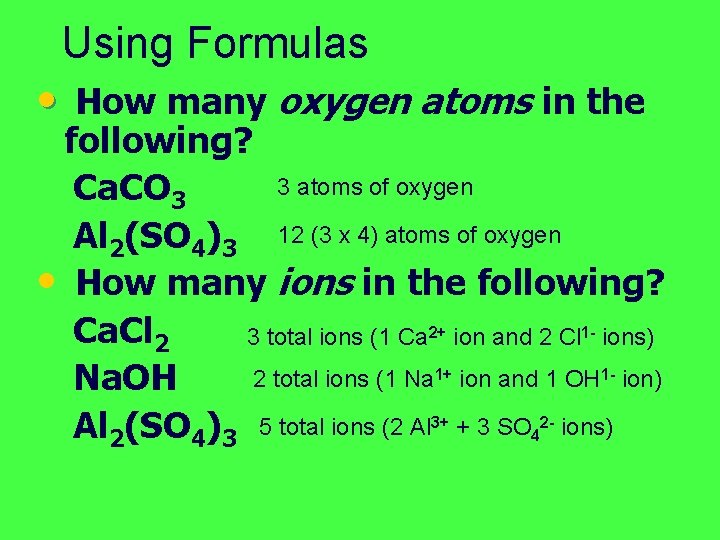
Using Formulas • How many oxygen atoms in the following? 3 atoms of oxygen Ca. CO 3 Al 2(SO 4)3 12 (3 x 4) atoms of oxygen • How many ions in the following? Ca. Cl 2 3 total ions (1 Ca 2+ ion and 2 Cl 1 - ions) 2 total ions (1 Na 1+ ion and 1 OH 1 - ion) Na. OH Al 2(SO 4)3 5 total ions (2 Al 3+ + 3 SO 42 - ions)

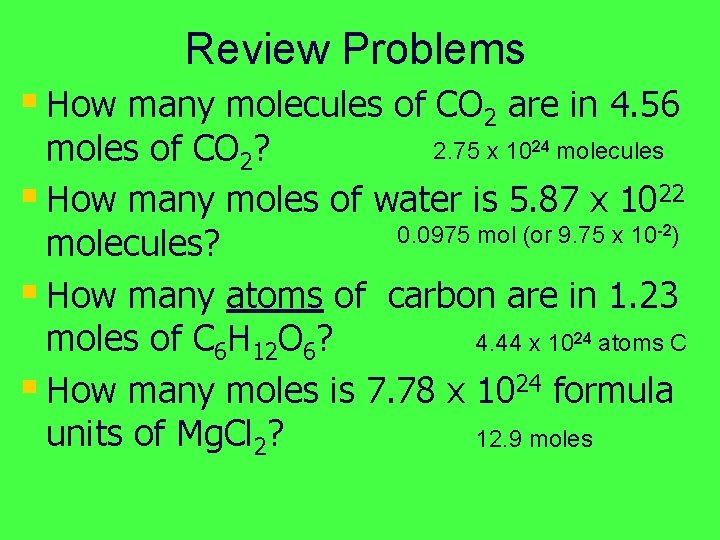
Review Problems § How many molecules of CO 2 are in 4. 56 2. 75 x 1024 molecules moles of CO 2? § How many moles of water is 5. 87 x 1022 0. 0975 mol (or 9. 75 x 10 -2) molecules? § How many atoms of carbon are in 1. 23 moles of C 6 H 12 O 6? 4. 44 x 1024 atoms C § How many moles is 7. 78 x 1024 formula units of Mg. Cl 2? 12. 9 moles

More Practice § How many grams is 2. 34 moles of carbon? 28. 1 grams C § How many moles of magnesium is 24. 31 g of Mg? 1. 000 mol Mg § How many atoms of lithium is 1. 00 g of Li? 8. 68 x 1022 atoms Li § How many grams is 3. 45 x 1022 atoms of U? 13. 6 grams U

