Chapter 10 Chemical Formulas and Equations Chemical Formulas














- Slides: 30

Chapter 10: Chemical Formulas and Equations

Chemical Formulas • Introduction – Empirical formula • The simplest whole number ratio of elements in a compound. • Ionic compounds are always shown as empirical formulas. – Molecular Formula • The actual numbers of atoms in a molecule. – Structural Formula • Show the relative arrangements of atoms in a molecule

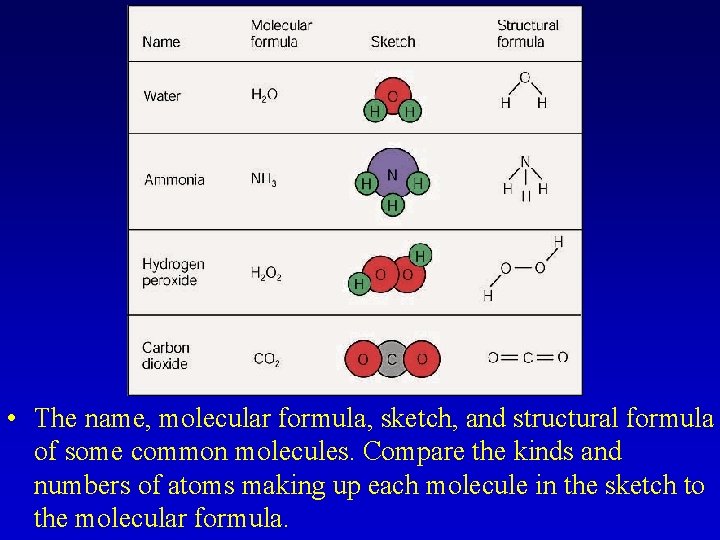
• The name, molecular formula, sketch, and structural formula of some common molecules. Compare the kinds and numbers of atoms making up each molecule in the sketch to the molecular formula.

Molecular and Formula Weights – The formula weight of a compound is the sum of all of the atomic weights of the atoms in a chemical formula. – The formula weight of an ionic compound is found by adding up all of the atomic weights of the atoms in the compound. – All compounds have a formula weight. – The molecular weight is the formula weight of a molecular compound.

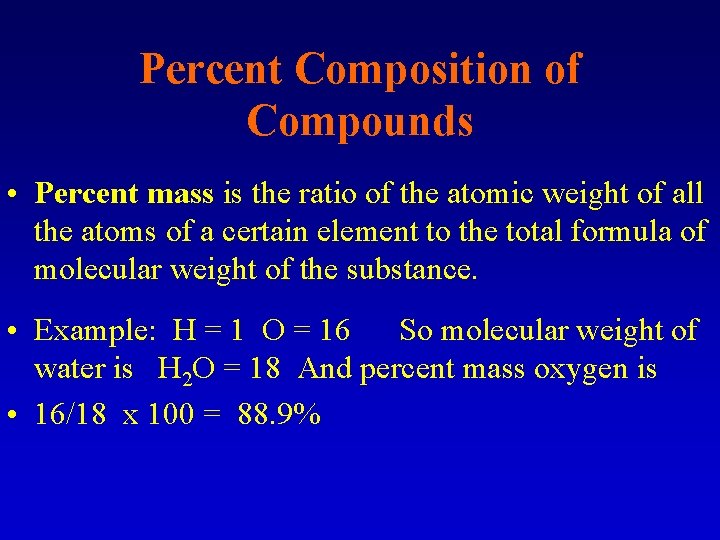
Percent Composition of Compounds • Percent mass is the ratio of the atomic weight of all the atoms of a certain element to the total formula of molecular weight of the substance. • Example: H = 1 O = 16 So molecular weight of water is H 2 O = 18 And percent mass oxygen is • 16/18 x 100 = 88. 9%

Chemical Equations • Introduction – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken. – Chemical reactions involve changes in matter, the making of new materials with new properties, or energy changes. – Chemical Reactions are described using a shorthand called a chemical equation.

