Chapter 7 Chemical Formulas Chemical Formulas and Names
















- Slides: 43

Chapter 7 Chemical Formulas

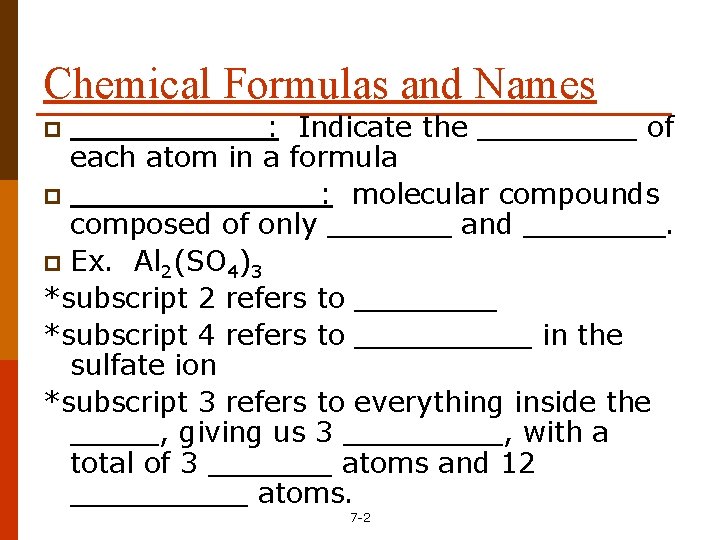
Chemical Formulas and Names ______: Indicate the _____ of each atom in a formula p _______: molecular compounds composed of only _______ and ____. p Ex. Al 2(SO 4)3 *subscript 2 refers to ____ *subscript 4 refers to _____ in the sulfate ion *subscript 3 refers to everything inside the _____, giving us 3 _____, with a total of 3 _______ atoms and 12 _____ atoms. p 7 -2

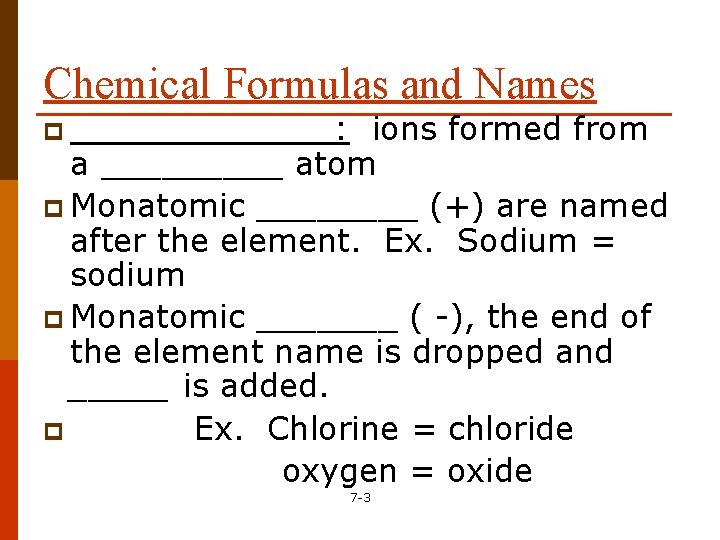
Chemical Formulas and Names p _______: ions formed from a _____ atom p Monatomic ____ (+) are named after the element. Ex. Sodium = sodium p Monatomic _______ ( -), the end of the element name is dropped and _____ is added. p Ex. Chlorine = chloride oxygen = oxide 7 -3

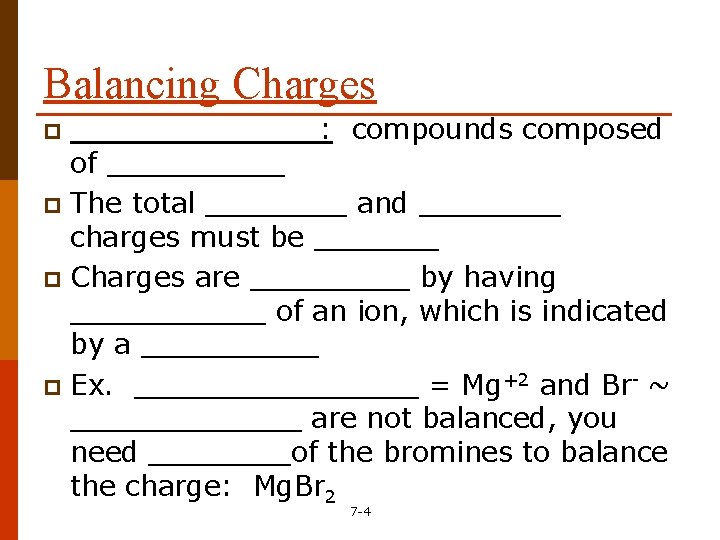
Balancing Charges _______: compounds composed of _____ p The total ____ and ____ charges must be _______ p Charges are _____ by having ______ of an ion, which is indicated by a _____ p Ex. ________ = Mg+2 and Br- ~ _______ are not balanced, you need ____of the bromines to balance the charge: Mg. Br 2 p 7 -4