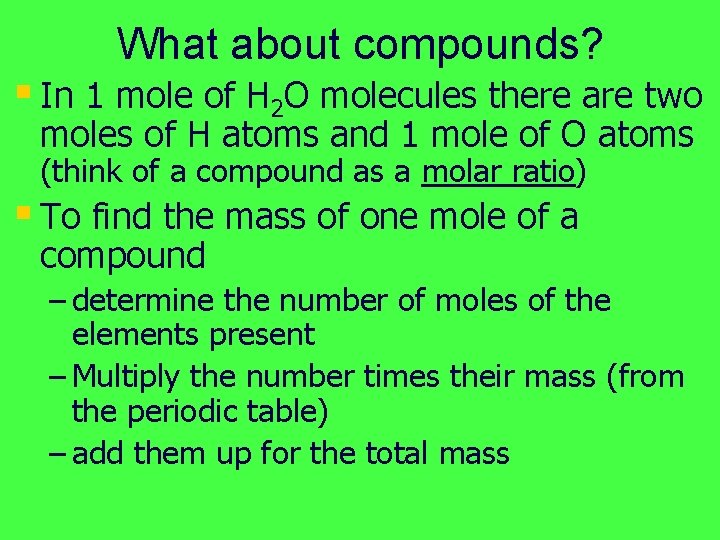
What about compounds? § In 1 mole of H 2 O molecules there are two moles of H atoms and 1 mole of O atoms (think of a compound as a molar ratio) § To find the mass of one mole of a compound – determine the number of moles of the elements present – Multiply the number times their mass (from the periodic table) – add them up for the total mass

Calculating Formula Mass Calculate the formula mass of magnesium carbonate, Mg. CO 3. 24. 31 g 12. 01 g 16. 00 g 24. 31 g/mol + 12. 01 g/mol + 3 x (16. 00 g/mol) = 84. 32 g/mol Thus, 84. 32 grams/mol is the formula mass for Mg. CO 3.

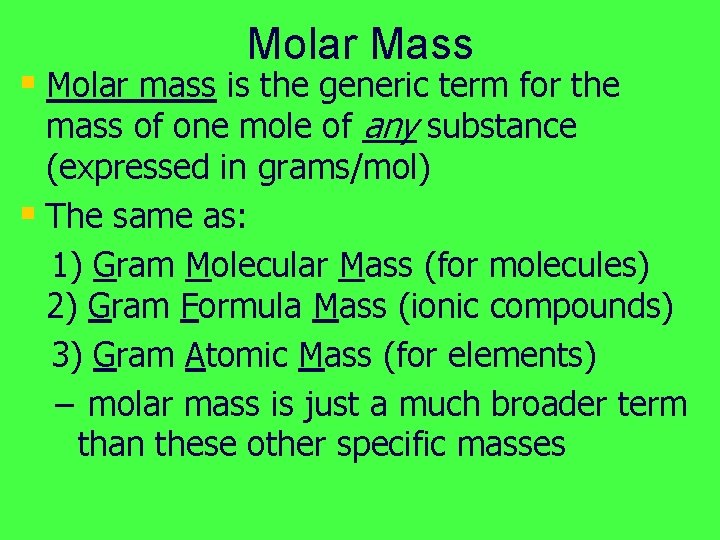
Molar Mass § Molar mass is the generic term for the mass of one mole of any substance (expressed in grams/mol) § The same as: 1) Gram Molecular Mass (for molecules) 2) Gram Formula Mass (ionic compounds) 3) Gram Atomic Mass (for elements) – molar mass is just a much broader term than these other specific masses

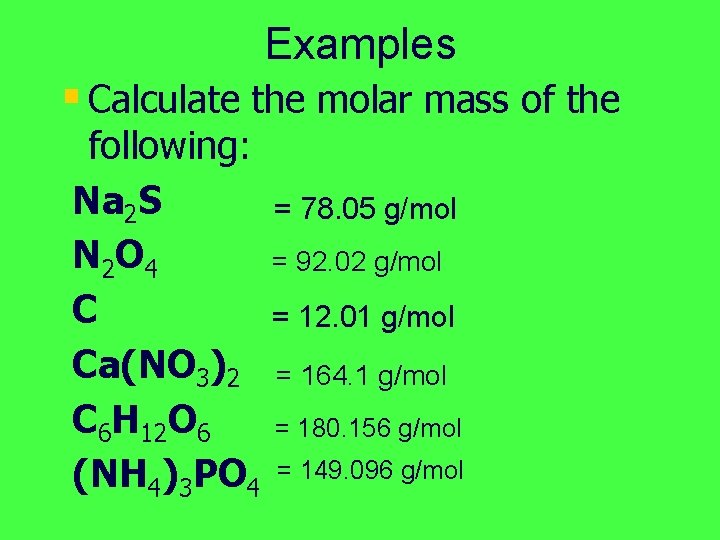
Examples § Calculate the molar mass of the following: Na 2 S N 2 O 4 C Ca(NO 3)2 C 6 H 12 O 6 (NH 4)3 PO 4 = 78. 05 g/mol = 92. 02 g/mol = 12. 01 g/mol = 164. 1 g/mol = 180. 156 g/mol = 149. 096 g/mol

Since Molar Mass is… § The number of grams in 1 mole of atoms, ions, or molecules, § We can make conversion factors from these. - To change between grams of a compound and moles of a compound.

