Chemical Names and Formulas Chapter 7 Chemical Formulas

























- Slides: 32

Chemical Names and Formulas Chapter 7

Chemical Formulas – number of each kind of atom Ex. C 8 H 18 , Al 2(SO 4)3

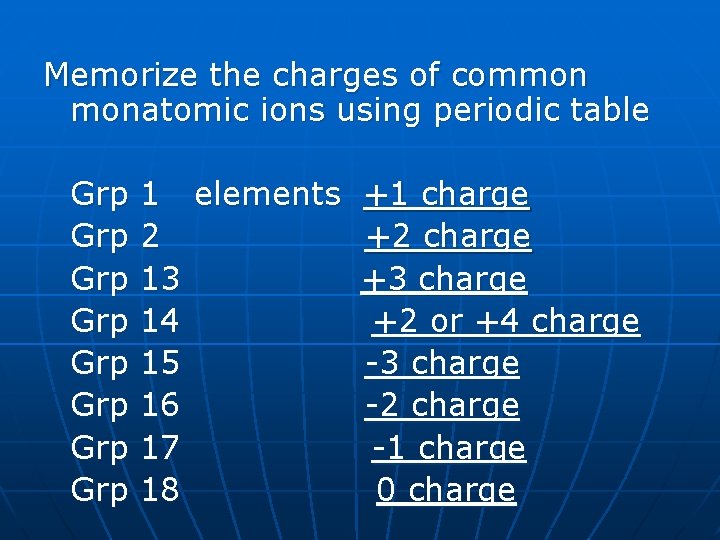
Memorize the charges of common monatomic ions using periodic table Grp Grp 1 elements +1 charge 2 +2 charge 13 +3 charge 14 +2 or +4 charge 15 -3 charge 16 -2 charge 17 -1 charge 18 0 charge

n n Grp 3 to Grp 12 Transition Metals have varying positive charge. These 3 ions do not vary in charge: • Ag+1, Cd+2, Zn+2 n H+1 OR H-1

Naming Monatomic Ions n Positive Ions – use element name and add word ion • Ex. K+1 potassium ion n Ions with varying positive charge have a roman number after the name to indicate the charge of the ion Ex. Fe+2 iron(II) ion OR Fe+3 iron(III) ion Pb+2 lead(II) ion OR Pb+4 lead(IV) ion

Negative Ions n n Negative Ions – use root name (drop last syllable) + -ide ion Examples: • F-1 fluoride ion • Note: H-1 hydride ion O-2 oxide ion

Binary Cmpds n Binary Cmpds – cmpds with only two different elements

Writing Ionic Cmpds Steps: 1. Write symbol and charge of positive ions first. 2. Write symbol and charge of negative ion next. 3. Use subscripts if more than one ion is needed to make the total charge of the cmpd zero.

Examples Write formula for each cmpd: 1. Aluminum oxide 2. Calcium chloride 3. Potassium sulfide 4. Gold (IV) oxide Prac. P 207 and p 209

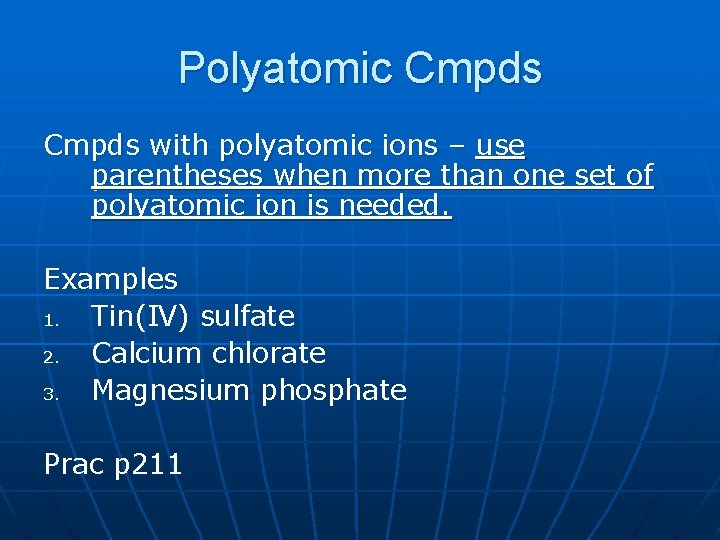
Polyatomic Cmpds with polyatomic ions – use parentheses when more than one set of polyatomic ion is needed. Examples 1. Tin(IV) sulfate 2. Calcium chlorate 3. Magnesium phosphate Prac p 211

Naming Ionic Cmpds Name cation, roman numeral, name anion (if needed) Examples: Name 1. Li. H 2. Na 2 SO 4 3. Mg. Cl 2 4. Cu. Cl 2 5. Al 2(SO 4)3

Binary Molecular Cmpd. n n Naming Binary Molecular Cmpd – only 2 elements in formula Memorize the Greek prefixes for one to ten p 212 Mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca 1 2 3 4 5 6 7 8 9 10

Molecular Cmpds. 1. 2. Less electronegative element (more metallic first), and prefix to element to show number of atoms. Omit mono- for the first element. Add prefix to root name of second element and add –ide ending.