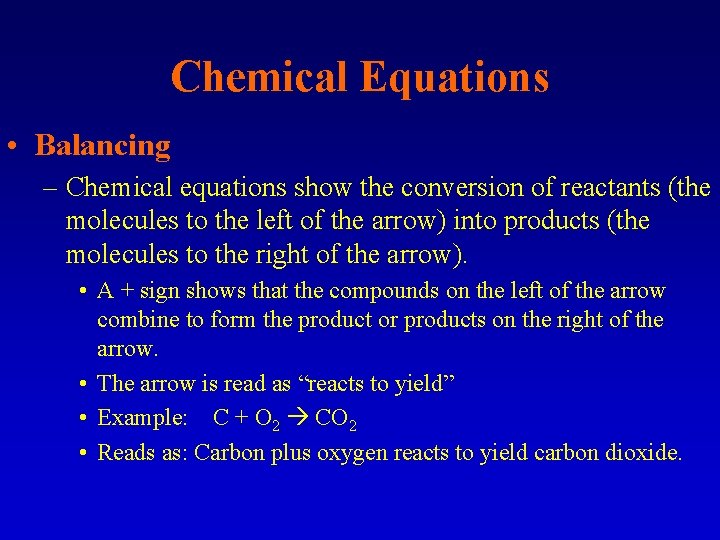
Chemical Equations • Balancing – Chemical equations show the conversion of reactants (the molecules to the left of the arrow) into products (the molecules to the right of the arrow). • A + sign shows that the compounds on the left of the arrow combine to form the product or products on the right of the arrow. • The arrow is read as “reacts to yield” • Example: C + O 2 CO 2 • Reads as: Carbon plus oxygen reacts to yield carbon dioxide.

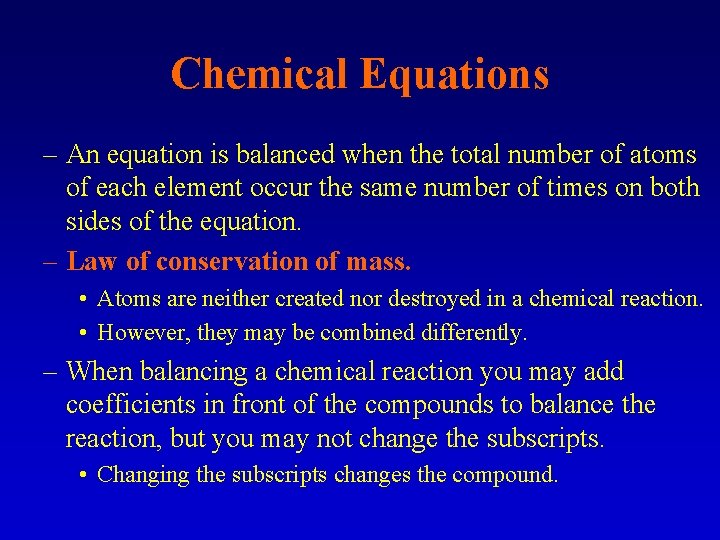
Chemical Equations – An equation is balanced when the total number of atoms of each element occur the same number of times on both sides of the equation. – Law of conservation of mass. • Atoms are neither created nor destroyed in a chemical reaction. • However, they may be combined differently. – When balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. • Changing the subscripts changes the compound.

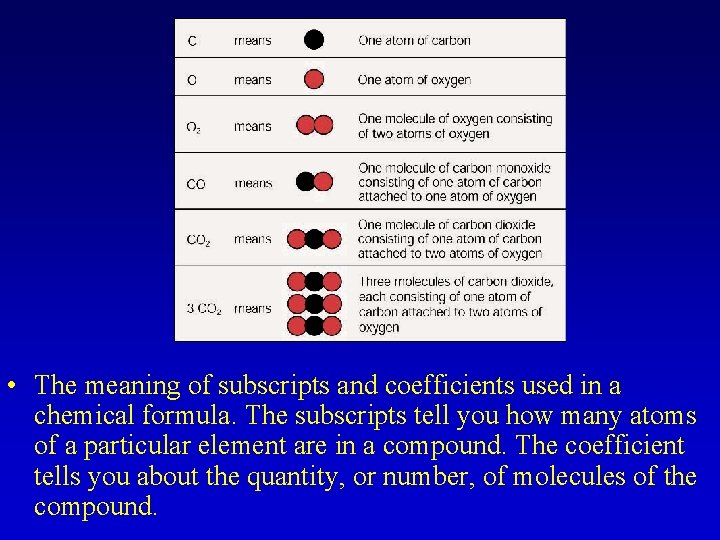
• The meaning of subscripts and coefficients used in a chemical formula. The subscripts tell you how many atoms of a particular element are in a compound. The coefficient tells you about the quantity, or number, of molecules of the compound.