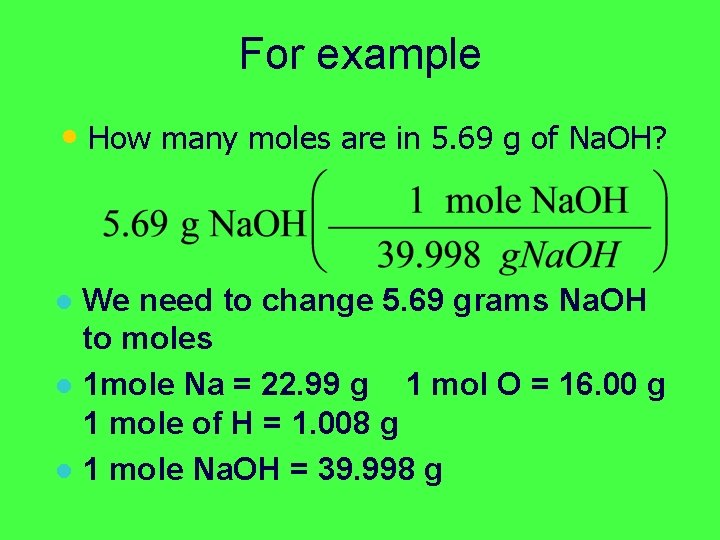
For example § How many moles are in 5. 69 g of Na. OH?
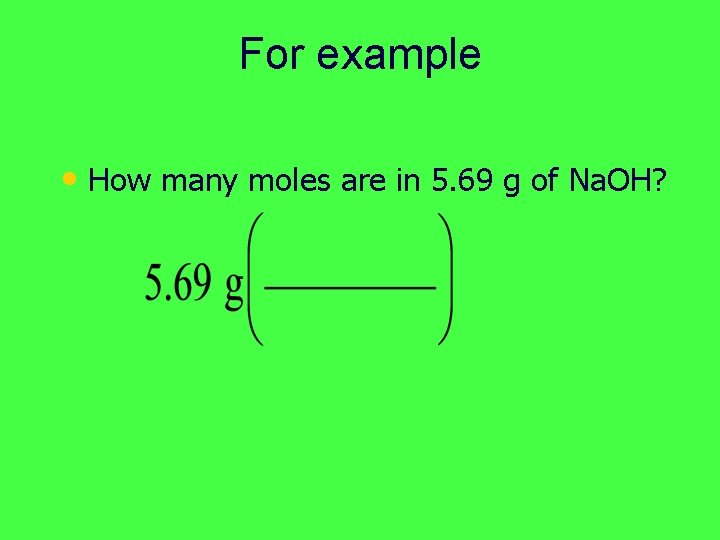
For example • How many moles are in 5. 69 g of Na. OH?

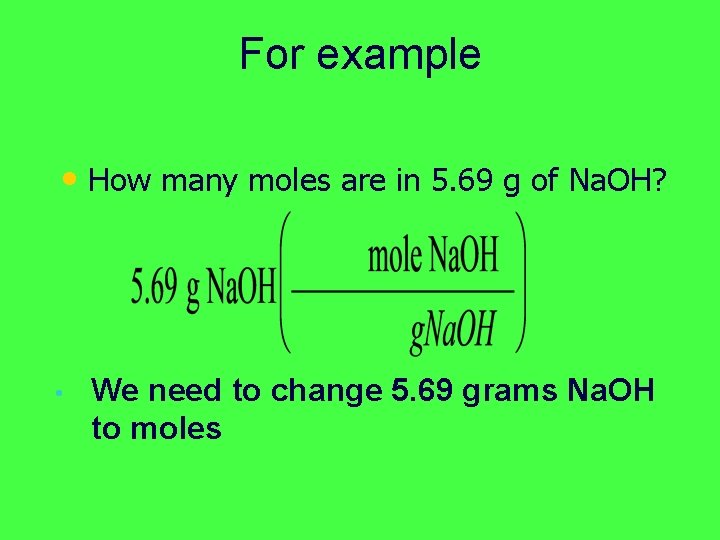
For example • How many moles are in 5. 69 g of Na. OH? • We need to change 5. 69 grams Na. OH to moles