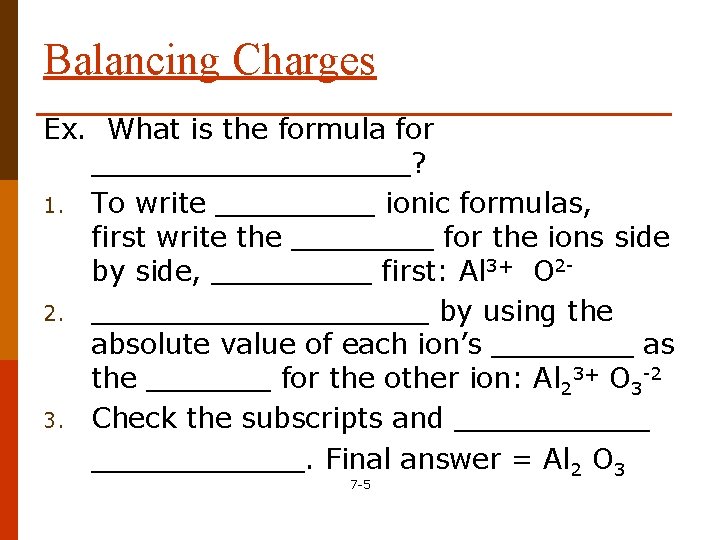
Balancing Charges Ex. What is the formula for _________? 1. To write _____ ionic formulas, first write the ____ for the ions side by side, _____ first: Al 3+ O 22. __________ by using the absolute value of each ion’s ____ as the _______ for the other ion: Al 23+ O 3 -2 3. Check the subscripts and ____________. Final answer = Al 2 O 3 7 -5

Practice 1) Write balanced formulas for the following binary ionic compounds: a) Sodium chloride: ____________ b) Zinc Oxide: ______________ c) Aluminum Bromide: ___________ a) Barium Phosphide: ___________ 7 -6

Practice 1) Write balanced formulas for the following binary ionic compounds: a) Sodium chloride: ____________ b) Zinc Oxide: ____________ c) Aluminum Bromide: ________ d) Barium Phosphide: _________ 7 -7

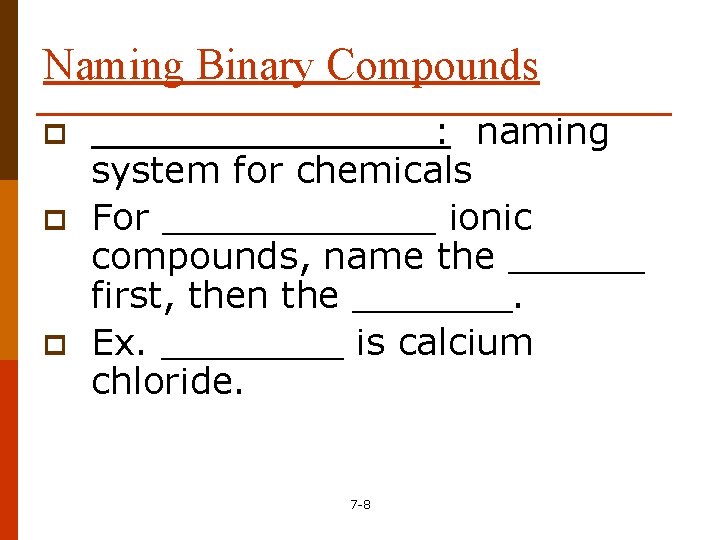
Naming Binary Compounds p p p ________: naming system for chemicals For ______ ionic compounds, name the ______ first, then the _______. Ex. ____ is calcium chloride. 7 -8

Practice a) What are the names of the following binary ionic compounds? Sr 3 N 2: __________ b) KI: ___________ c) Li 2 S: __________ 2) 7 -9

Practice a) What are the names of the following binary ionic compounds? Sr 3 N 2: b) KI: c) Li 2 S: 2) 7 -10

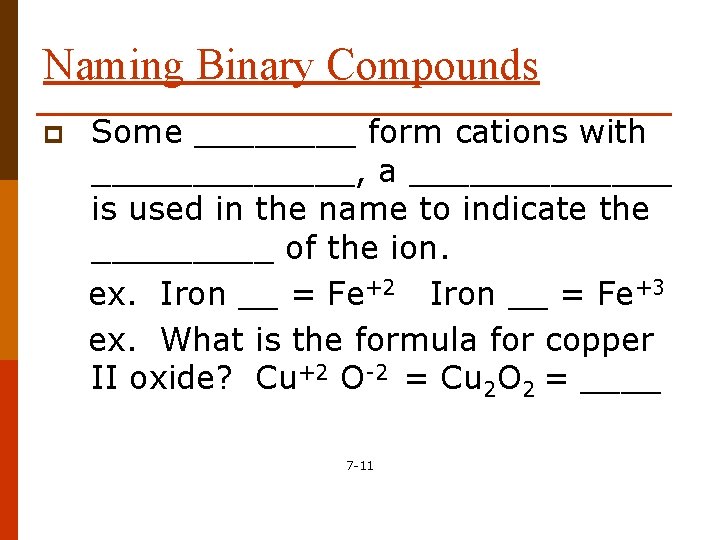
Naming Binary Compounds p Some ____ form cations with _______, a _______ is used in the name to indicate the _____ of the ion. ex. Iron __ = Fe+2 Iron __ = Fe+3 ex. What is the formula for copper II oxide? Cu+2 O-2 = Cu 2 O 2 = ____ 7 -11

