Molecules Compounds Chemical Reactions Overview Compounds Formulas Ionic
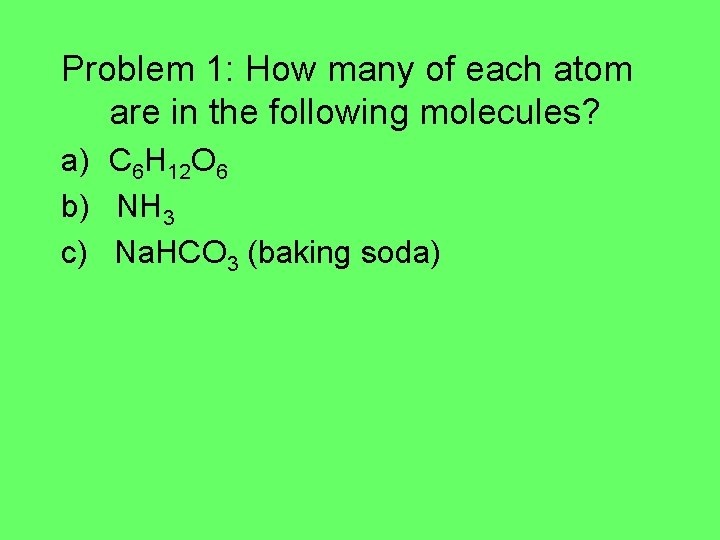




















- Slides: 101

Molecules, Compounds & Chemical Reactions Overview • Compounds – Formulas – Ionic compounds – Molecular (covalent) compounds • Molecular weight/molar mass • Covalent Bonds – Bonding models: Lewis, VSEPR, etc. – Molecular geometry and chemical properties • Chemical reactions – Balancing equations – Molar relationships

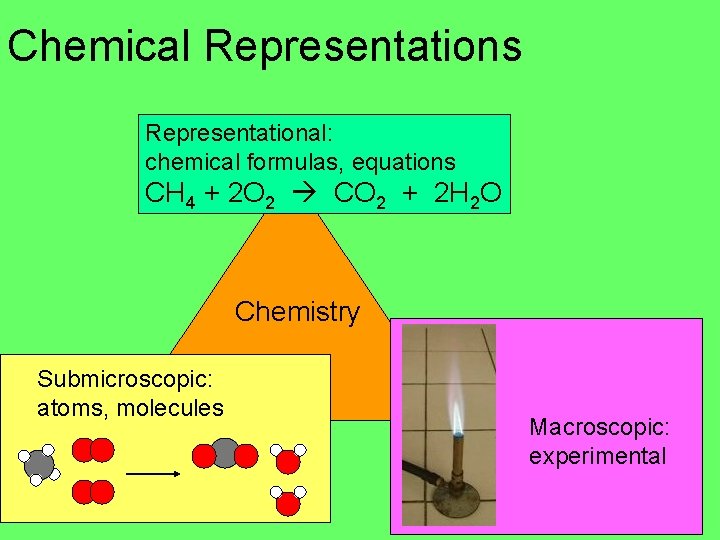
Chemical Representations Representational: chemical formulas, equations CH 4 + 2 O 2 CO 2 + 2 H 2 O Chemistry Submicroscopic: atoms, molecules Macroscopic: experimental

Compounds • Atoms are typically bound together in molecules • Noble gases exist as individual atoms, most other elements found as compounds • More stable – Forming a bond: releases energy, less stable to more stable – Breaking a bond: requires energy, more stable to less stable (misconception)

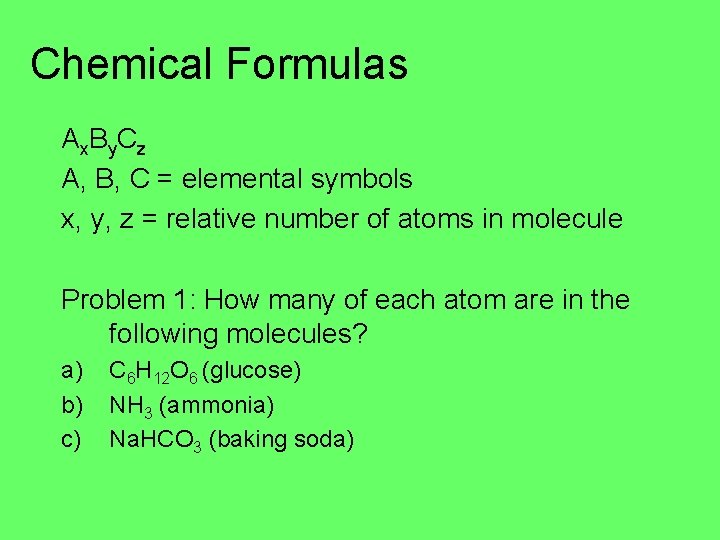
Chemical Formulas Ax. By. Cz A, B, C = elemental symbols x, y, z = relative number of atoms in molecule Problem 1: How many of each atom are in the following molecules? a) b) c) C 6 H 12 O 6 (glucose) NH 3 (ammonia) Na. HCO 3 (baking soda)
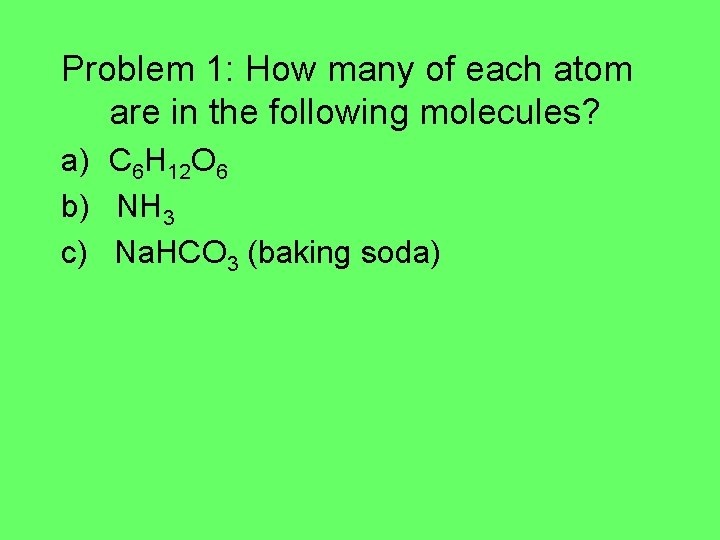
Problem 1: How many of each atom are in the following molecules? a) C 6 H 12 O 6 b) NH 3 c) Na. HCO 3 (baking soda)

Problem 1: How many of each atom are in the following molecules? a) C 6 H 12 O 6 6 carbons, 12 hydrogens, 6 oxygens b) NH 3 1 nitrogen, 3 hydrogens c) Na. HCO 3 (baking soda) 1 sodium, 1 hydrogen, 1 carbon, 3 oxygens

Ionic Compounds • Ionic compounds are made up of ions held together by electrostatic forces: ionic bonds • Cation (+) & anion (–) • Cation (metal) + anion (nonmetal: monatomic or polyatomic) Na+ + Cl– Na. Cl

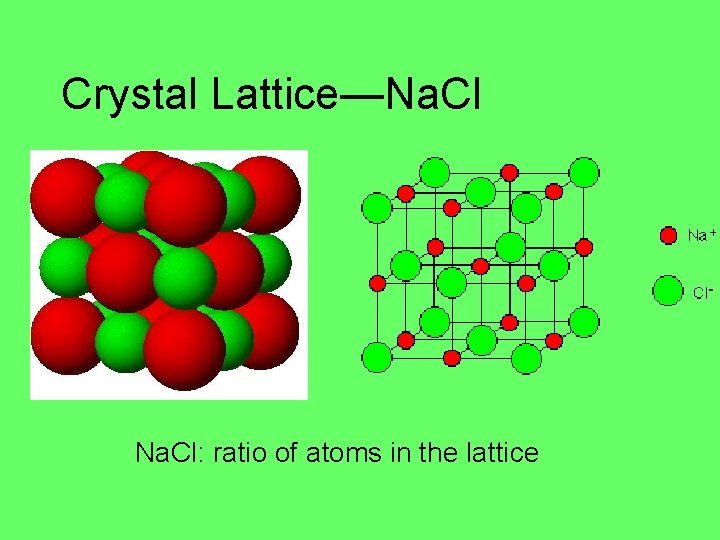
Crystal Lattice—Na. Cl: ratio of atoms in the lattice

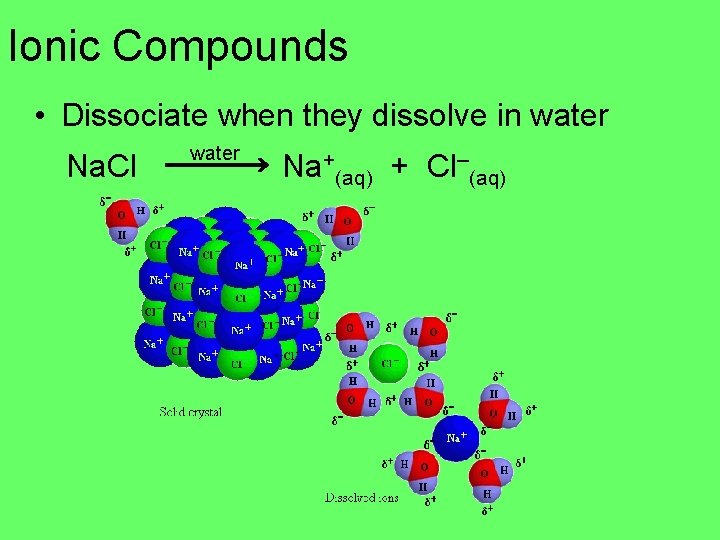
Ionic Compounds • Dissociate when they dissolve in water Na. Cl water Na+(aq) + Cl–(aq)


Ionic Compounds Monatomic ions: Cation: group number Li+, Na+, K+, Rb+, Cs+ Mg 2+, Ca 2+ Al 3+ Transition metals often variable: Cu 2+, Cu+ (copper II and copper I) and Fe 3+, Fe 2+ (iron III and iron II)

Ionic Compounds Monatomic ions: Anion: 8 – group number add to electrons to make 8 (octet) O O 2– oxide S S 2– sulfide Cl Cl– chloride P P 3– phosphide

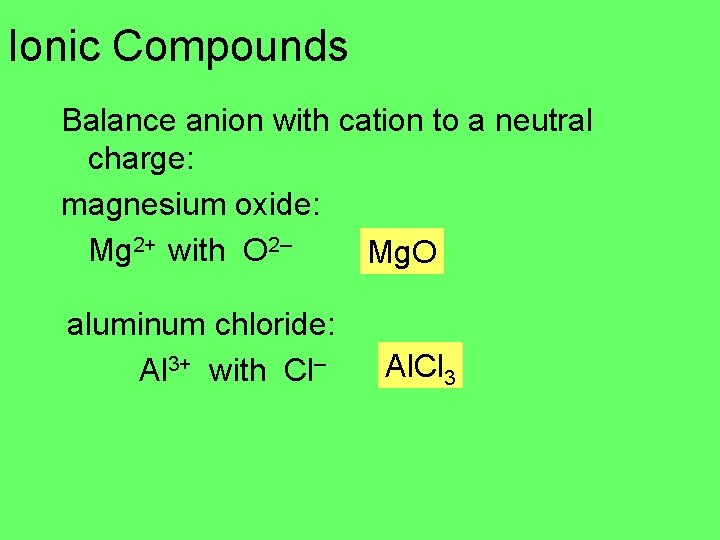
Ionic Compounds Balance anion with cation to a neutral charge: magnesium oxide: Mg 2+ with O 2– Mg. O aluminum chloride: Al 3+ with Cl– Al. Cl 3

Problem 2 Write the ionic formulas for each of the following: a) The compound that magnesium makes with chlorine b) The compound that Fe 3+ makes with oxygen c) Sodium fluoride d) Cesium oxide e) Copper (I) sulfide

Problem 2 Write the ionic formulas for each of the following: a) The compound that magnesium makes with chlorine Mg. Cl 2 b) The compound that Fe 3+ makes with oxygen Fe 2 O 3 c) Sodium fluoride Na. F d) Cesium oxide Cs 2 O e) Copper (I) sulfide Cu 2 S

Polyatomic Ions CO 32– carbonate ion Travels as a unit (Texans) Na 2 CO 3 sodium carbonate Fe 2(CO 3)3 iron (III) carbonate

Common Polyatomic Ions nitrate NO 3– nitrite NO 2– sulfate SO 42– sulfite SO 32– phosphate PO 43– carbonate CO 32– hydroxide OH– ammonium NH 4+

Problem 3: Write the ionic formulas for each of the following: a) Magnesium phosphate b) Ammonium carbonate c) Sodium sulfite Problem 4: Write the names for the following: a) Na. NO 3 b) K 2 SO 4 c) Fe. PO 4

Problem 3: Write the ionic formulas for each of the following: a) Magnesium phosphate Mg 2+ with PO 43– Mg 3(PO 4)2 b) Ammonium carbonate NH 4+ with CO 32– (NH 4)2 CO 3 c) Sodium sulfite Na+ with SO 32– Na 2 SO 3