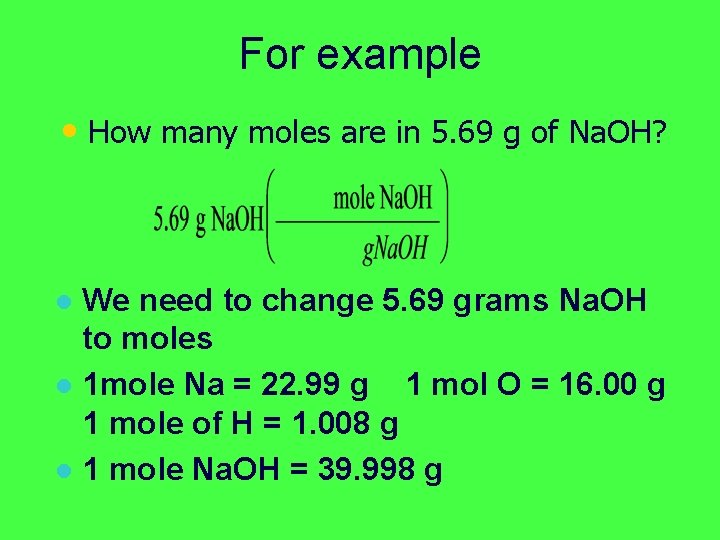
For example • How many moles are in 5. 69 g of Na. OH? We need to change 5. 69 grams Na. OH to moles l 1 mole Na = 22. 99 g 1 mol O = 16. 00 g 1 mole of H = 1. 008 g l

For example • How many moles are in 5. 69 g of Na. OH? We need to change 5. 69 grams Na. OH to moles l 1 mole Na = 22. 99 g 1 mol O = 16. 00 g 1 mole of H = 1. 008 g l 1 mole Na. OH = 39. 998 g l

For example • How many moles are in 5. 69 g of Na. OH? We need to change 5. 69 grams Na. OH to moles l 1 mole Na = 22. 99 g 1 mol O = 16. 00 g 1 mole of H = 1. 008 g l 1 mole Na. OH = 39. 998 g l

For example • How many moles are in 5. 69 g of Na. OH? We need to change 5. 69 grams Na. OH to moles l 1 mole Na = 22. 99 g 1 mol O = 16. 00 g 1 mole of H = 1. 008 g l 1 mole Na. OH = 39. 998 g l

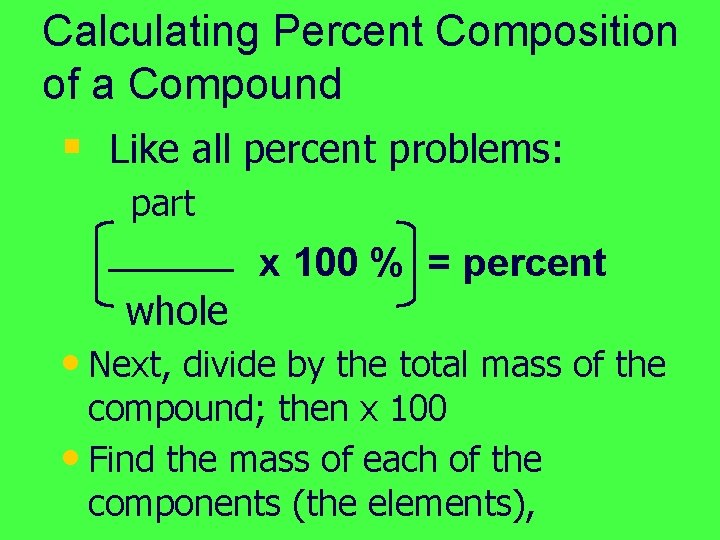
Calculating Percent Composition of a Compound § Like all percent problems: part whole x 100 % = percent • Next, divide by the total mass of the compound; then x 100 • Find the mass of each of the components (the elements),

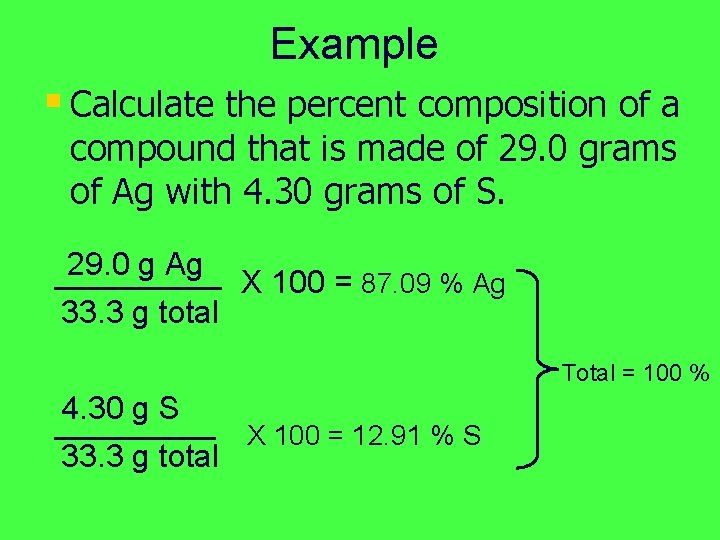
Example § Calculate the percent composition of a compound that is made of 29. 0 grams of Ag with 4. 30 grams of S. 29. 0 g Ag X 100 = 87. 09 % Ag 33. 3 g total Total = 100 % 4. 30 g S X 100 = 12. 91 % S 33. 3 g total