Practice 3) a) b) 4) a) b) What are the formulas for the following binary ionic compounds? Iron III sulfide: ______ Copper I oxide: ______ What are the names of the following binary ionic compounds? Cr. F 2: ___________ Pb 3 N 4: __________ 7 -12

Practice 3) a) b) 4) a) b) What are the formulas for the following binary ionic compounds? Iron III sulfide: _______ Copper I oxide: _______ What are the names of the following binary ionic compounds? Cr. F 2: ___________ Pb 3 N 4: __________ 7 -13

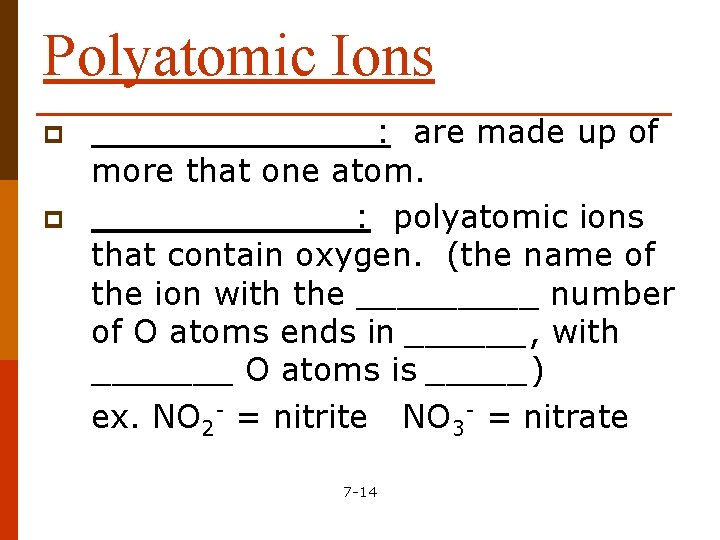
Polyatomic Ions p p _______: are made up of more that one atom. _______: polyatomic ions that contain oxygen. (the name of the ion with the _____ number of O atoms ends in ______, with _______ O atoms is _____) ex. NO 2 - = nitrite NO 3 - = nitrate 7 -14

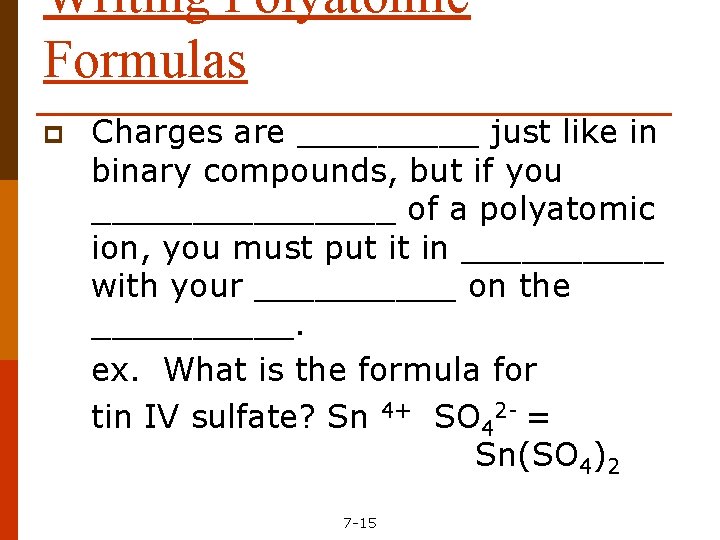
Writing Polyatomic Formulas p Charges are _____ just like in binary compounds, but if you ________ of a polyatomic ion, you must put it in _____ with your _____ on the _____. ex. What is the formula for tin IV sulfate? Sn 4+ SO 42 - = Sn(SO 4)2 7 -15

Practice 5) a) b) c) d) e) Write the formulas for the following ionic compounds: Sodium iodide: _______ Lithium nitrate: _______ Copper II sulfate: ______ Sodium Carbonate: _____ Potassium perchlorate: _______ 7 -16

Practice 5) a) b) c) d) e) Write the formulas for the following ionic compounds: Sodium iodide: _______ Lithium nitrate: _______ Copper II sulfate: _______ Sodium Carbonate: ______ Potassium perchlorate: _____ 7 -17

Practice 6)Write the names for the following ionic compounds: a) Ca(OH)2__________ b) KCl. O 3: ___________ c) NH 4 OH: ___________ d) Fe 2(Cr. O 4)3: __________ e) KCl. O: ____________ 7 -18

Practice 6)Write the names for the following ionic compounds: a) Ca(OH)2: __________ _ b) KCl. O 3: ____________ c) NH 4 OH: ___________ d) Fe 2(Cr. O 4)3: __________ e) KCl. O: ____________ 7 -19