Steps in Balancing Equations – Four basic steps to balancing a chemical equation. • Write the correct formula for the reactants and the products in an unbalanced equation. • Inventory the number of each kind of atom on both sides of the unbalanced equation. • Determine where to place coefficients in front of formulas to balance the equation. • Take another inventory to determine if: – The numbers of atoms on both sides are balanced. – The coefficients are the lowest possible ratios.

Balancing Equations – When balancing equations remember the following: • Atoms are neither lost nor gained during a chemical reaction. • A correct formula of a compound cannot be changed by altering the number or placement of subscripts. • A coefficient in front of a formula multiplies everything in the formula by that number.

Balancing Equations: Tips – A general approach to balancing reactions is: • Look first to formulas of compounds with the most atoms and try to balance the atoms or compounds they were formed from or decomposed to. • Polyatomic ions that appear on both sides of the equation should be balanced as independent units with their charge. • Try both the “Crossover technique” and the use of “fractional coefficients”

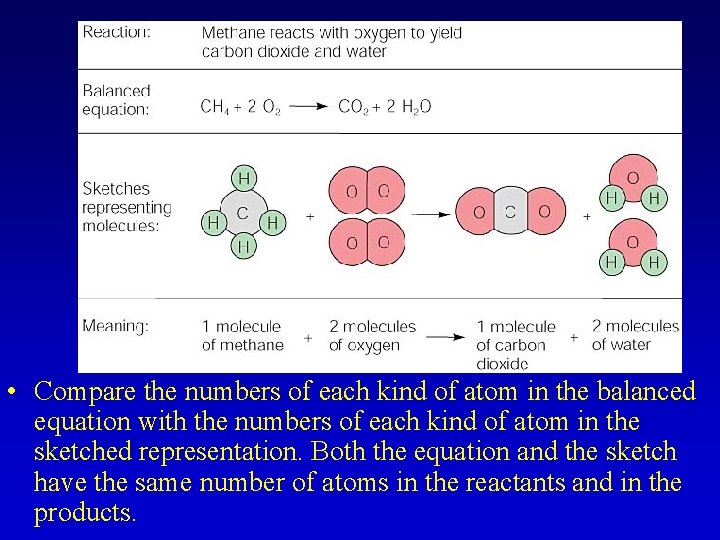
• Compare the numbers of each kind of atom in the balanced equation with the numbers of each kind of atom in the sketched representation. Both the equation and the sketch have the same number of atoms in the reactants and in the products.

Symbols Used in Equations – Conventions • gas (g) • Liquid (l) • Aqueous solution (aq) • Escaping gas ( ) • Solid formation ( ) • Change of temperature ( )

Oxidation-reduction reactions – An oxidation reduction reaction is one in which electrons are transferred between atoms. – Oxidation is the loss of electrons – Reduction is the gain of electrons – Oxidizing agents are substances which take electrons away from other atoms. • An oxidizing agent is reduced when it oxidizes another atom – Reducing agents are substances which donate electrons to other substances. • A reducing agent is oxidized in the process of reducing another atom.

• Oxidizing agents take electrons from other substances that are being oxidized. Oxygen and chlorine are commonly used, strong oxidizing agents.