Getting it from the formula § If we know the formula, assume you have 1 mole, § then you know the mass of the elements and the whole compound (these values come from the periodic table!).

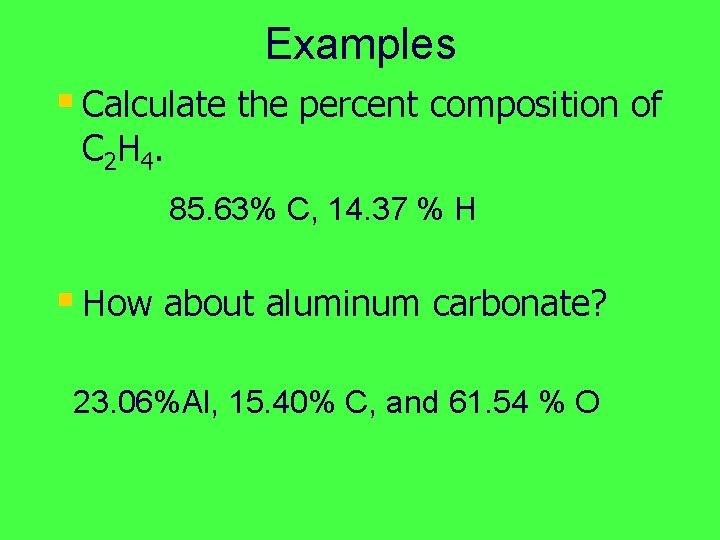
Examples § Calculate the percent composition of C 2 H 4. 85. 63% C, 14. 37 % H § How about aluminum carbonate? 23. 06%Al, 15. 40% C, and 61. 54 % O

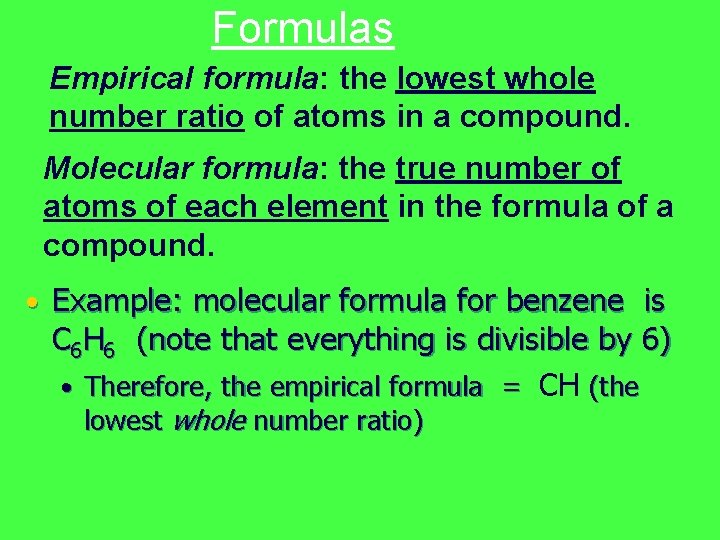
Formulas Empirical formula: the lowest whole number ratio of atoms in a compound. Molecular formula: the true number of atoms of each element in the formula of a compound. • Example: molecular formula for benzene is C 6 H 6 (note that everything is divisible by 6) • Therefore, the empirical formula = CH (the lowest whole number ratio)