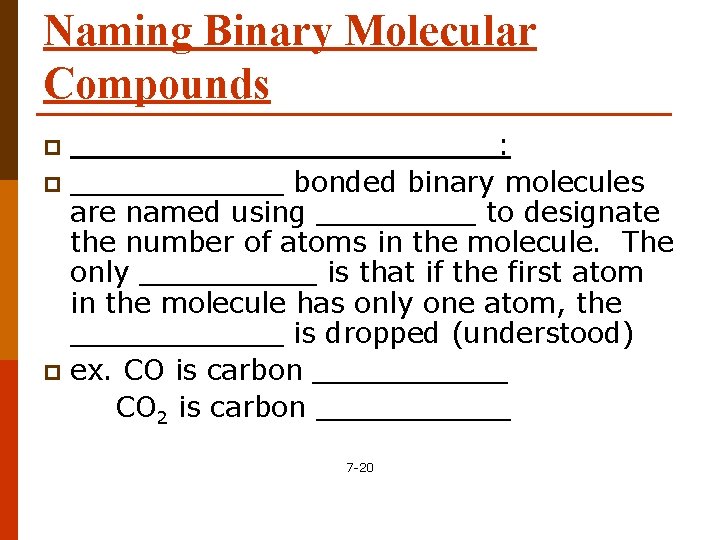
Naming Binary Molecular Compounds ____________: p ______ bonded binary molecules are named using _____ to designate the number of atoms in the molecule. The only _____ is that if the first atom in the molecule has only one atom, the ______ is dropped (understood) p ex. CO is carbon ______ CO 2 is carbon ______ p 7 -20

Practice 7) Name to following binary molecular compounds using the prefix system: a) H 20: _______________ b) N 2 O 3: ______________ c) P 4 O 10: ______________ 8) Write the formula for the following binary molecular compounds: a) Sulfur dioxide: _____________ b) Phosphorous pentabromide: ________ c) Carbon tetrachloride: ___________ 7 -21

Practice 7) Name to following binary molecular compounds using the prefix system: a) H 20: ________________ b) N 2 O 3: ________________ c) P 4 O 10: ________________ 8) Write the formula for the following binary molecular compounds: a) Sulfur dioxide: _____________ b) Phosphorous pentabromide: ________ c) Carbon tetrachloride: __________ 7 -22

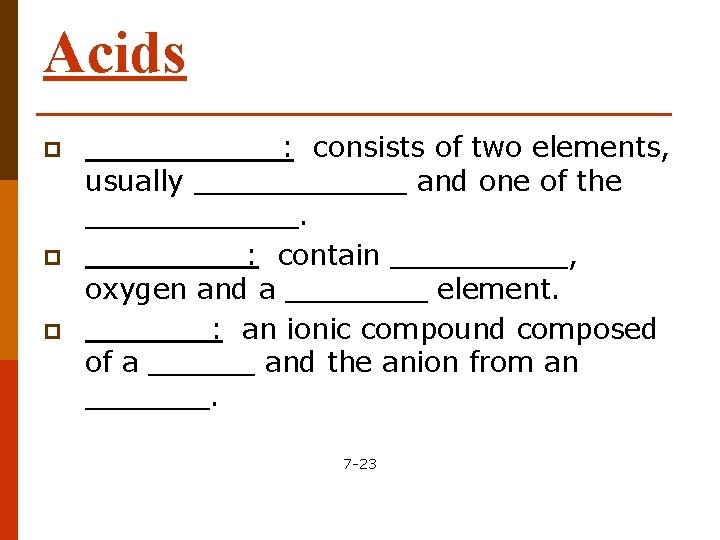
Acids p p p ______: consists of two elements, usually ______ and one of the ______: contain _____, oxygen and a ____ element. _______: an ionic compound composed of a ______ and the anion from an _______. 7 -23

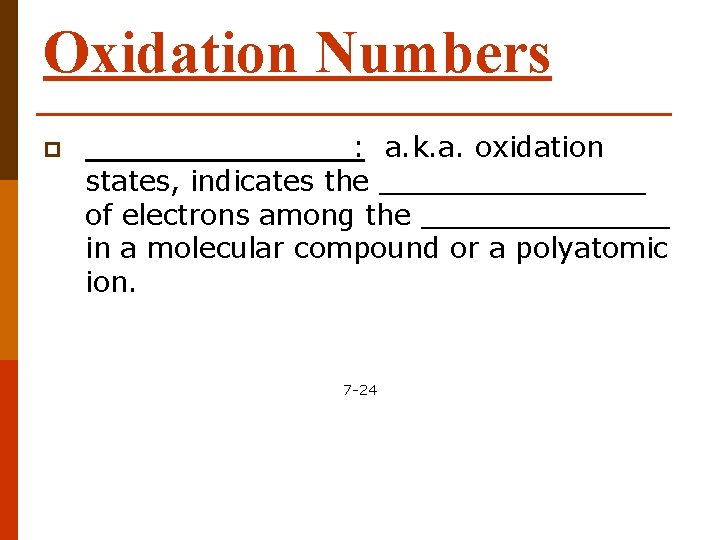
Oxidation Numbers p ________: a. k. a. oxidation states, indicates the ________ of electrons among the _______ in a molecular compound or a polyatomic ion. 7 -24