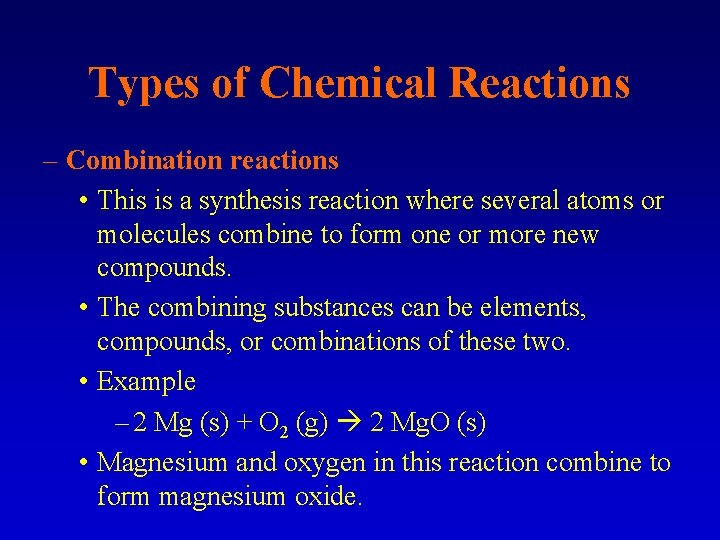
Types of Chemical Reactions – Combination reactions • This is a synthesis reaction where several atoms or molecules combine to form one or more new compounds. • The combining substances can be elements, compounds, or combinations of these two. • Example – 2 Mg (s) + O 2 (g) 2 Mg. O (s) • Magnesium and oxygen in this reaction combine to form magnesium oxide.

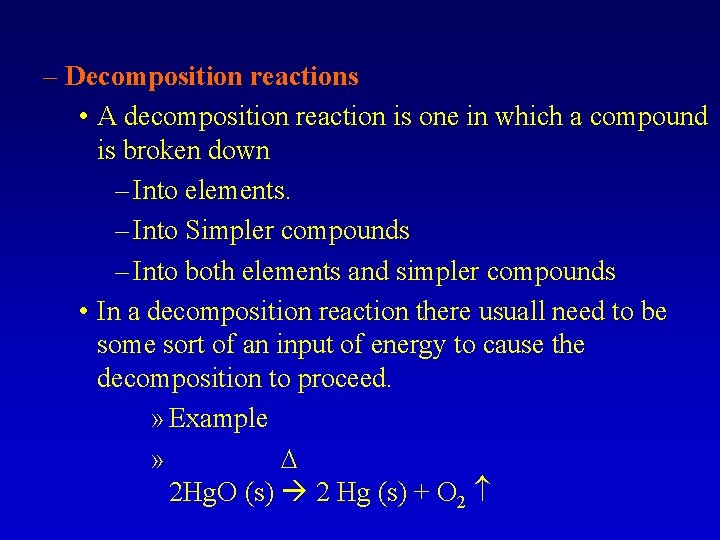
– Decomposition reactions • A decomposition reaction is one in which a compound is broken down – Into elements. – Into Simpler compounds – Into both elements and simpler compounds • In a decomposition reaction there usuall need to be some sort of an input of energy to cause the decomposition to proceed. » Example » 2 Hg. O (s) 2 Hg (s) + O 2

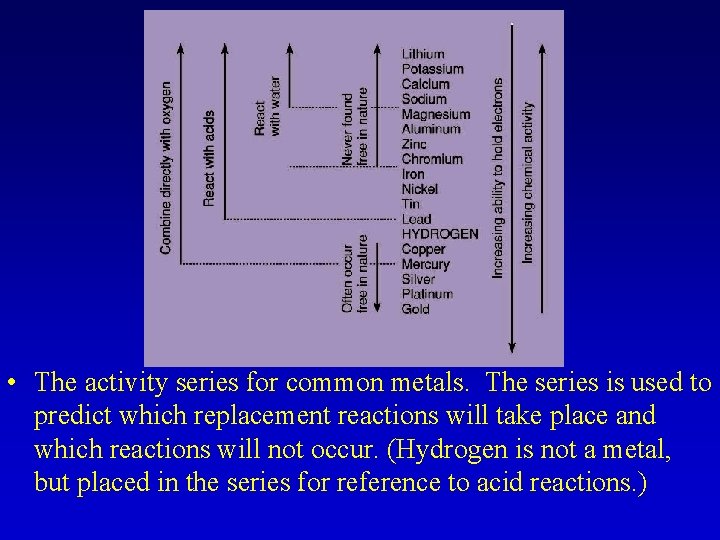
• The activity series for common metals. The series is used to predict which replacement reactions will take place and which reactions will not occur. (Hydrogen is not a metal, but placed in the series for reference to acid reactions. )