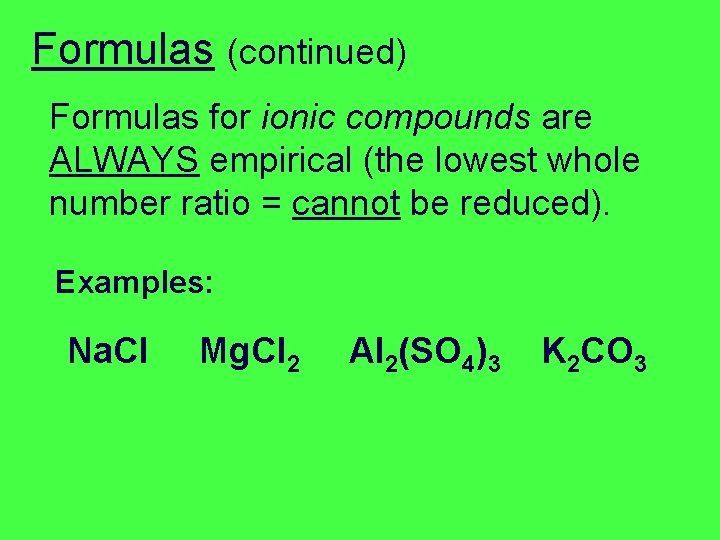
Formulas (continued) Formulas for ionic compounds are ALWAYS empirical (the lowest whole number ratio = cannot be reduced). Examples: Na. Cl Mg. Cl 2 Al 2(SO 4)3 K 2 CO 3

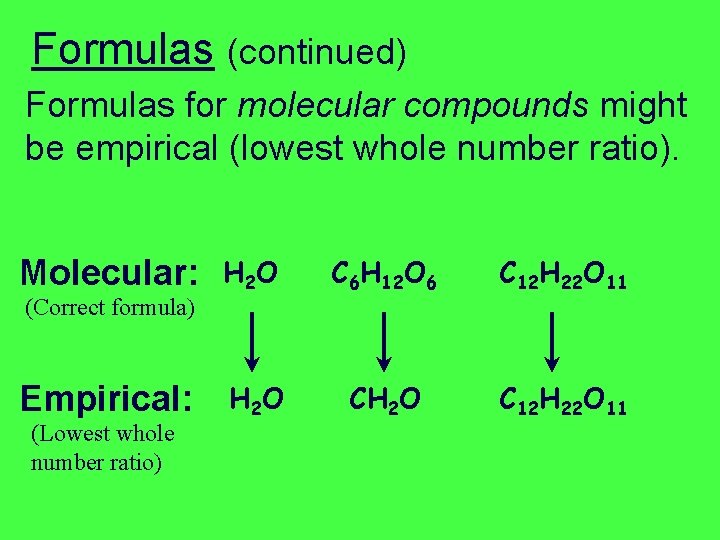
Formulas (continued) Formulas for molecular compounds might be empirical (lowest whole number ratio). Molecular: (Correct formula) Empirical: (Lowest whole number ratio) H 2 O C 6 H 12 O 6 C 12 H 22 O 11 H 2 O CH 2 O C 12 H 22 O 11

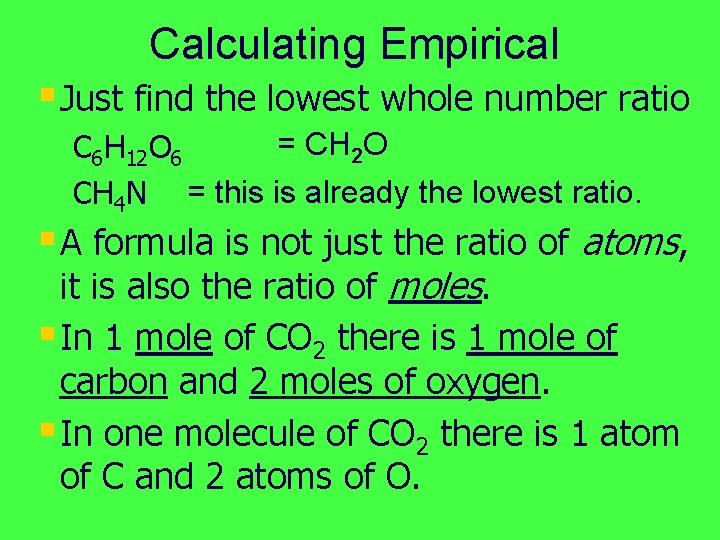
Calculating Empirical § Just find the lowest whole number ratio = CH 2 O C 6 H 12 O 6 CH 4 N = this is already the lowest ratio. § A formula is not just the ratio of atoms, it is also the ratio of moles. § In 1 mole of CO 2 there is 1 mole of carbon and 2 moles of oxygen. § In one molecule of CO 2 there is 1 atom of C and 2 atoms of O.