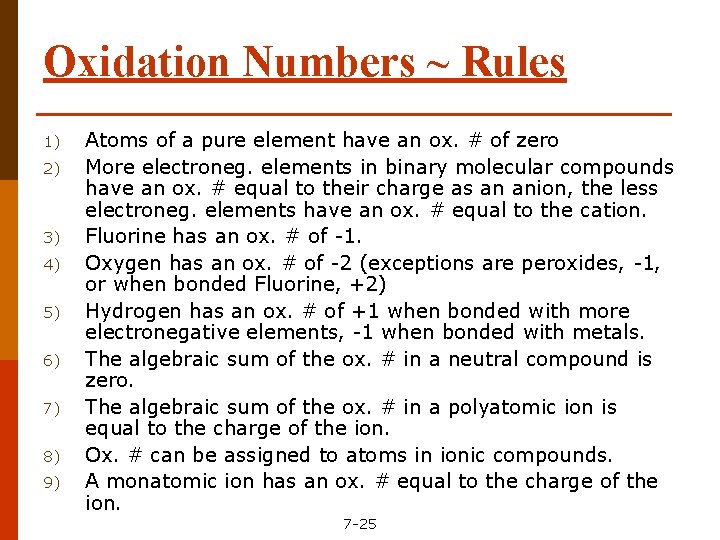
Oxidation Numbers ~ Rules 1) 2) 3) 4) 5) 6) 7) 8) 9) Atoms of a pure element have an ox. # of zero More electroneg. elements in binary molecular compounds have an ox. # equal to their charge as an anion, the less electroneg. elements have an ox. # equal to the cation. Fluorine has an ox. # of -1. Oxygen has an ox. # of -2 (exceptions are peroxides, -1, or when bonded Fluorine, +2) Hydrogen has an ox. # of +1 when bonded with more electronegative elements, -1 when bonded with metals. The algebraic sum of the ox. # in a neutral compound is zero. The algebraic sum of the ox. # in a polyatomic ion is equal to the charge of the ion. Ox. # can be assigned to atoms in ionic compounds. A monatomic ion has an ox. # equal to the charge of the ion. 7 -25

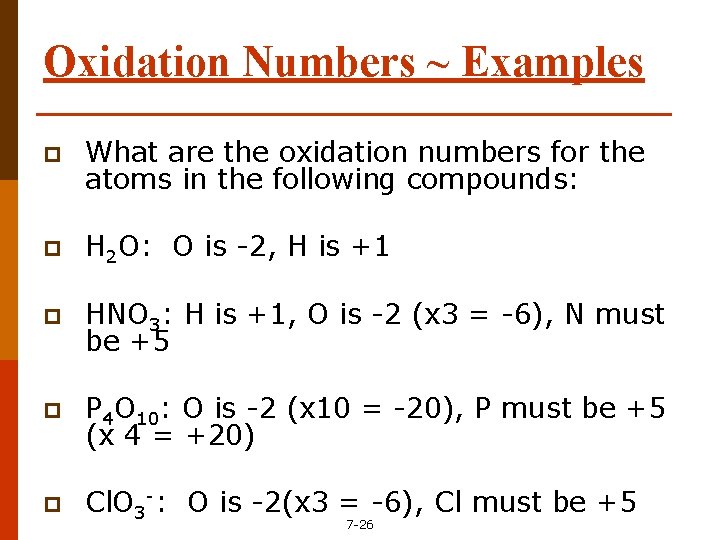
Oxidation Numbers ~ Examples p What are the oxidation numbers for the atoms in the following compounds: p H 2 O: O is -2, H is +1 p HNO 3: H is +1, O is -2 (x 3 = -6), N must be +5 p P 4 O 10: O is -2 (x 10 = -20), P must be +5 (x 4 = +20) p Cl. O 3 -: O is -2(x 3 = -6), Cl must be +5 7 -26

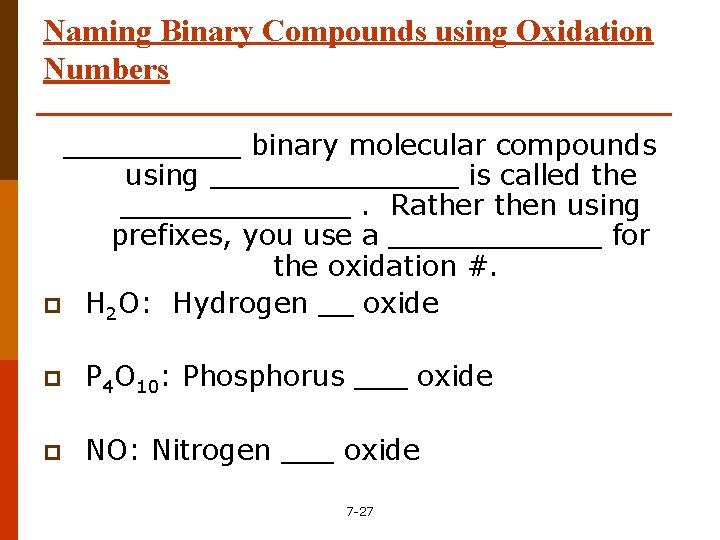
Naming Binary Compounds using Oxidation Numbers _____ binary molecular compounds using _______ is called the _______. Rather then using prefixes, you use a ______ for the oxidation #. p H 2 O: Hydrogen __ oxide p P 4 O 10: Phosphorus ___ oxide p NO: Nitrogen ___ oxide 7 -27