Problem 4: Write the names for the following: a) Na. NO 3 sodium nitrate b) K 2 SO 4 potassium sulfate c) Fe. PO 4 iron (III) phosphate

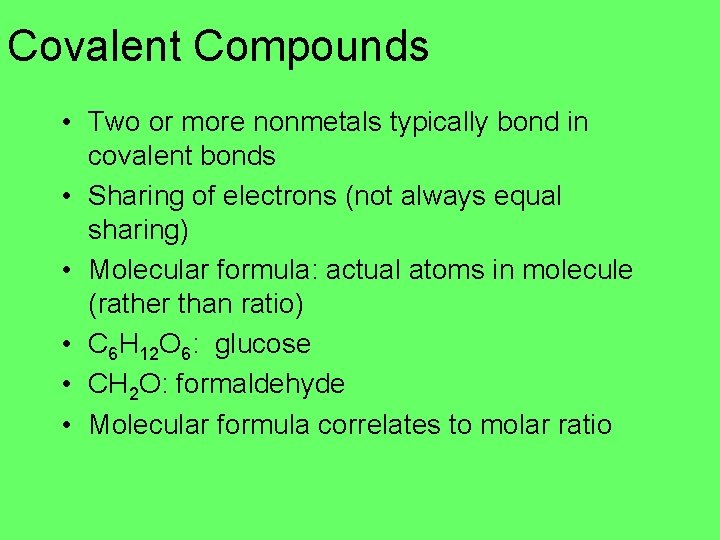
Covalent Compounds • Two or more nonmetals typically bond in covalent bonds • Sharing of electrons (not always equal sharing) • Molecular formula: actual atoms in molecule (rather than ratio) • C 6 H 12 O 6: glucose • CH 2 O: formaldehyde • Molecular formula correlates to molar ratio

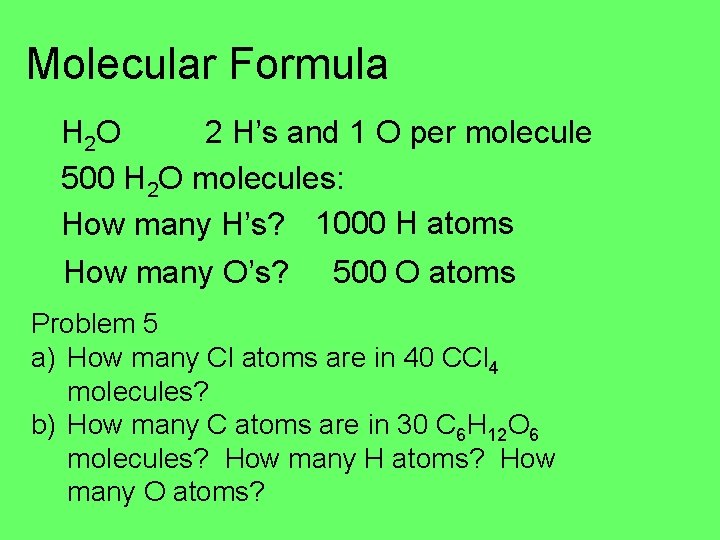
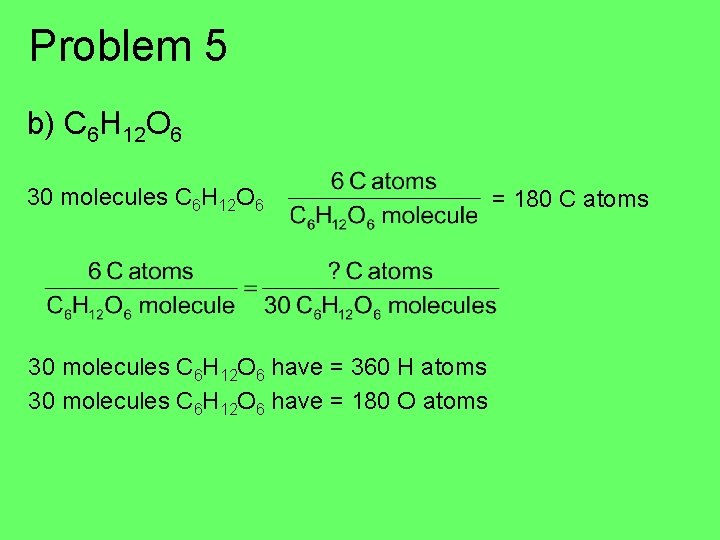
Molecular Formula H 2 O 2 H’s and 1 O per molecule 500 H 2 O molecules: How many H’s? 1000 H atoms How many O’s? 500 O atoms Problem 5 a) How many Cl atoms are in 40 CCl 4 molecules? b) How many C atoms are in 30 C 6 H 12 O 6 molecules? How many H atoms? How many O atoms?

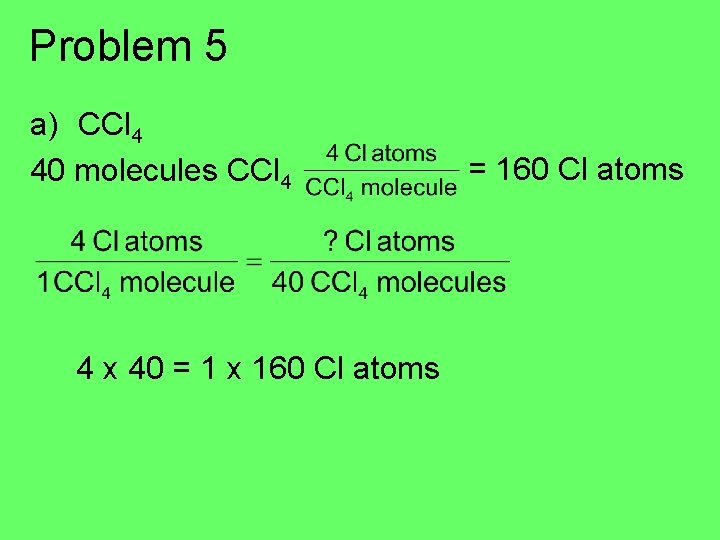
Problem 5 a) CCl 4 40 molecules CCl 4 4 x 40 = 1 x 160 Cl atoms = 160 Cl atoms

Problem 5 b) C 6 H 12 O 6 30 molecules C 6 H 12 O 6 have = 360 H atoms 30 molecules C 6 H 12 O 6 have = 180 O atoms = 180 C atoms

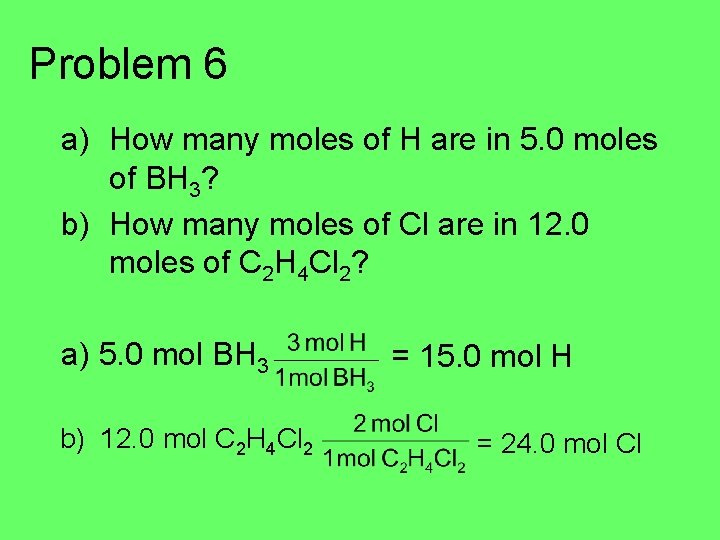
Problem 6 a) How many moles of H are in 5. 0 moles of BH 3? b) How many moles of Cl are in 12. 0 moles of C 2 H 4 Cl 2?

Problem 6 a) How many moles of H are in 5. 0 moles of BH 3? b) How many moles of Cl are in 12. 0 moles of C 2 H 4 Cl 2? a) 5. 0 mol BH 3 b) 12. 0 mol C 2 H 4 Cl 2 = 15. 0 mol H = 24. 0 mol Cl

Covalent Bonding • Sharing of valence electrons • Non-metals • Many models – Lewis structures: number and types of bonds – VSEPR (valence shell electron pair repulsion): empirical model that predicts molecular geometry – Valence Bond model: describes nature of bonds and predicts reactivity – Molecular Orbital theory: gold standard of understanding bonding, but requires high level of mathematics (calculus, group theory)

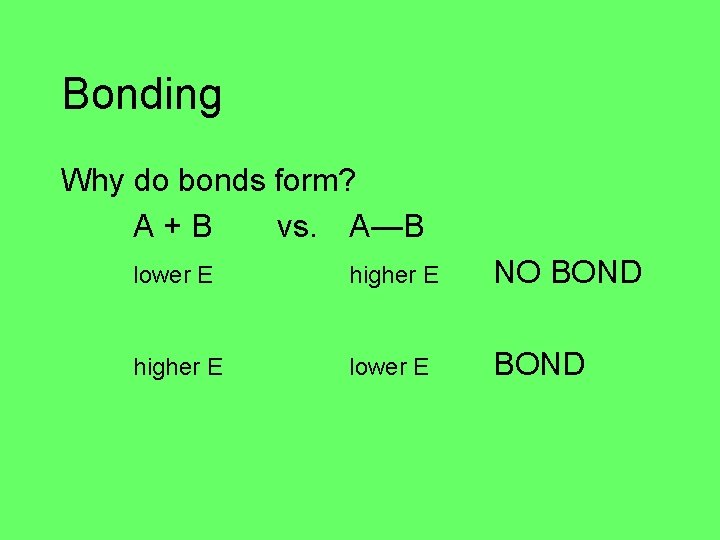
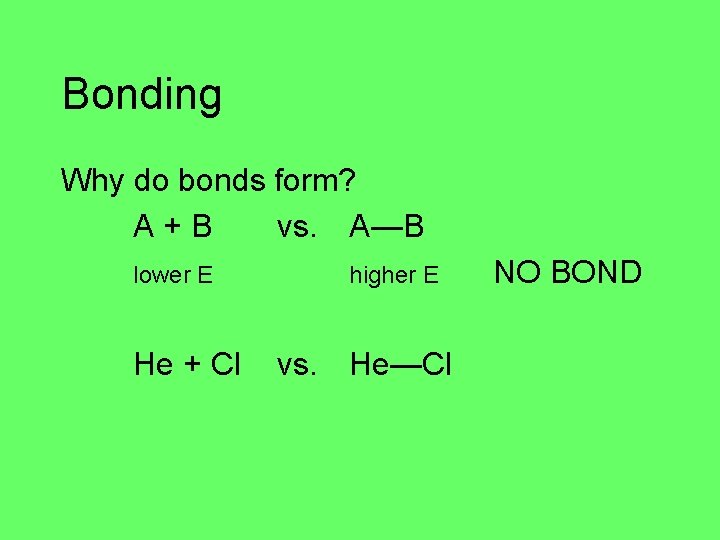
Bonding Why do bonds form? A+B vs. A—B lower E higher E NO BOND higher E lower E BOND

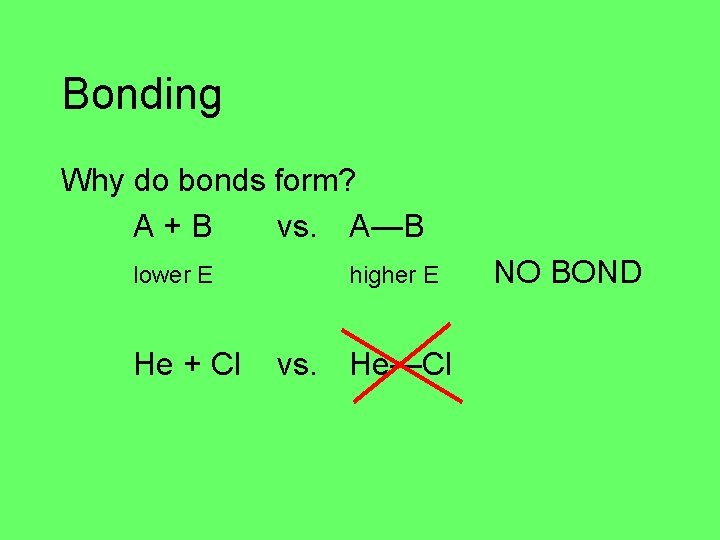
Bonding Why do bonds form? A+B vs. A—B lower E He + Cl higher E vs. He—Cl NO BOND

Bonding Why do bonds form? A+B vs. A—B lower E He + Cl higher E vs. He—Cl NO BOND