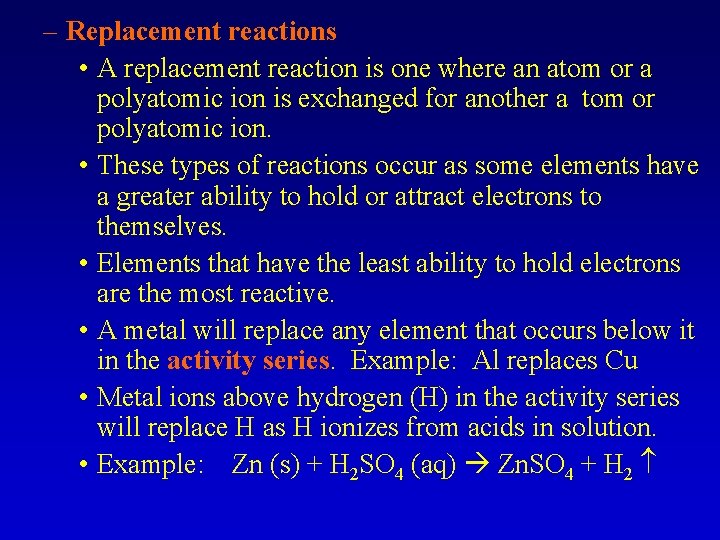
– Replacement reactions • A replacement reaction is one where an atom or a polyatomic ion is exchanged for another a tom or polyatomic ion. • These types of reactions occur as some elements have a greater ability to hold or attract electrons to themselves. • Elements that have the least ability to hold electrons are the most reactive. • A metal will replace any element that occurs below it in the activity series. Example: Al replaces Cu • Metal ions above hydrogen (H) in the activity series will replace H as H ionizes from acids in solution. • Example: Zn (s) + H 2 SO 4 (aq) Zn. SO 4 + H 2

• This shows a reaction between metallic aluminum and the blue solution of copper (II) chloride. Aluminum is above copper in the activity series, and aluminum replaces the copper ions from the solution as copper is deposited as a metal. The aluminum loses electrons to the copper and forms aluminum ions in solution.

– Ion exchange reactions • An ion exchange reaction is a reaction that takes place when the ion of one compound interacts with the ions of another compound forming any of the following: – A precipitate; a solid that comes out of the solution – A gas – Water • Example • 3 Ca(OH)2 (aq) + Al 2(SO 4)3 (aq) 3 Ca. SO 4 (aq) + 2 Al(OH)3

Information from Chemical Equations – Information from a balanced chemical equation tells us information about: • Atoms • Molecules • Atomic weights. – The coefficients in the balanced reaction is the number of atoms or molecules involve in the reaction.

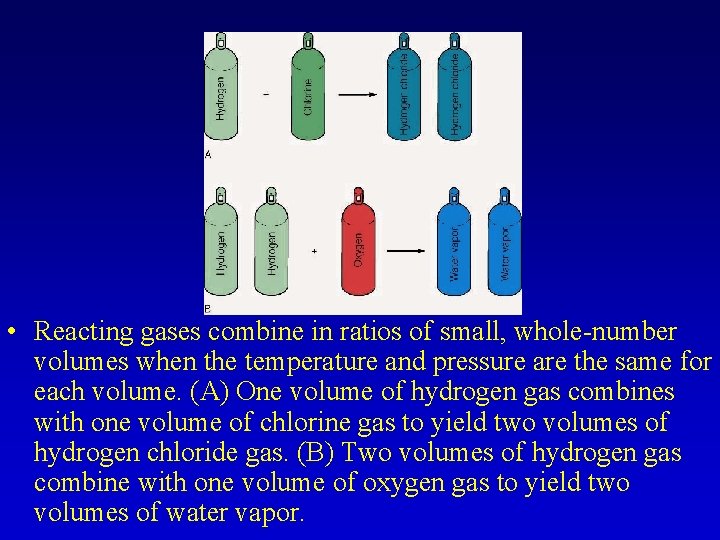
– In 1808, Gay-Lussac determined that gases combine in small, whole number volumes when the temperature and pressure were held constant. • This is the Law of Combining Volumes. – Avogadro proposed an explanation for the law of combining volumes in 1811. • It was proposed that gases at the same temperature contained the same number of molecules. – This had two implications for the coefficients in a balanced equation • The coefficients represent the number of molecules of each substance • It also represents the ratios of the combining volumes.