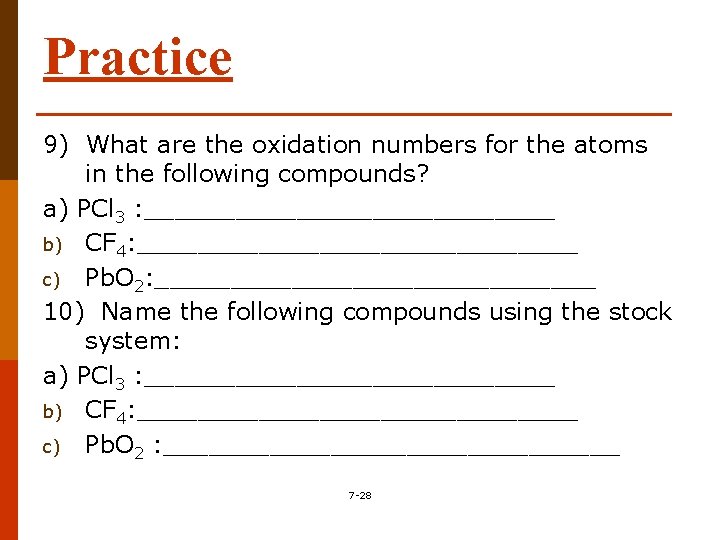
Practice 9) What are the oxidation numbers for the atoms in the following compounds? a) PCl 3 : ______________ b) CF 4: _______________ c) Pb. O 2: _______________ 10) Name the following compounds using the stock system: a) PCl 3 : ______________ b) CF 4: _______________ c) Pb. O 2 : _______________ 7 -28

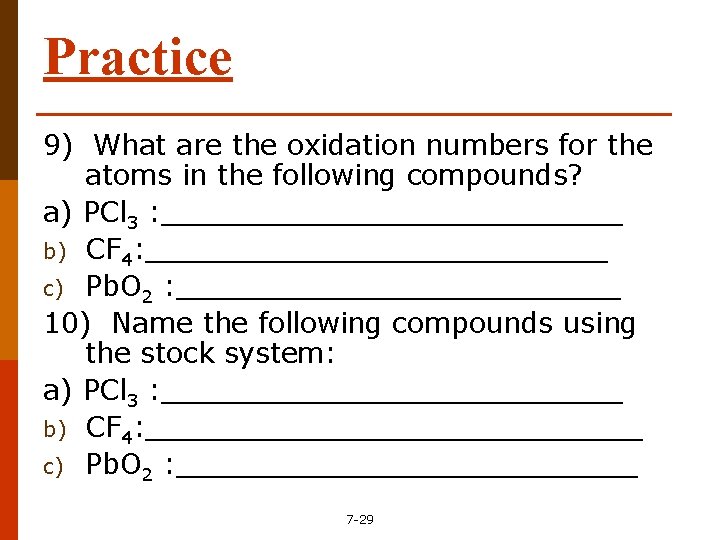
Practice 9) What are the oxidation numbers for the atoms in the following compounds? a) PCl 3 : _____________ b) CF 4: _____________ c) Pb. O 2 : _____________ 10) Name the following compounds using the stock system: a) PCl 3 : _____________ b) CF 4: ______________ c) Pb. O 2 : _____________ 7 -29

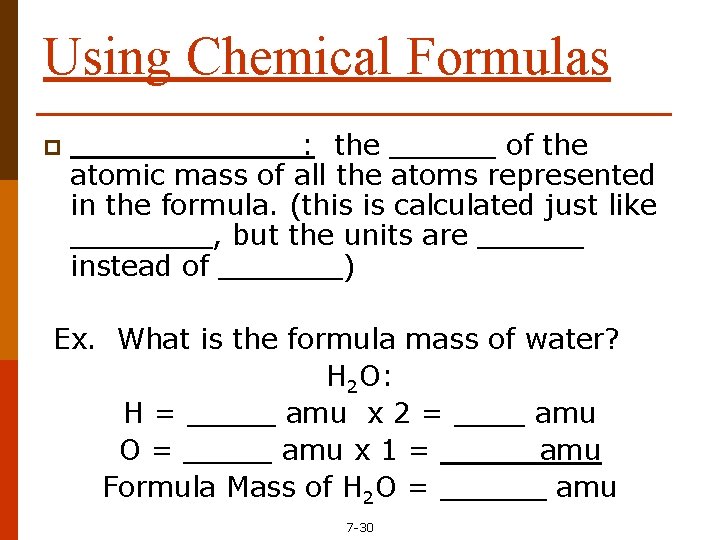
Using Chemical Formulas p _______: the ______ of the atomic mass of all the atoms represented in the formula. (this is calculated just like ____, but the units are ______ instead of _______) Ex. What is the formula mass of water? H 2 O: H = _____ amu x 2 = ____ amu O = _____ amu x 1 = _____ amu Formula Mass of H 2 O = ______ amu 7 -30

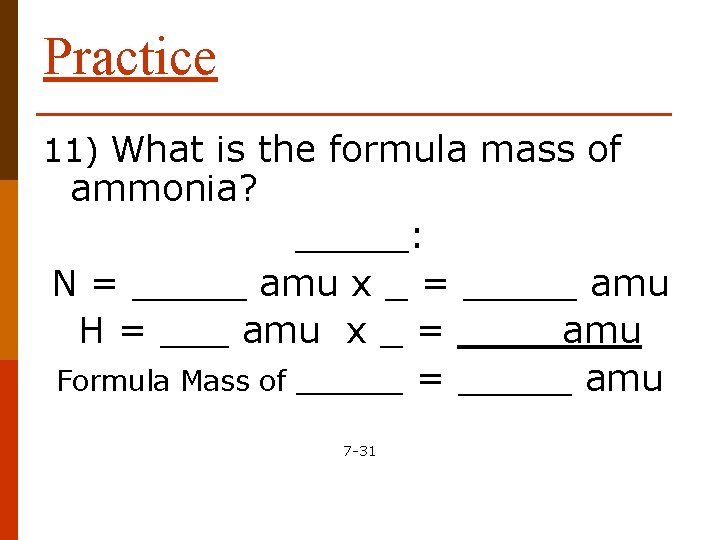
Practice 11) What is the formula mass of ammonia? _____: N = _____ amu x _ = _____ amu H = ___ amu x _ = ____ amu Formula Mass of ______ = _____ amu 7 -31