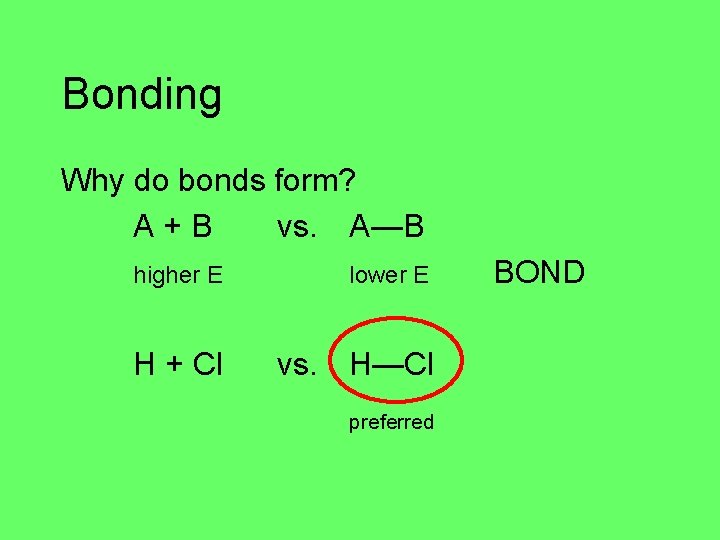
Bonding Why do bonds form? A+B vs. A—B higher E lower E H + Cl vs. H—Cl preferred BOND

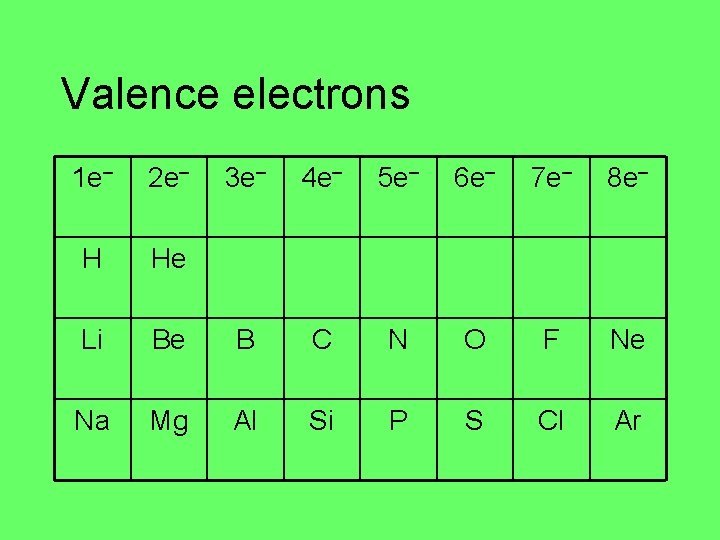
Valence electrons 1 e– 2 e– 3 e– 4 e– 5 e– 6 e– 7 e– 8 e– H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar

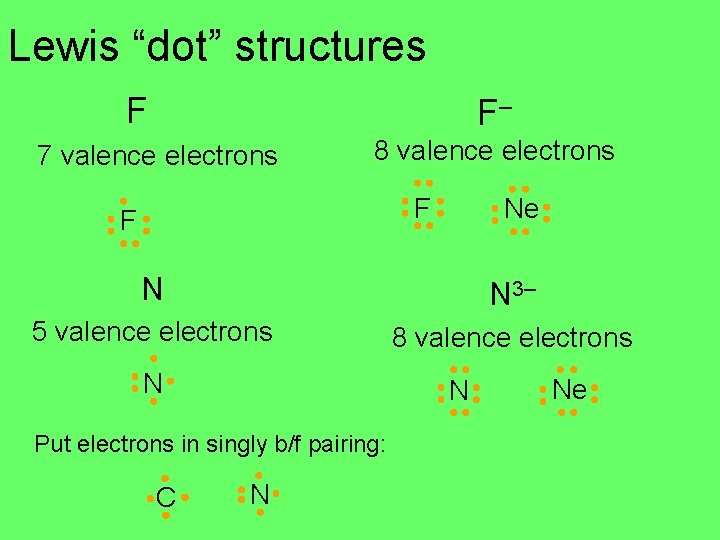
Lewis “dot” structures F F– 7 valence electrons 8 valence electrons Ne F F N N 3– 5 valence electrons 8 valence electrons N N Put electrons in singly b/f pairing: C N Ne

Problem 7 How many valence electrons would you expect each of the following to have? Draw a Lewis structure for each one. a) Sr b) Se c) I (iodine) d) K e) Cs

Problem 7 a) Sr: 2 valence electrons Sr b) Se: 6 valence electrons Se c) I: 7 valence electrons I d) K: 1 valence electron K e) Cs: 1 valence electron Cs

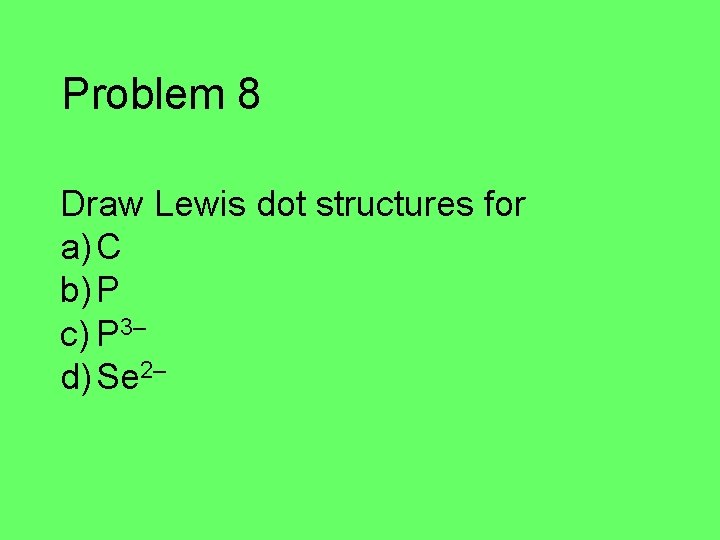
Problem 8 Draw Lewis dot structures for a) C b) P c) P 3– d) Se 2–

Problem 8 a) C: 4 valence electrons C b) P: 5 valence electrons P c) P 3–: 8 valence electrons P d) Se 2 -: 8 valence electrons Se

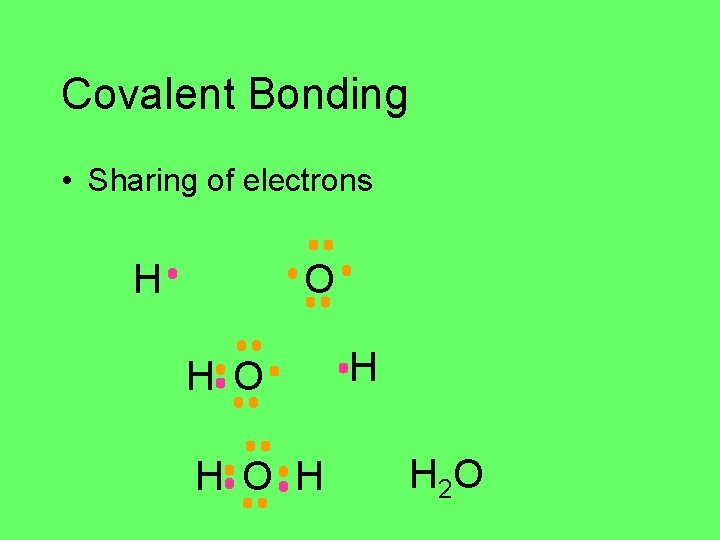
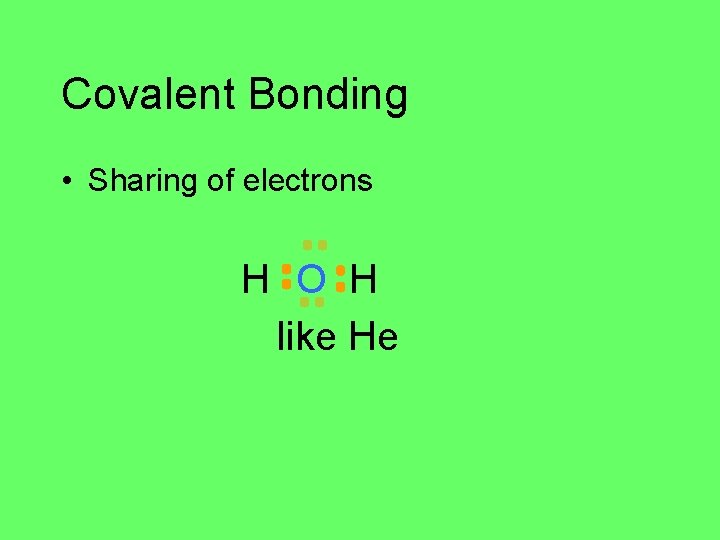
Covalent Bonding • Sharing of electrons H O H O H H H 2 O

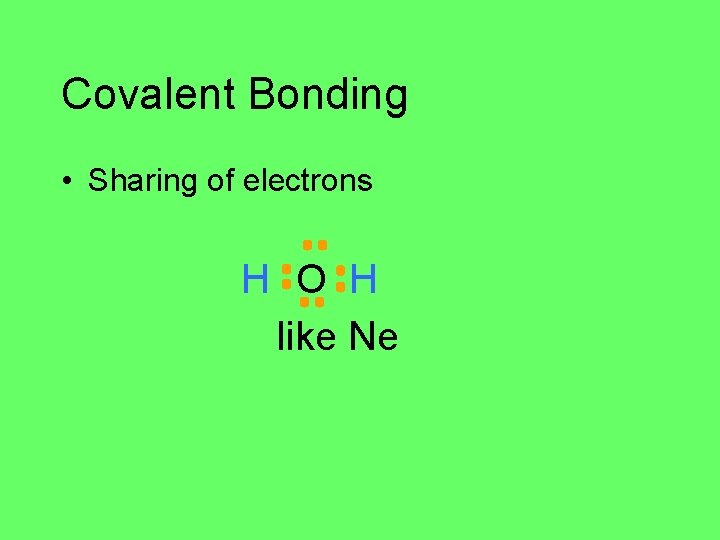
Covalent Bonding • Sharing of electrons H O H like Ne

Covalent Bonding • Sharing of electrons H O H like He

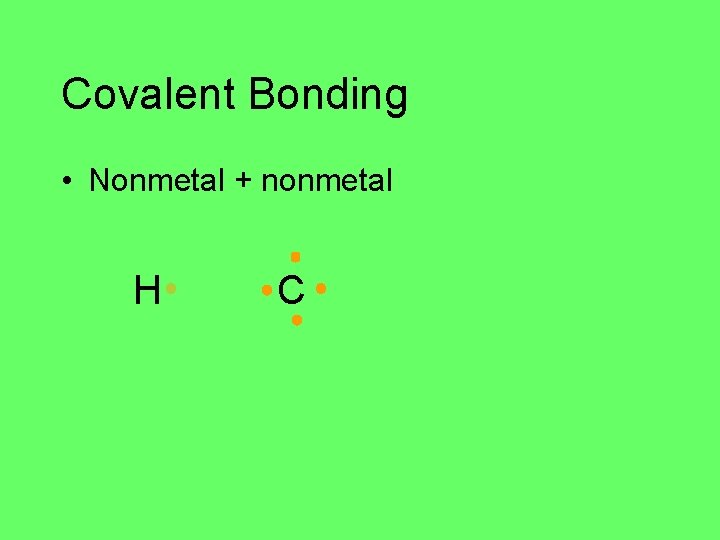
Covalent Bonding • Nonmetal + nonmetal H C

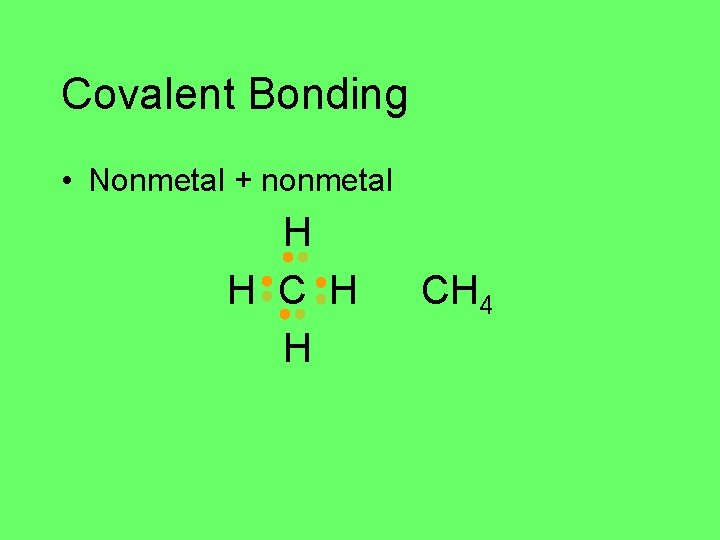
Covalent Bonding • Nonmetal + nonmetal H H CH 4