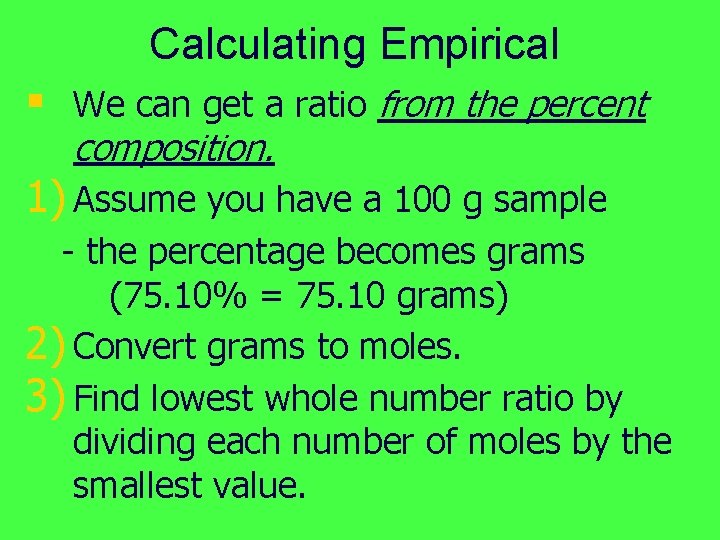
Calculating Empirical § We can get a ratio from the percent composition. 1) Assume you have a 100 g sample - the percentage becomes grams (75. 10% = 75. 10 grams) 2) Convert grams to moles. 3) Find lowest whole number ratio by dividing each number of moles by the smallest value.

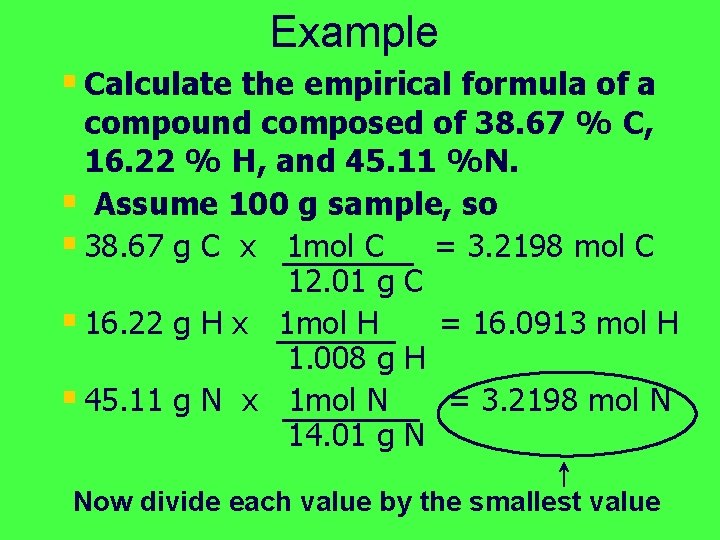
Example § Calculate the empirical formula of a compound composed of 38. 67 % C, 16. 22 % H, and 45. 11 %N. § Assume 100 g sample, so § 38. 67 g C x 1 mol C = 3. 2198 mol C 12. 01 g C § 16. 22 g H x 1 mol H = 16. 0913 mol H 1. 008 g H § 45. 11 g N x 1 mol N = 3. 2198 mol N 14. 01 g N Now divide each value by the smallest value

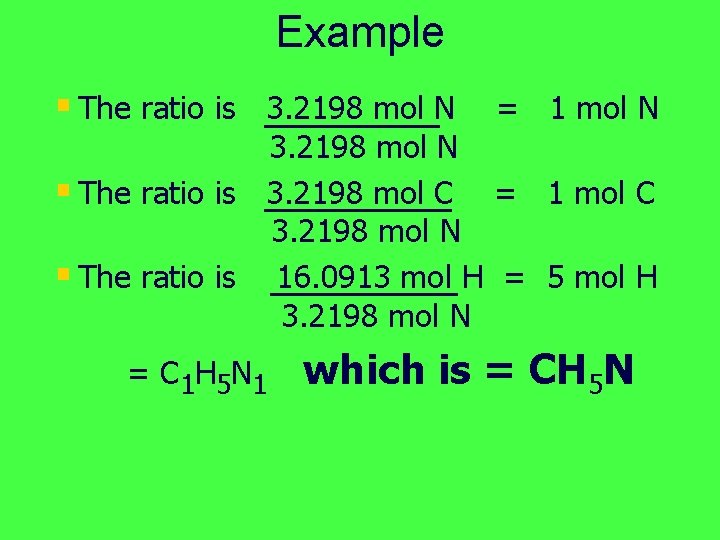
Example § The ratio is 3. 2198 mol N = 1 mol N 3. 2198 mol N § The ratio is 3. 2198 mol C = 1 mol C 3. 2198 mol N § The ratio is 16. 0913 mol H = 5 mol H 3. 2198 mol N = C 1 H 5 N 1 which is = CH 5 N