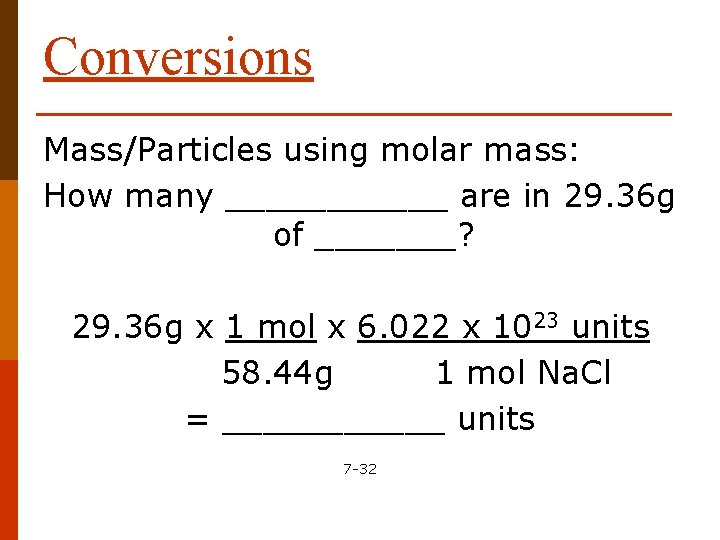
Conversions Mass/Particles using molar mass: How many ______ are in 29. 36 g of _______? 29. 36 g x 1 mol x 6. 022 x 1023 units 58. 44 g 1 mol Na. Cl = ______ units 7 -32

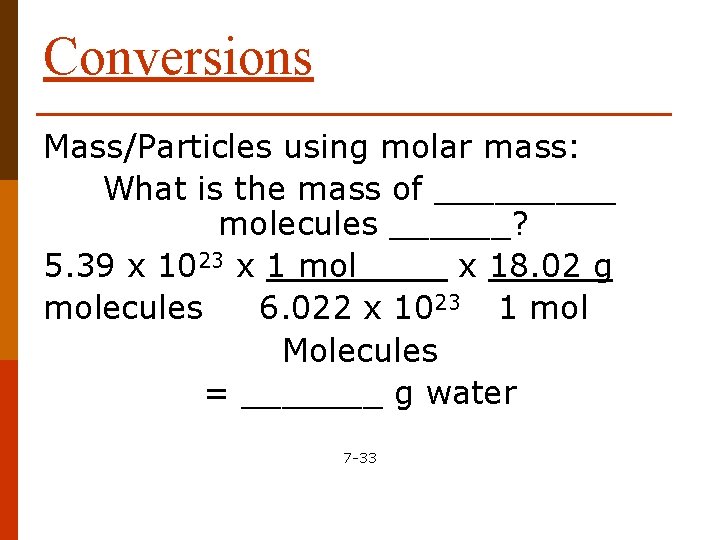
Conversions Mass/Particles using molar mass: What is the mass of _____ molecules ______? 5. 39 x 1023 x 1 mol x 18. 02 g molecules 6. 022 x 1023 1 mol Molecules = _______ g water 7 -33

Practice How many atoms of hydrogen are in 35. 67 g of H 2 O? 12) What is the mass of 7. 89 x 1024 formula units of Ba. Cl 2? 13) 7 -34

Practice How many atoms of hydrogen are in 35. 67 g of H 2 O? 12) 13) 7 -35

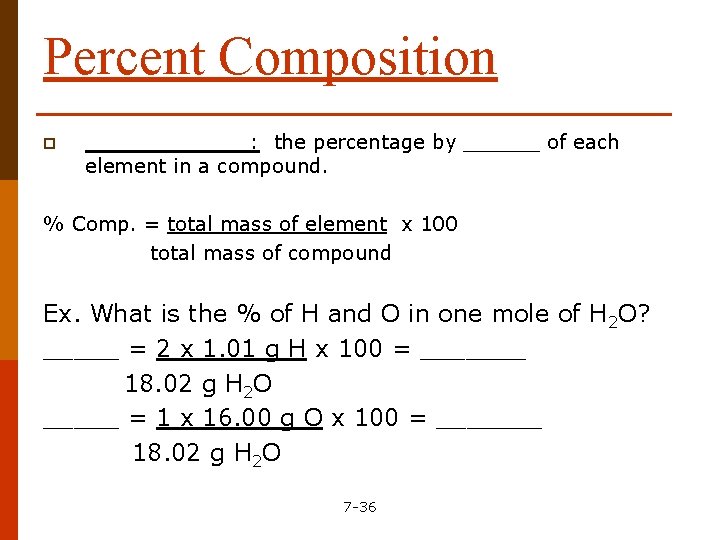
Percent Composition p _______: the percentage by ______ of each element in a compound. % Comp. = total mass of element x 100 total mass of compound Ex. What is the % of H and O in one mole of H 2 O? _____ = 2 x 1. 01 g H x 100 = _______ 18. 02 g H 2 O _____ = 1 x 16. 00 g O x 100 = _______ 18. 02 g H 2 O 7 -36