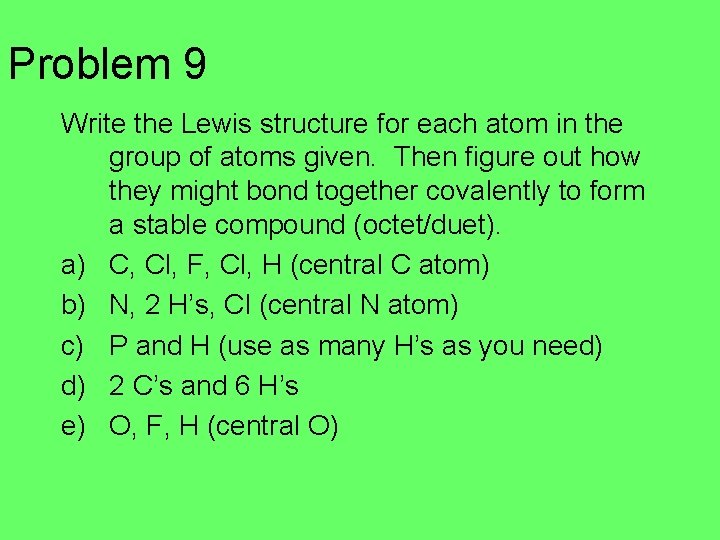
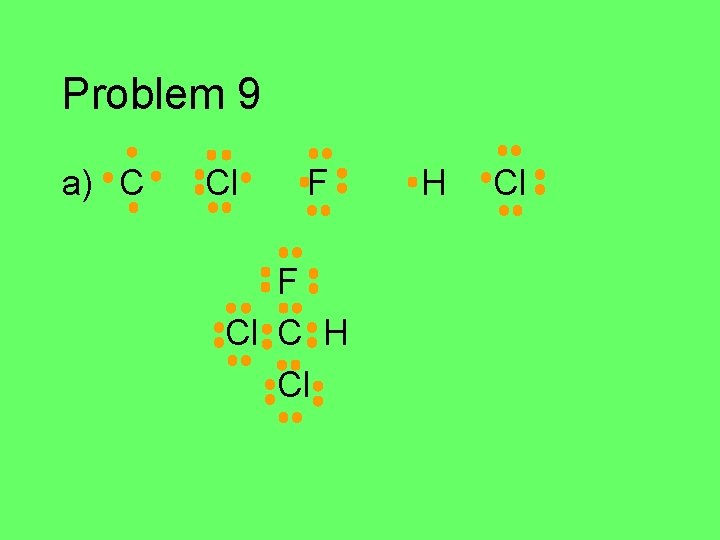
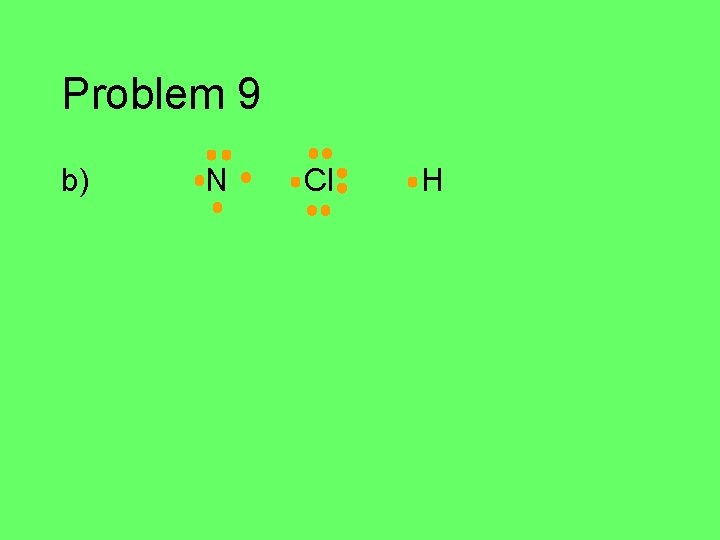
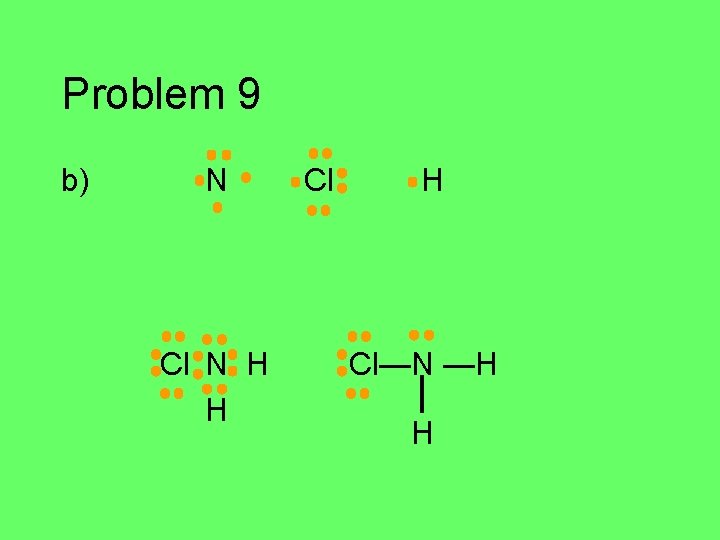
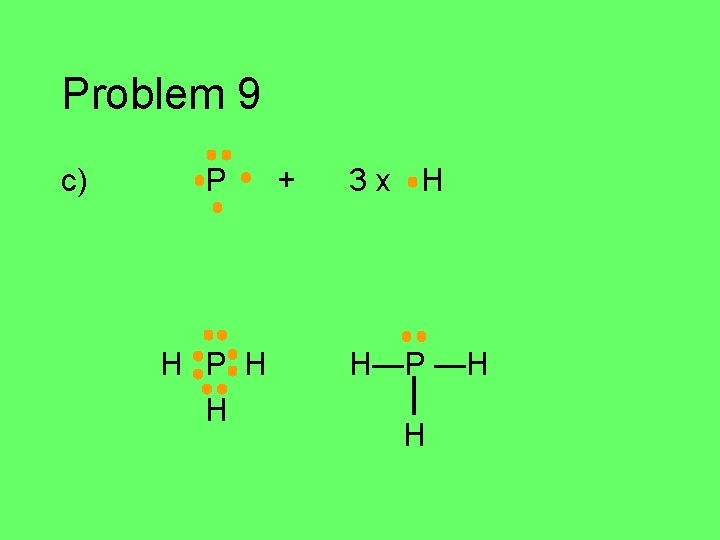
Problem 9 Write the Lewis structure for each atom in the group of atoms given. Then figure out how they might bond together covalently to form a stable compound (octet/duet). a) C, Cl, F, Cl, H (central C atom) b) N, 2 H’s, Cl (central N atom) c) P and H (use as many H’s as you need) d) 2 C’s and 6 H’s e) O, F, H (central O)

Problem 9 a) C Cl F F Cl C H Cl

Problem 9 b) N Cl H

Problem 9 b) N Cl N H H Cl—N —H H

Problem 9 c) P H H + 3 x H H—P —H H

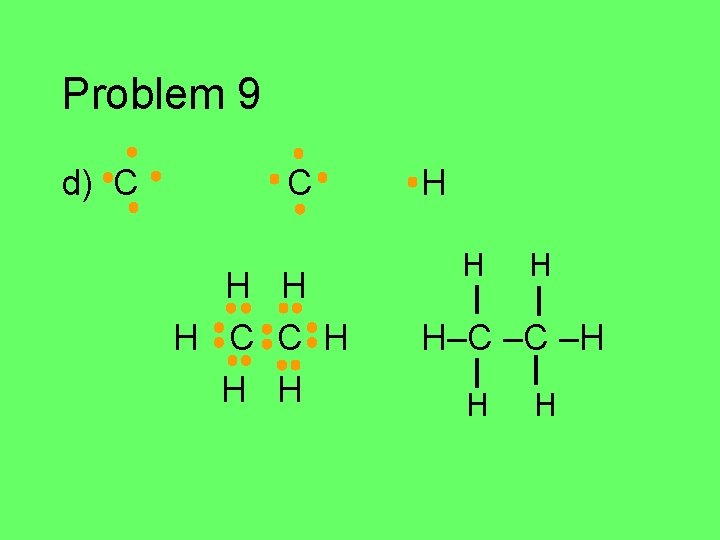
Problem 9 d) C C H H H H H–C –C –H H H

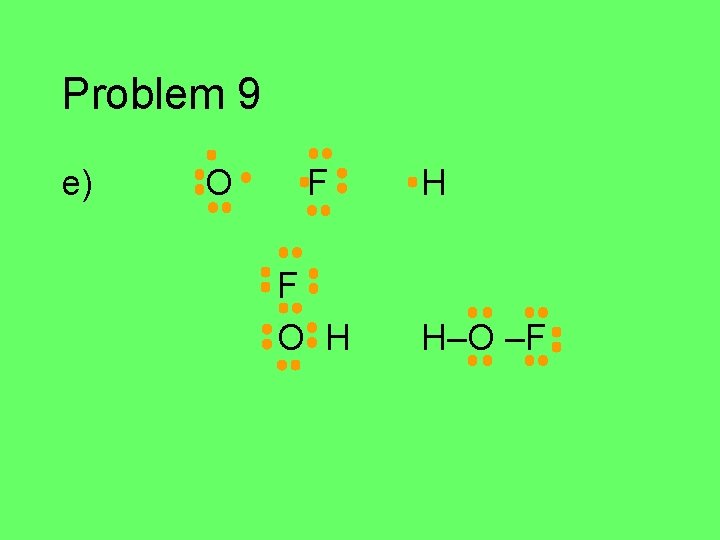
Problem 9 e) O F F O H H H–O –F

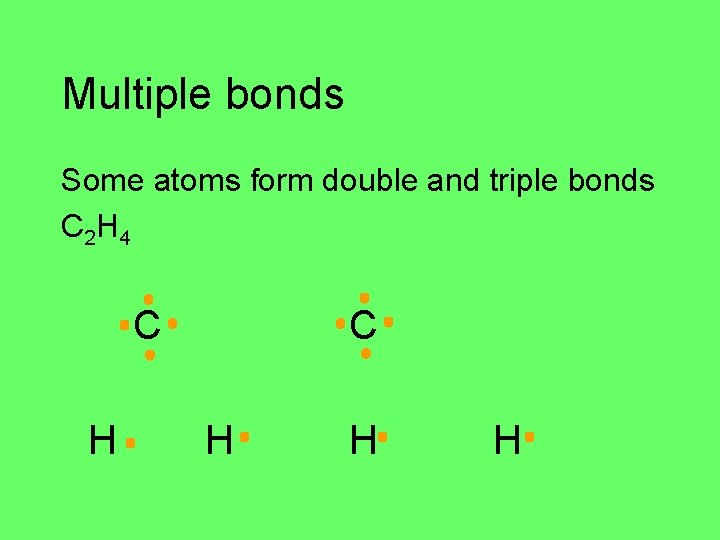
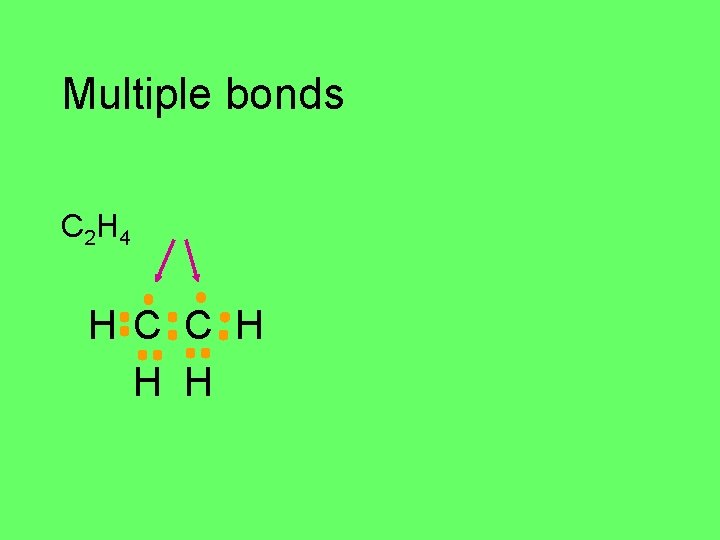
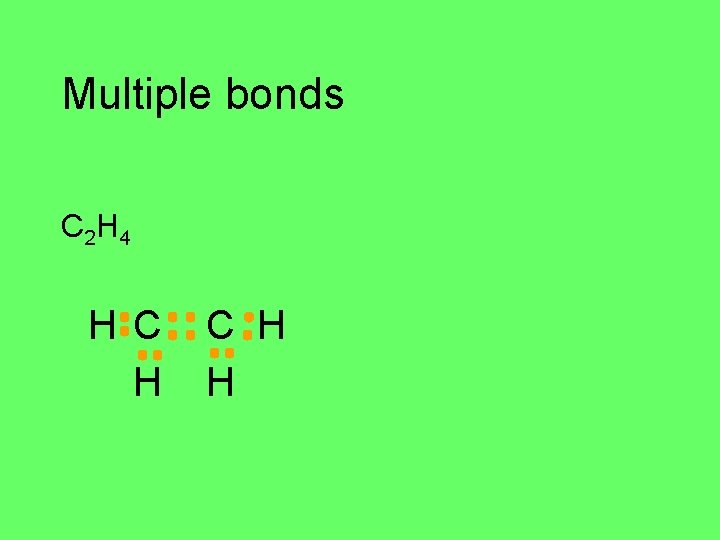
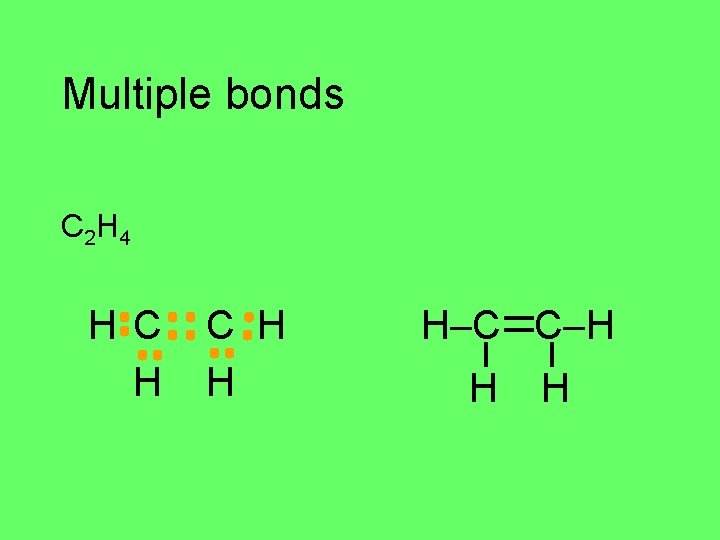
Multiple bonds Some atoms form double and triple bonds C 2 H 4 C H H H