Examples: Name 1. P 4 O 10 2. N 2 O 3. NO 4. NO 2 5. N 2 O 3 6. N 2 O 4 7. N 2 O 5 Prac p 213

Covalent Network Cmpds n n Atoms covalently bonded to its neighbors in a 3 D network Ex. Si. C Illustrate

Acids n Acid – compounds with the hydrogen ion, H+1 , first in the formula

Hydrogen Anion -ate -ide Ex. Hydrogen sulfate hydrogen sulfide Section Review p 215 Acid Name -ic acid -ous acid hydro-ic acid ________ (note: ur added in for S and added in for P)

Using Chemical Formulas n n Formula mass – sum of all masses of atoms in formula Ex: formula mass of: a. Fe b. O 2 c. H 2 O d. C 12 H 22 O 11 Practice p 222

Molar Mass n n Molar Mass – mass in grams of one mole of a substance Ex. Determine the molar mass of: a. Fe b. O 2 c. H 2 O d. C 12 H 22 O 11 Practice p 223

Formula Mass Vs. Molar Mass n Note: The only difference between formula mass and molar mass is the unit of amu and grams.

Mass to Moles Problem: 1. Convert 2. 34 X 103 g of carbon to moles. 2. Convert 14. 6 g of H 2 O to moles. Prac. P 226 #1

Mass to Number of Particles Problem: 3. Convert 2. 052 X 10 -2 moles of carbon to atoms. 4. 5. Change 340. 1 moles of water to molecules. How many formula units is 2. 985 X 10 -3 moles of Na. Cl?

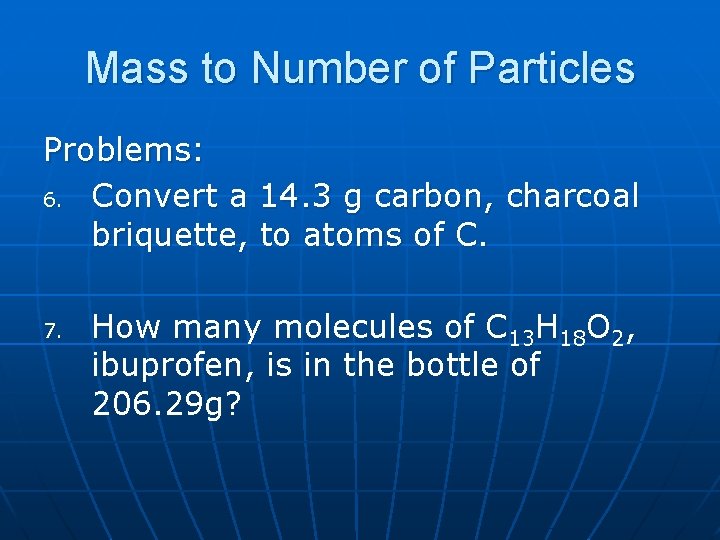
Mass to Number of Particles Problems: 6. Convert a 14. 3 g carbon, charcoal briquette, to atoms of C. 7. How many molecules of C 13 H 18 O 2, ibuprofen, is in the bottle of 206. 29 g?

8. How much of table salt must be measured out to give 4. 82 X 1025 formula units of Na. Cl? Prac p 226 #2, 3

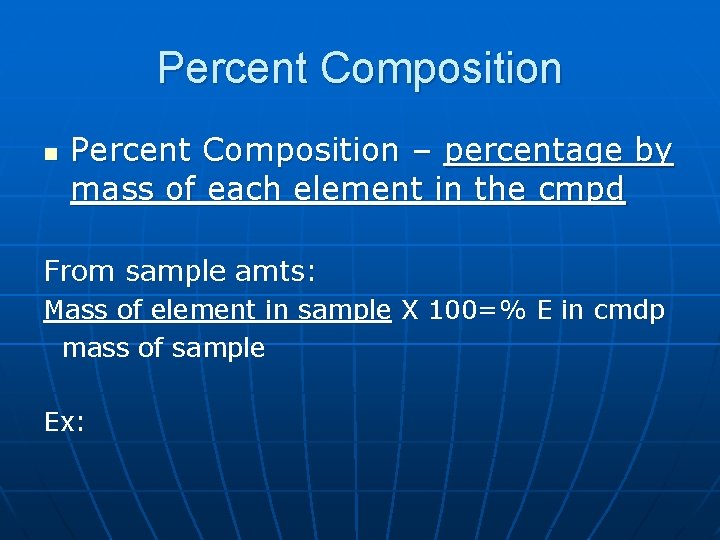
Percent Composition n Percent Composition – percentage by mass of each element in the cmpd From sample amts: Mass of element in sample X 100=% E in cmdp mass of sample Ex:

From formula: Mass of E in 1 mol of cmpd X 100=% E in cmdp molar mass of cmpd Prac. Find the % composition of Cu 2 S. Prac p 228