Practice 14) What is the percent comp. of HCl. O 3? 7 -37

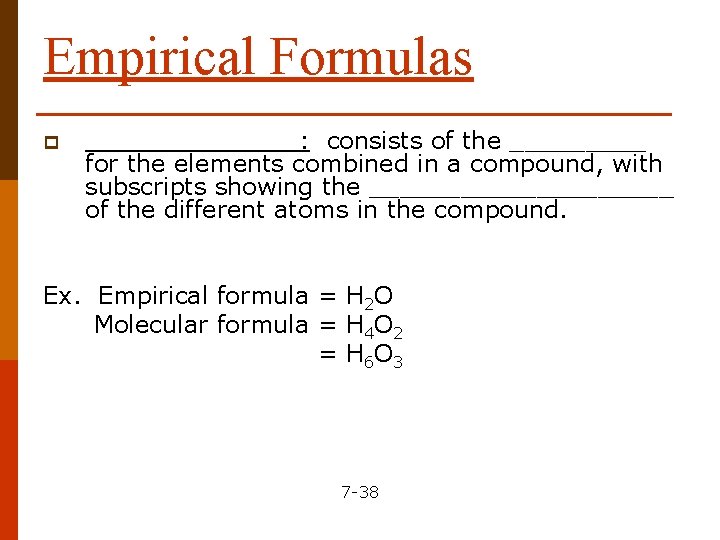
Empirical Formulas p _______: consists of the _____ for the elements combined in a compound, with subscripts showing the __________ of the different atoms in the compound. Ex. Empirical formula = H 2 O Molecular formula = H 4 O 2 = H 6 O 3 7 -38

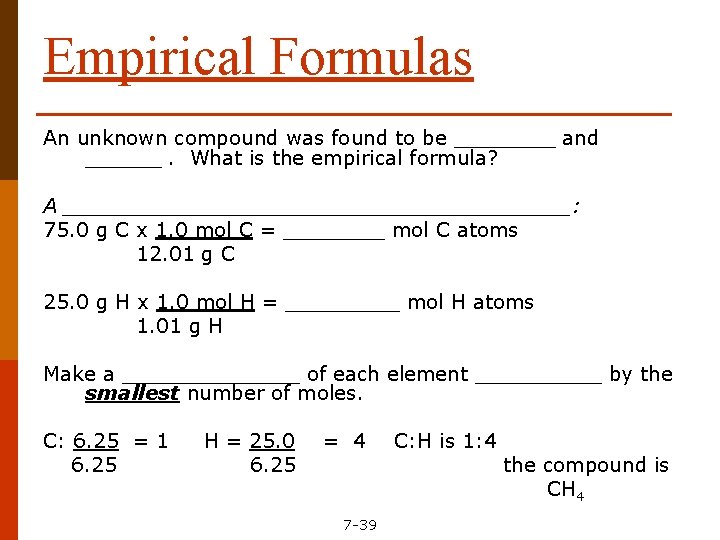
Empirical Formulas An unknown compound was found to be ____ and ______. What is the empirical formula? A ____________________: 75. 0 g C x 1. 0 mol C = ____ mol C atoms 12. 01 g C 25. 0 g H x 1. 0 mol H = _____ mol H atoms 1. 01 g H Make a _______ of each element _____ by the smallest number of moles. C: 6. 25 = 1 6. 25 H = 25. 0 6. 25 = 4 7 -39 C: H is 1: 4 the compound is CH 4

Empirical Formulas 15) Now you try one: determine the empirical formula for a compound containing 7. 30 g Na, 5. 08 g S and 7. 62 g O.

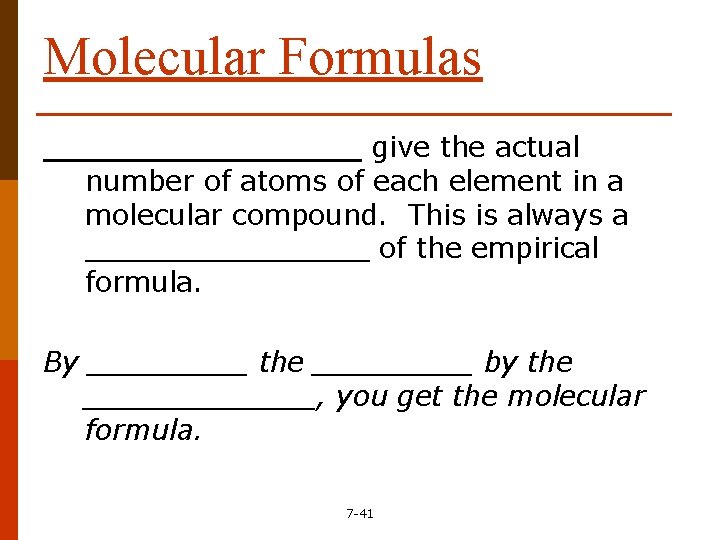
Molecular Formulas ________ give the actual number of atoms of each element in a molecular compound. This is always a ________ of the empirical formula. By _____ the _____ by the _______, you get the molecular formula. 7 -41

Practice 16) Find the molecular formula for a compound that contains 4. 90 g N and 11. 2 g O with a molar mass of 92. 0 g/mol. 7 -42

Ch. 7 The End!