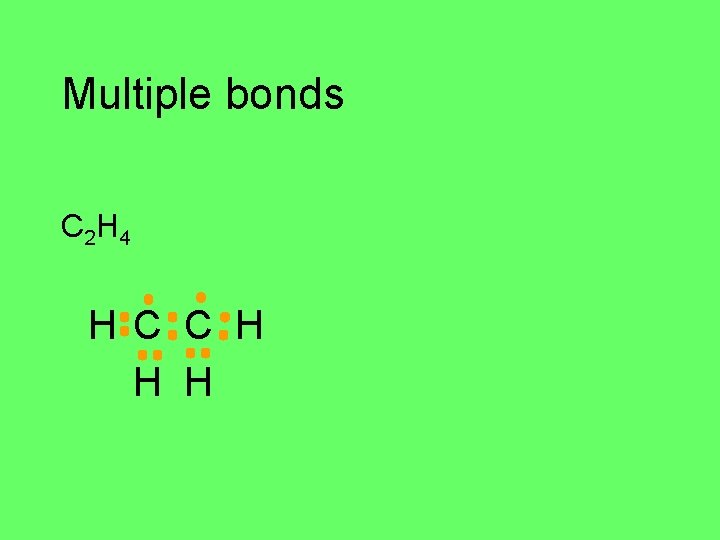
Multiple bonds C 2 H 4 H C C H H H

Multiple bonds C 2 H 4 H C C H H H

Multiple bonds C 2 H 4 H C H H

Multiple bonds C 2 H 4 H C H H H–C C–H H H

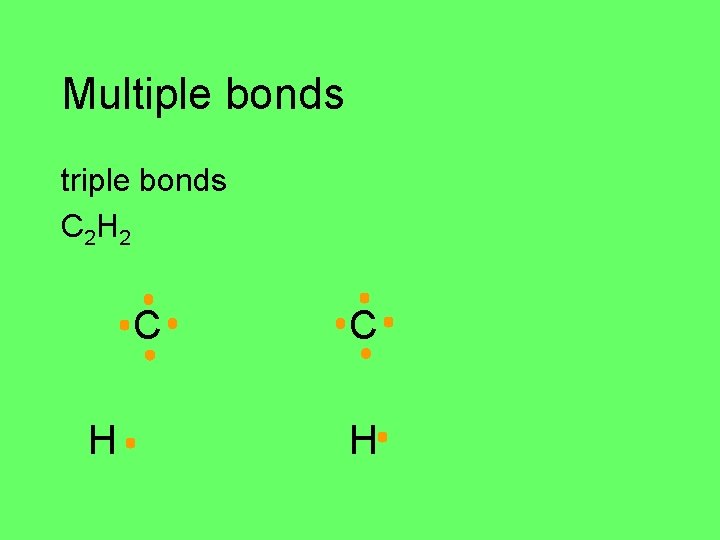
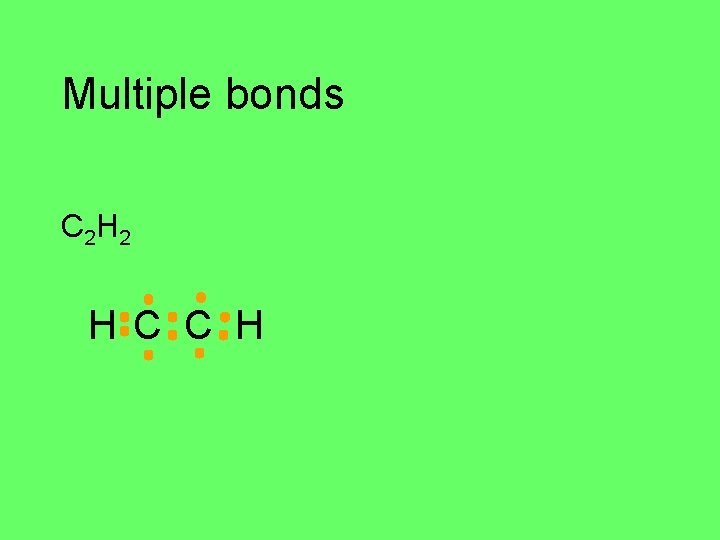
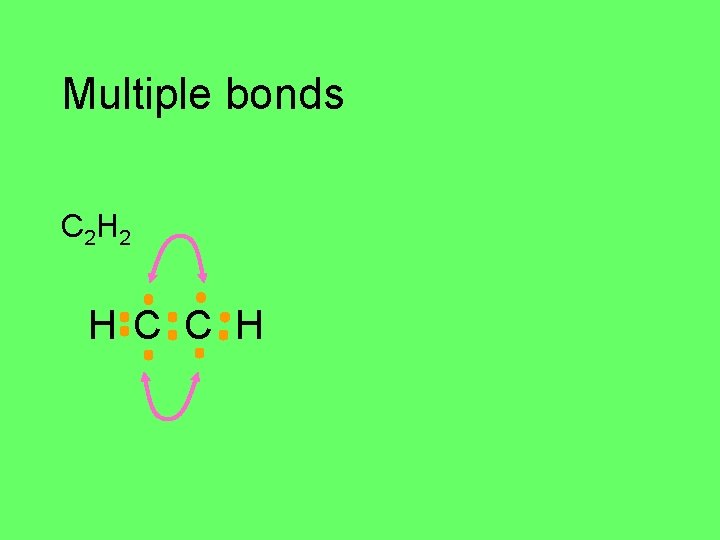
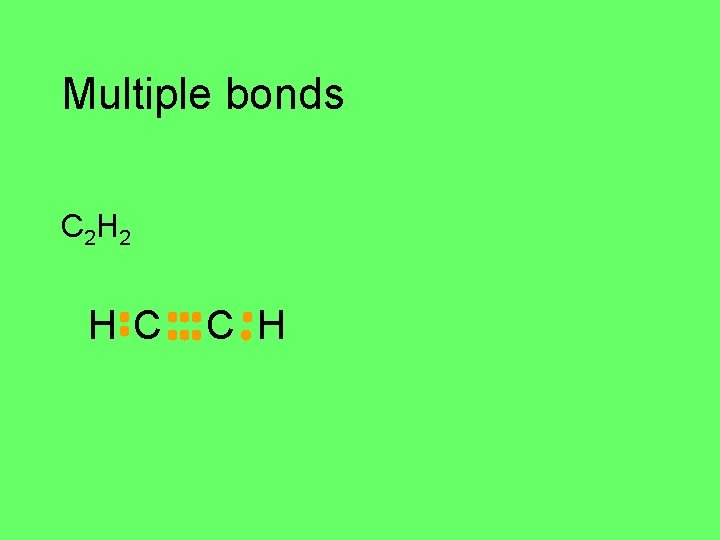
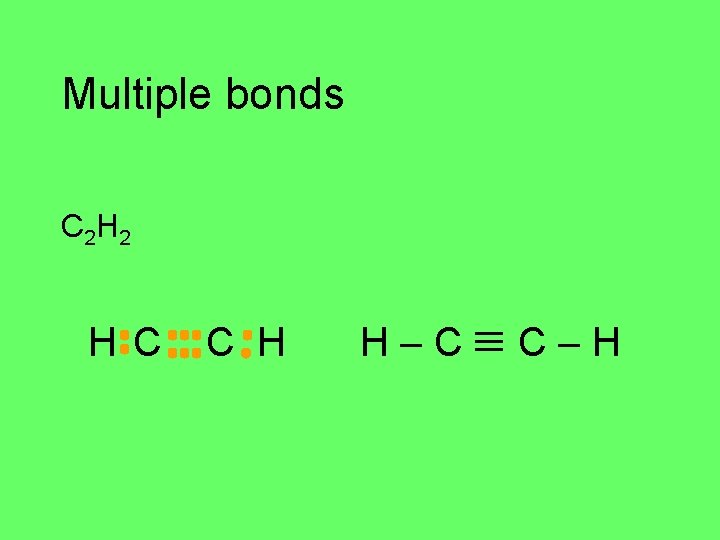
Multiple bonds triple bonds C 2 H 2 C H

Multiple bonds C 2 H 2 H C C H

Multiple bonds C 2 H 2 H C C H

Multiple bonds C 2 H 2 H C C H

Multiple bonds C 2 H 2 H C C H H–C C–H

Covalent Bonds Atom… has… C, Si N, P O, S, Se F, Cl, Br, I 4 valence electrons 5 valence electrons 6 valence electrons 7 valence electrons typically forms… 4 bonds 3 bonds 2 bonds 1 bond