, • Reacting gases combine in ratios of small, whole-number volumes when the temperature and pressure are the same for each volume. (A) One volume of hydrogen gas combines with one volume of chlorine gas to yield two volumes of hydrogen chloride gas. (B) Two volumes of hydrogen gas combine with one volume of oxygen gas to yield two volumes of water vapor.

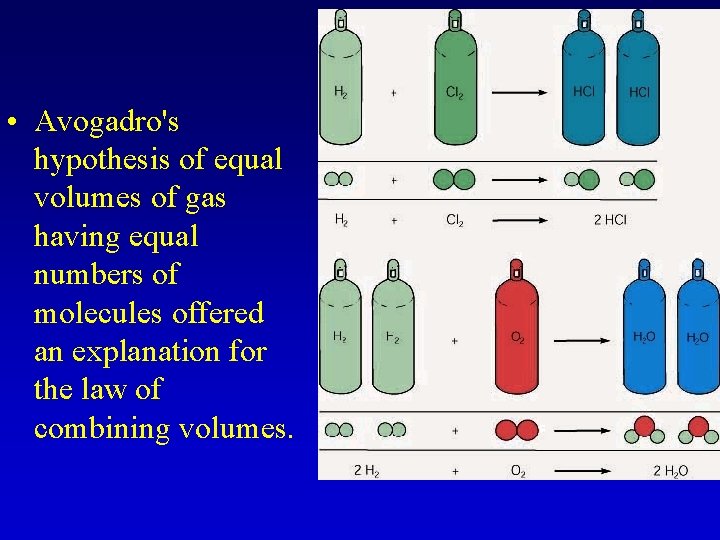
• Avogadro's hypothesis of equal volumes of gas having equal numbers of molecules offered an explanation for the law of combining volumes.

Measurements used with Equations – We use a mole concept to bring together the concepts of counting numbers and atomic weights of elements. – The mole is derived from the following information. • Atomic weights are an average of the relative masses of all of the isotopes of the given element. • The number of C-12 atoms in exactly 12. 00 g is 6. 02 X 1023. – This called Avogadro’s number. • An amount of a substance that contains Avogadro’s number of atoms, ions, molecules, or any other unit is called a mole. • A mole of C-12 atoms is defined as having a mass of exactly 12. 00 g, a mass that is equal to its atomic weight.

Weights – The gram atomic weight of an element is the mass in grams of one mole of an element that is numerically equal to its atomic weight. – The gram formula weight of a compound is the mass in grams of one mole of the compound that is numerically equal to its formula weight. • The gram formula weight of a compound is the sum total of all the individual atomic weights in the formula. – The gram molecular weight is the gram formula weight of a molecular compound.

Quantitative uses of Equations – A balanced chemical equation can be used to interpret the: • Molecular ratio of reactants to products. • Mole ratio of the reactants to products. • Mass ratio of the reactants to products.

Quantitative uses of Equations – The molecular ratio leads to the concept of the mole ratio since any number of molecules can react as long as they are in the correct ratio. – Since 6. 02 X 1023 molecules is the number of particles in a mole, the coefficients could therefore represent the number of moles involved in a chemical reaction. – The gram formula weight of a compound is the mass in grams of one mole that is numerically equal to its formula weight. • The equation also describes the mass ratios of the reactants to products.