Practice § A compound is 43. 64 % P and 56. 36 % O. What is the empirical formula? § P 2 O 5 § Caffeine is 49. 48% C, 5. 15% H, 28. 87% N and 16. 49% O. What is its empirical formula? § C 4 H 5 N 2 O

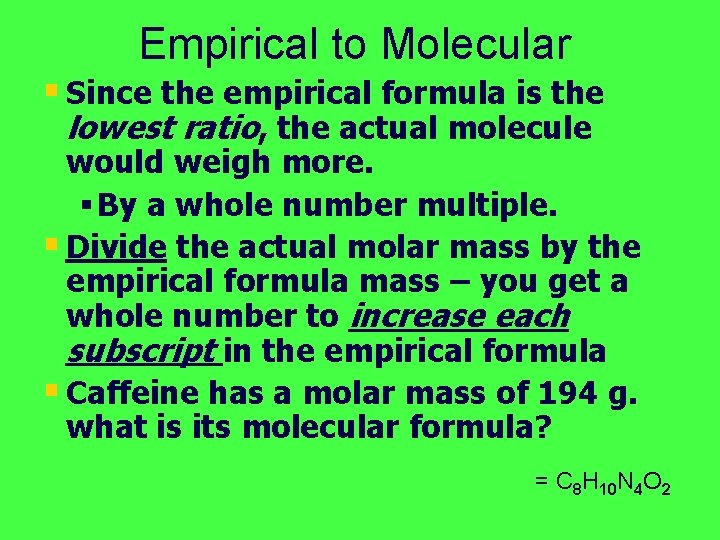
Empirical to Molecular § Since the empirical formula is the lowest ratio, the actual molecule would weigh more. § By a whole number multiple. § Divide the actual molar mass by the empirical formula mass – you get a whole number to increase each subscript in the empirical formula § Caffeine has a molar mass of 194 g. what is its molecular formula? = C 8 H 10 N 4 O 2

Hydrates • Hydrate: a compound that has a specific number of water molecules bound to its atoms • You can determine the formula of a hydrate by heating it until the water is gone, and analyzing the data

Ba. Cl 2 ? H 2 O • Mass of the compound before heating (hydrate): 5. 00 g • Mass after heating (anhydrous): 4. 26 g • Find the mass of the water lost by subtracting 5. 00 g – 4. 26 g = 0. 74 g H 2 O • The mass of just the Ba. Cl 2 is 4. 26 g

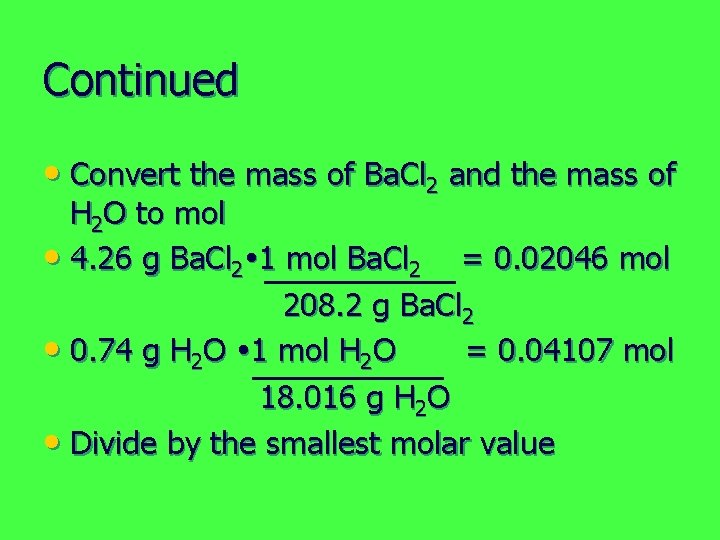
Continued • Convert the mass of Ba. Cl 2 and the mass of H 2 O to mol • 4. 26 g Ba. Cl 2 1 mol Ba. Cl 2 = 0. 02046 mol 208. 2 g Ba. Cl 2 • 0. 74 g H 2 O 1 mol H 2 O = 0. 04107 mol 18. 016 g H 2 O • Divide by the smallest molar value

Continued • 0. 02046 mol Ba. Cl 2 = 1 0. 02046 mol Ba. Cl 2 • 0. 04107 mol H 2 O = 2 0. 02046 mol Ba. Cl 2 • Formula of the hydrate: Ba. Cl 2 2 H 2 O

Another Practice • Fe. PO 4 ? H 2 O • Mass before heating: 7. 61 g • Mass after heating: 5. 15 g • Mass of water: 2. 46 g • Formula of the hydrate: Fe. PO 4 4 H 2 O