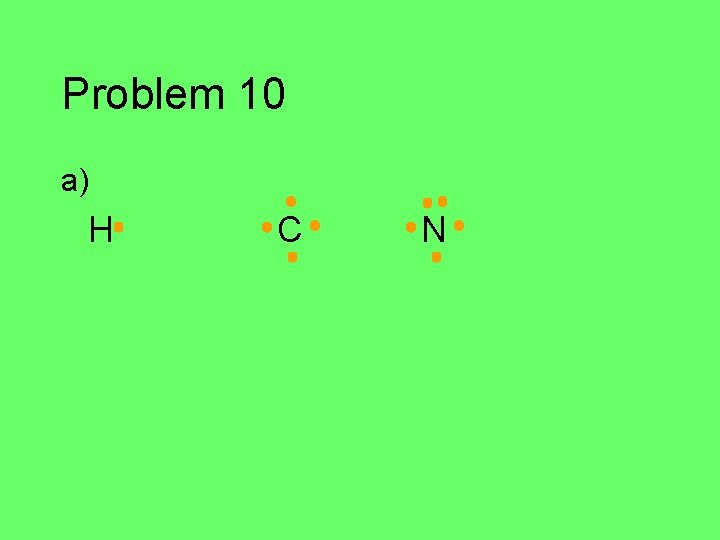
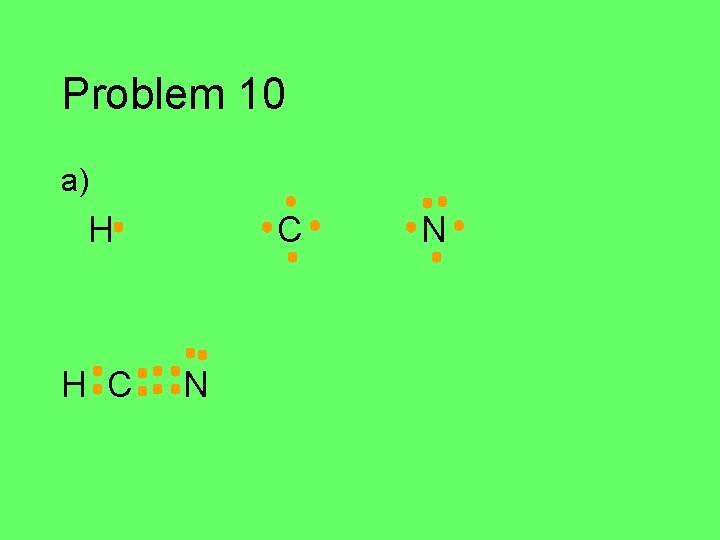
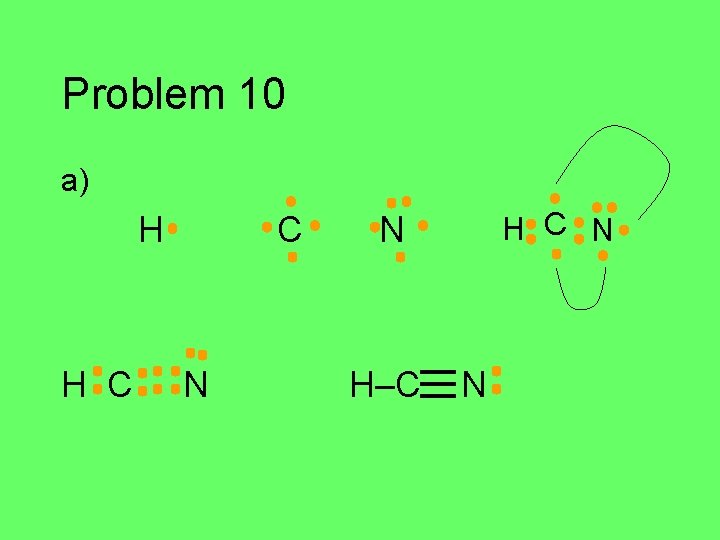
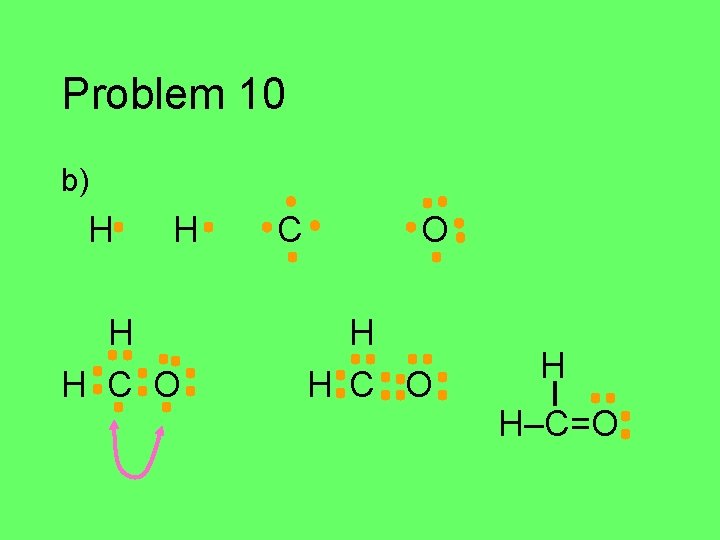
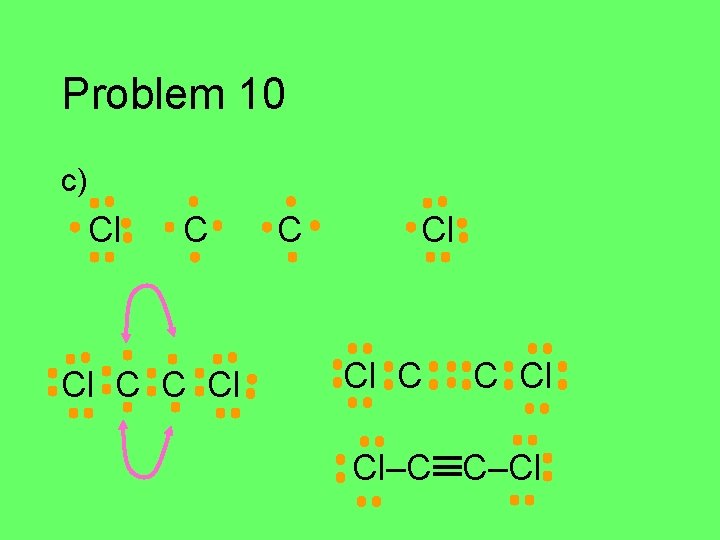
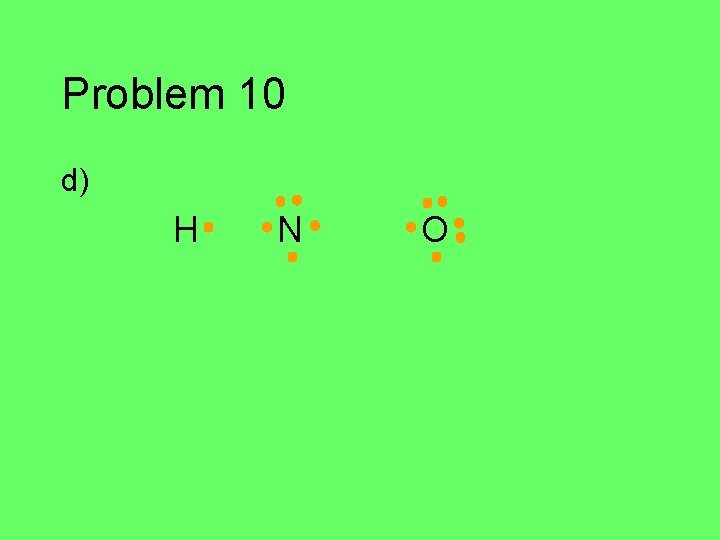
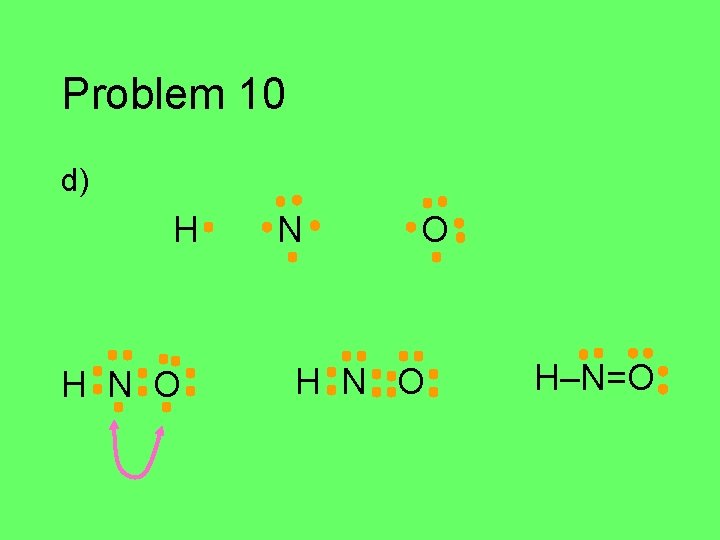
Problem 10 Draw the Lewis structure for each of the atoms in the formula below. Then draw the Lewis structure for the molecule. a) HCN (central C) b) CH 2 O (central C) c) C 2 Cl 2 d) HNO (central N)

Problem 10 a) H C N

Problem 10 a) H H C C N N

Problem 10 a) H H C C N H C N N H–C N

Problem 10 b) H H C O H H–C=O

Problem 10 c) Cl C C Cl Cl–C C–Cl

Problem 10 d) H N O

Problem 10 d) H H N O H–N=O

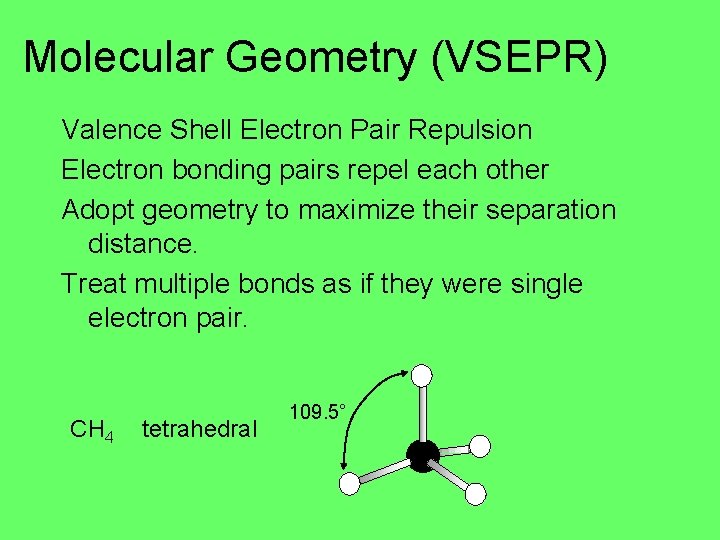
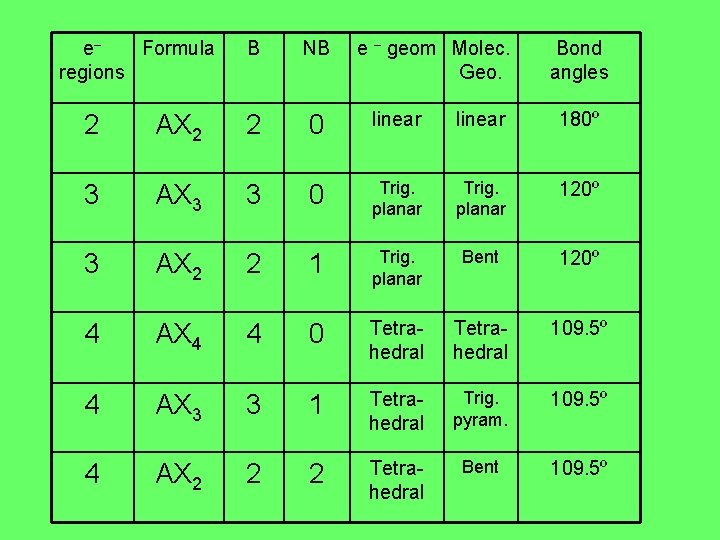
Molecular Geometry (VSEPR) Valence Shell Electron Pair Repulsion Electron bonding pairs repel each other Adopt geometry to maximize their separation distance. Treat multiple bonds as if they were single electron pair. CH 4 tetrahedral 109. 5°

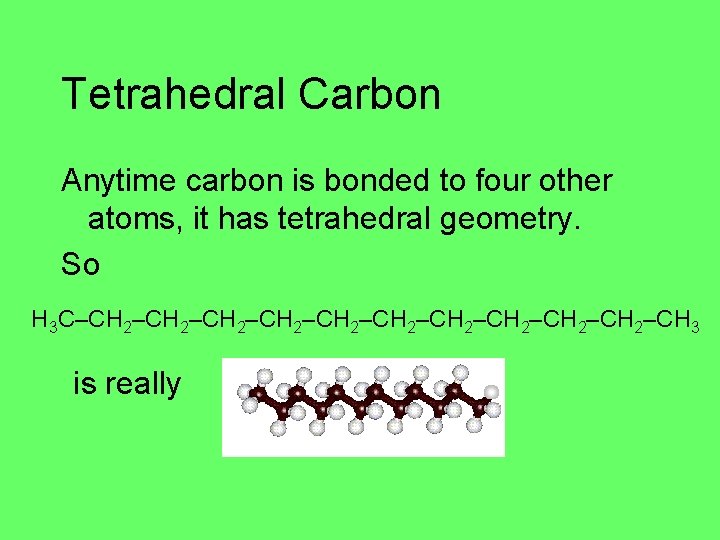
Tetrahedral Carbon Anytime carbon is bonded to four other atoms, it has tetrahedral geometry. So H 3 C–CH 2–CH 2–CH 2–CH 3 is really

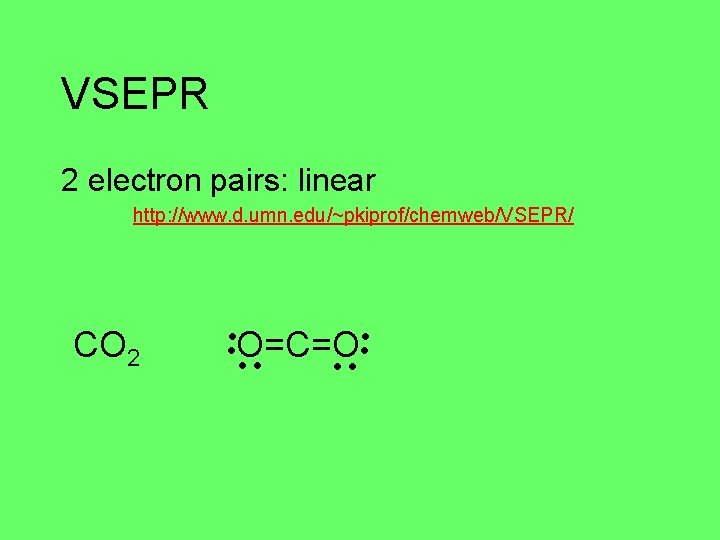
VSEPR 2 electron pairs: linear http: //www. d. umn. edu/~pkiprof/chemweb/VSEPR/ CO 2 O=C=O

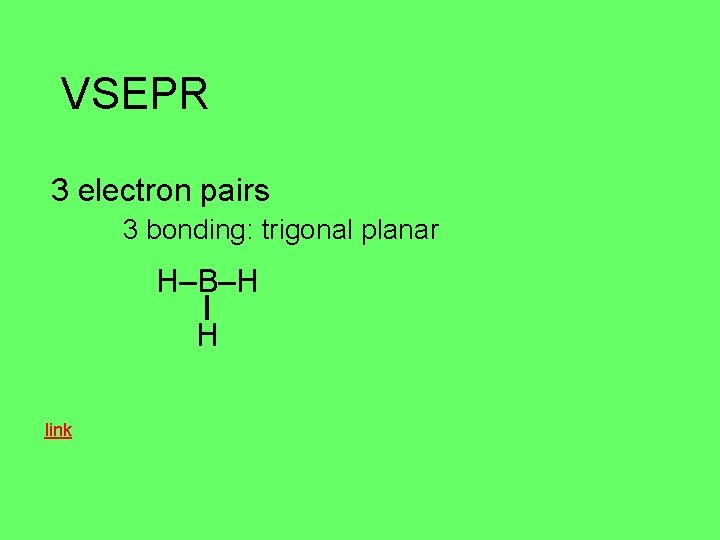
VSEPR 3 electron pairs 3 bonding: trigonal planar H–B–H H link