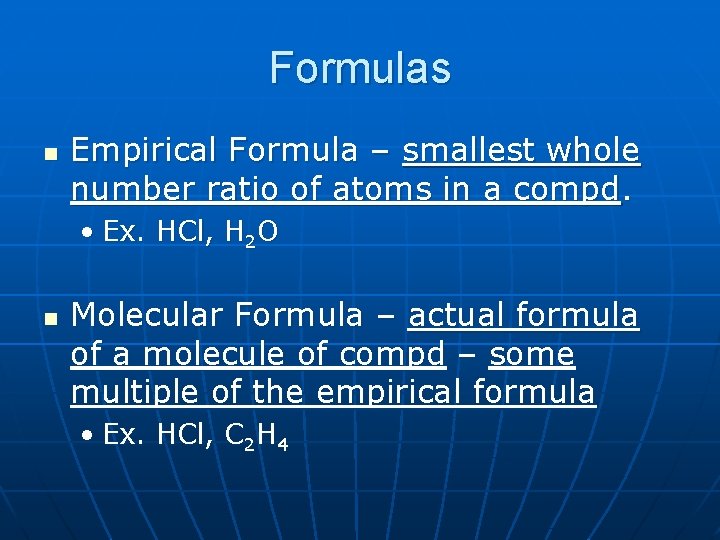
Formulas n Empirical Formula – smallest whole number ratio of atoms in a compd. • Ex. HCl, H 2 O n Molecular Formula – actual formula of a molecule of compd – some multiple of the empirical formula • Ex. HCl, C 2 H 4

Determine which is empirical and molecular formulas: 1. 2. 3. 4. 5. CH 4 P 2 O 5 NH 3 C 6 H 6 Ca(OH)2

Determining Empirical Formulas from Quantitative Analysis: 1. 2. 3. Convert % info to grams. Convert grams to moles of element Get whole number ratios by dividing by smallest # of moles.

Ex. Find the empirical formula of a compd with 32. 38% Na, 22. 65% S, and 44. 99% O. Prac p 231

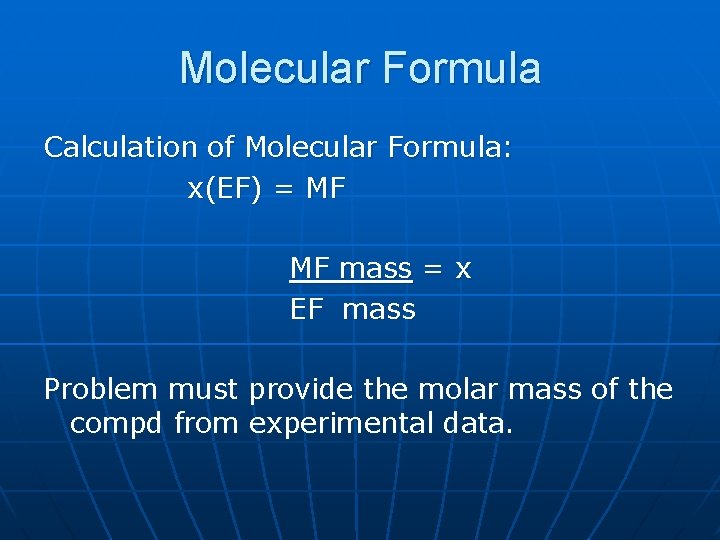
Molecular Formula Calculation of Molecular Formula: x(EF) = MF MF mass = x EF mass Problem must provide the molar mass of the compd from experimental data.

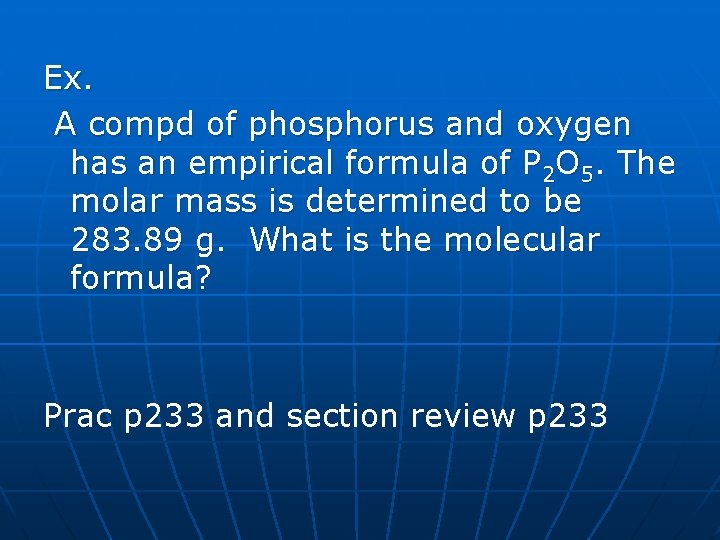
Ex. A compd of phosphorus and oxygen has an empirical formula of P 2 O 5. The molar mass is determined to be 283. 89 g. What is the molecular formula? Prac p 233 and section review p 233