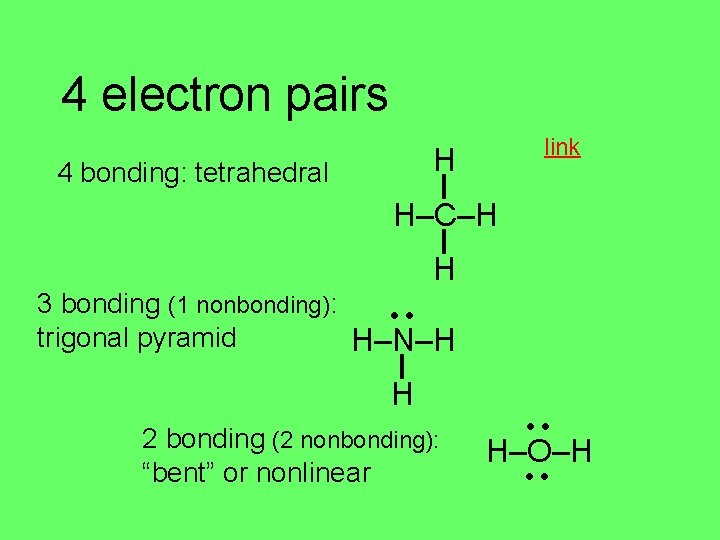
4 electron pairs link H 4 bonding: tetrahedral H–C–H H 3 bonding (1 nonbonding): trigonal pyramid H–N–H H 2 bonding (2 nonbonding): “bent” or nonlinear H–O–H

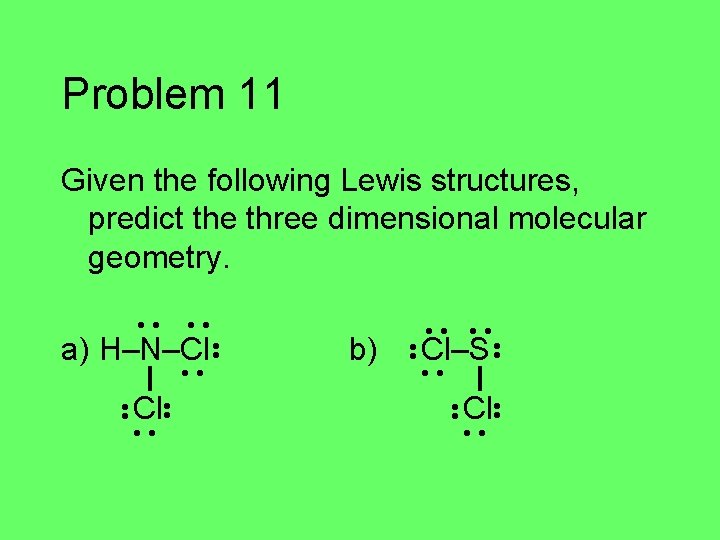
Problem 11 Given the following Lewis structures, predict the three dimensional molecular geometry. • • a) H–N–Cl • • Cl • • b) • • Cl–S • • Cl • •

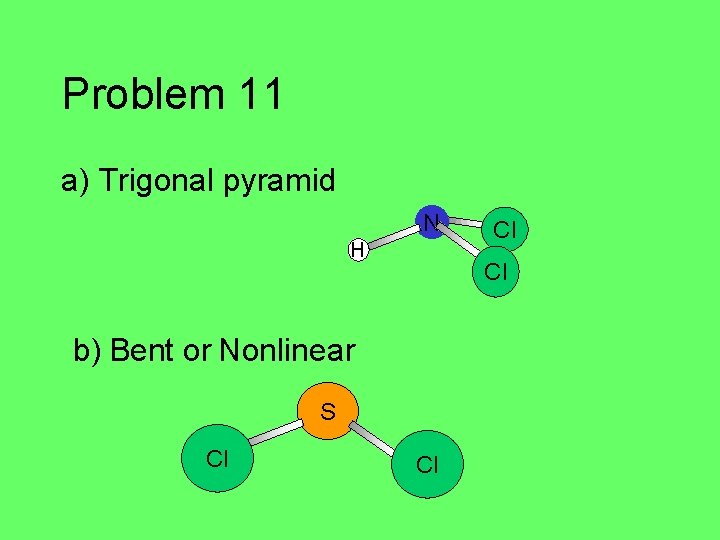
Problem 11 a) Trigonal pyramid N H Cl b) Bent or Nonlinear S Cl Cl Cl

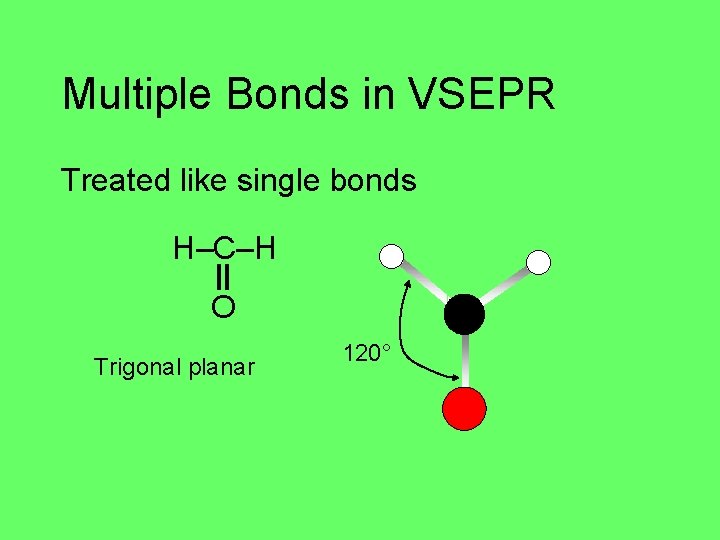
Multiple Bonds in VSEPR Treated like single bonds H–C–H O Trigonal planar 120°

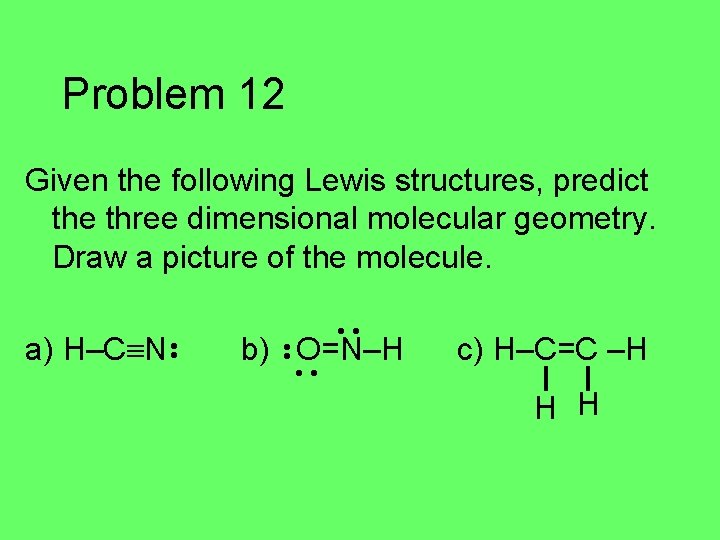
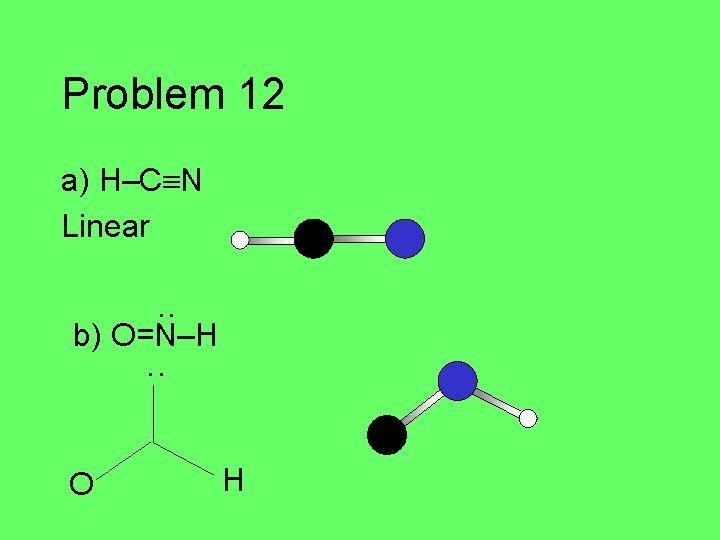
Problem 12 Given the following Lewis structures, predict the three dimensional molecular geometry. Draw a picture of the molecule. a) H–C N • • b) • • O=N–H • • c) H–C=C –H H H

Problem 12 a) H–C N Linear ·· b) O=N–H ·· O H

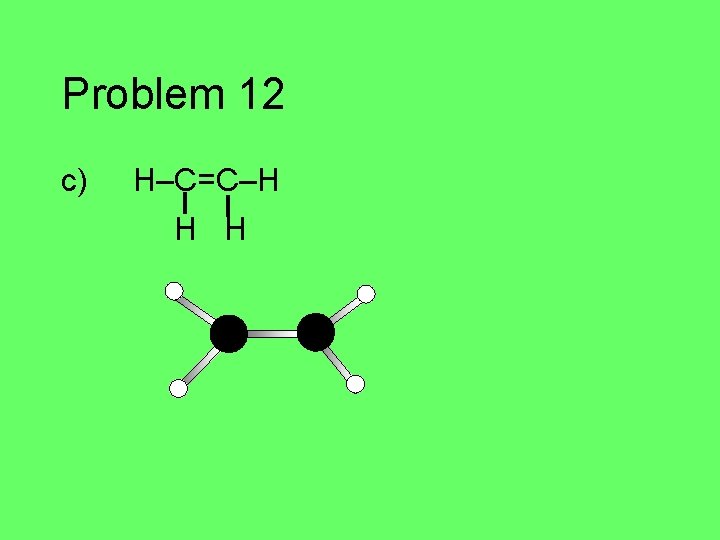
Problem 12 c) H–C=C–H H H

e– Formula regions B NB e – geom Molec. Geo. Bond angles 2 AX 2 2 0 linear 180º 3 AX 3 3 0 Trig. planar 120º 3 AX 2 2 1 Trig. planar Bent 120º 4 AX 4 4 0 Tetrahedral 109. 5º 4 AX 3 3 1 Tetrahedral Trig. pyram. 109. 5º 4 AX 2 2 2 Tetrahedral Bent 109. 5º

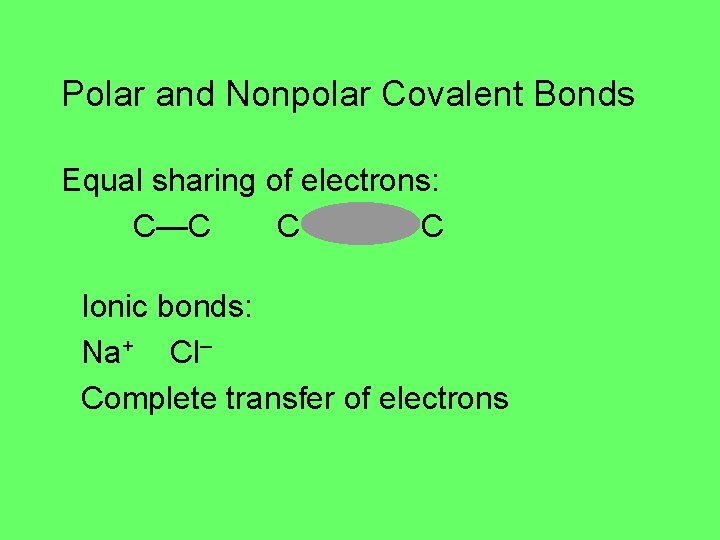
Polar and Nonpolar Covalent Bonds Equal sharing of electrons: C—C C C Ionic bonds: Na+ Cl– Complete transfer of electrons

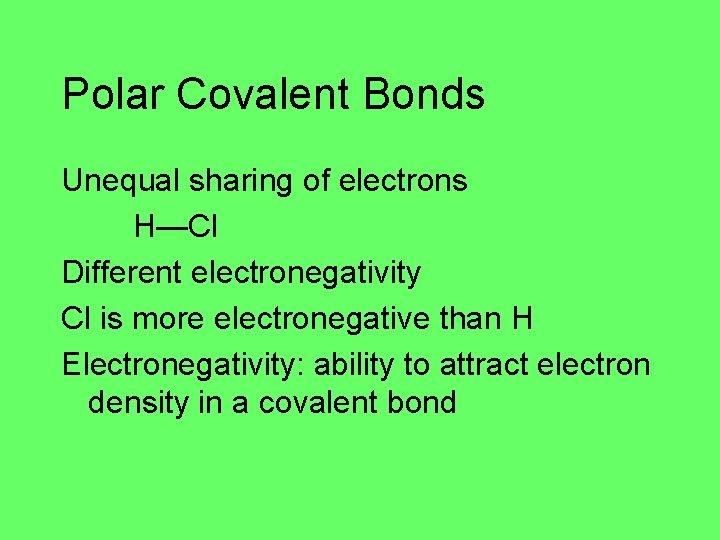
Polar Covalent Bonds Unequal sharing of electrons H—Cl Different electronegativity Cl is more electronegative than H Electronegativity: ability to attract electron density in a covalent bond

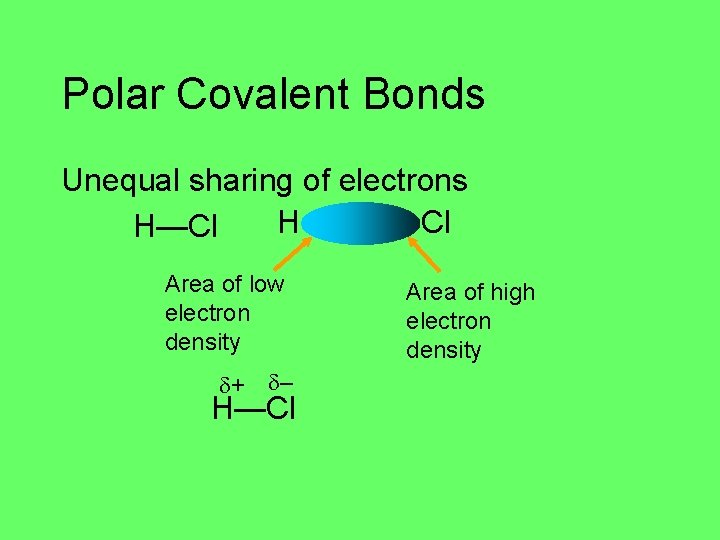
Polar Covalent Bonds Unequal sharing of electrons H Cl H—Cl Area of low electron density + – H—Cl Area of high electron density

Polar Covalent Bonds

Problem 13 Which of the following pairs of atoms is the more electronegative? P or F Sr or Si Se or Cs

Problem 13 Which of the following pairs of atoms is the more electronegative? P or F Sr or Si Se or Cs

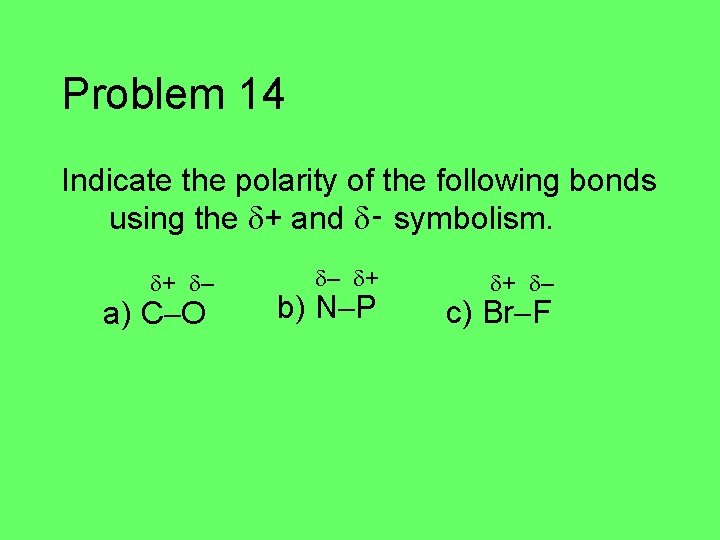
Problem 14 Indicate the polarity of the following bonds using the + and ‑ symbolism. a) C O b) N P c) Br F

Problem 14 Indicate the polarity of the following bonds using the + and ‑ symbolism. + – a) C O – + b) N P + – c) Br F

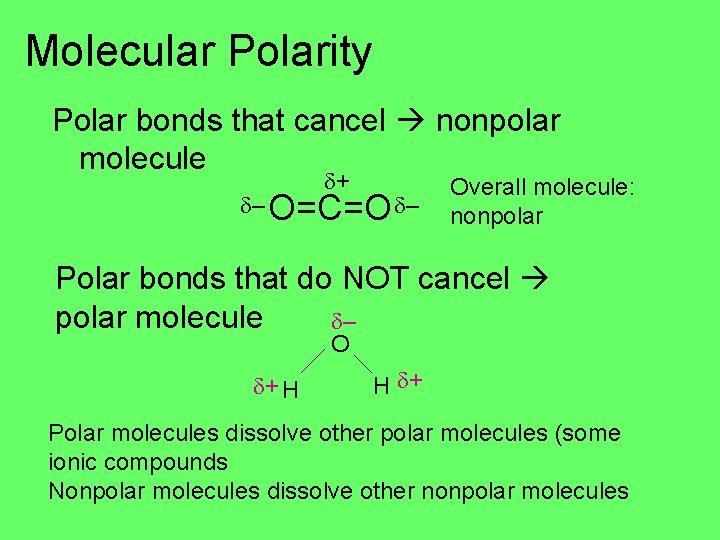
Molecular Polarity Polar bonds that cancel nonpolar molecule + – O=C=O – Overall molecule: nonpolar Polar bonds that do NOT cancel polar molecule – O + H H + Polar molecules dissolve other polar molecules (some ionic compounds Nonpolar molecules dissolve other nonpolar molecules

Chemical Reactions • Chemical reactions occur when bonds break and reform in different arrangements • Can absorb or release heat • Overall energy needs to be “downhill” (more stable products)

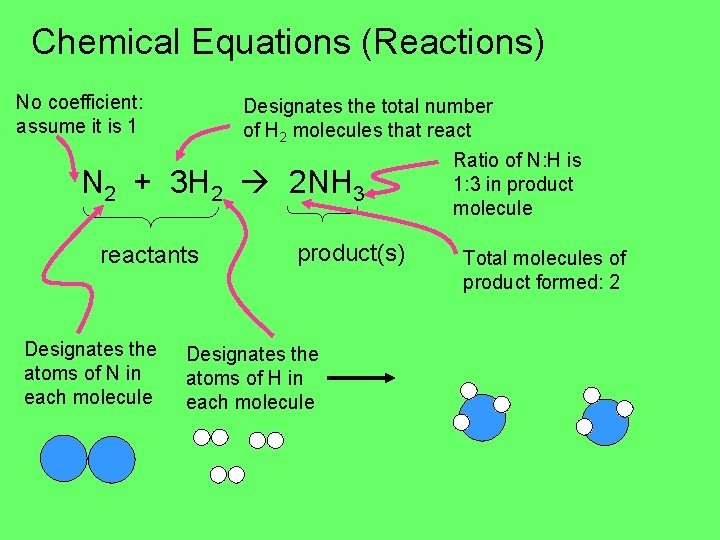
Chemical Equations (Reactions) No coefficient: assume it is 1 Designates the total number of H 2 molecules that react N 2 + 3 H 2 2 NH 3 reactants Designates the atoms of N in each molecule product(s) Designates the atoms of H in each molecule Ratio of N: H is 1: 3 in product molecule Total molecules of product formed: 2

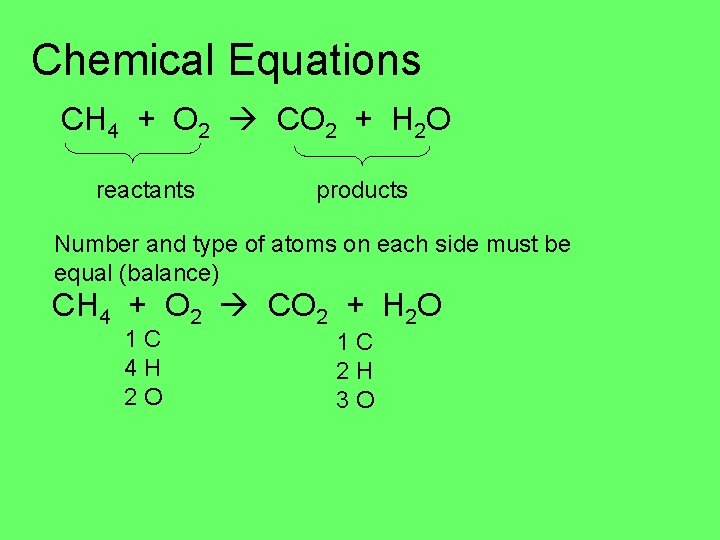
Chemical Equations CH 4 + O 2 CO 2 + H 2 O reactants products Number and type of atoms on each side must be equal (balance) CH 4 + O 2 CO 2 + H 2 O 1 C 4 H 2 O 1 C 2 H 3 O

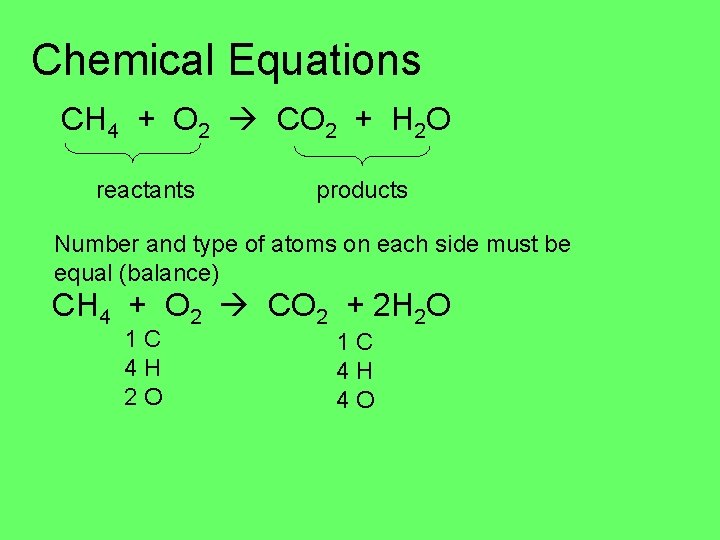
Chemical Equations CH 4 + O 2 CO 2 + H 2 O reactants products Number and type of atoms on each side must be equal (balance) CH 4 + O 2 CO 2 + 2 H 2 O 1 C 4 H 4 O

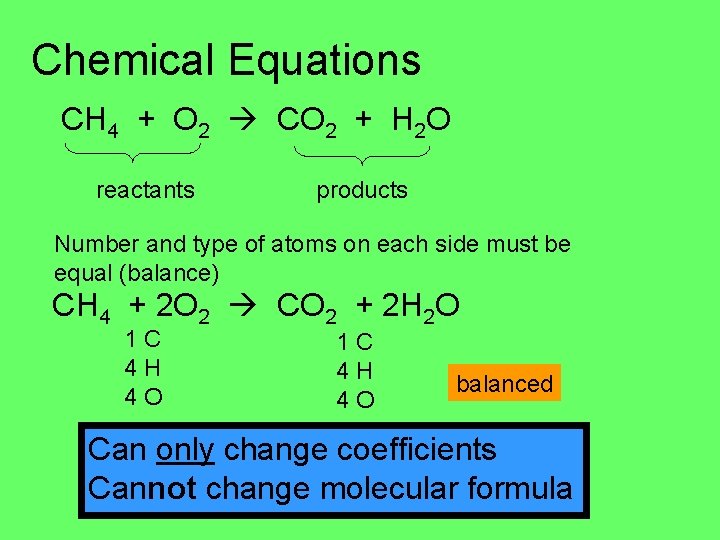
Chemical Equations CH 4 + O 2 CO 2 + H 2 O reactants products Number and type of atoms on each side must be equal (balance) CH 4 + 2 O 2 CO 2 + 2 H 2 O 1 C 4 H 4 O balanced Can only change coefficients Cannot change molecular formula

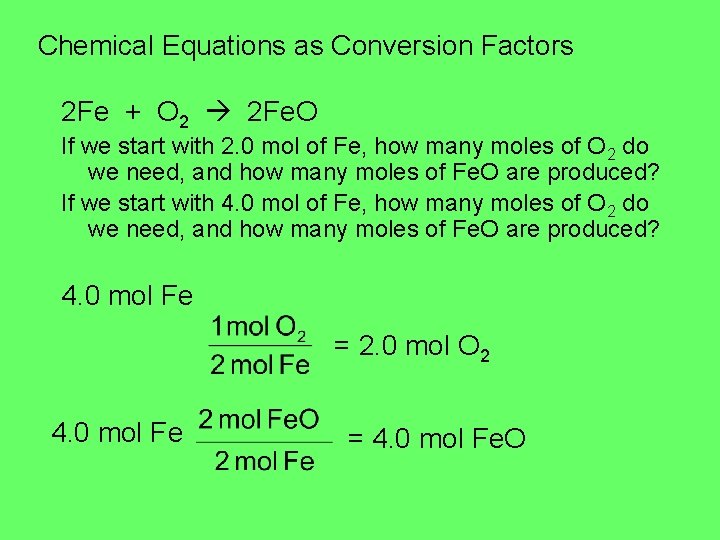
Chemical Equations as Conversion Factors 2 Fe + O 2 2 Fe. O If we start with 2. 0 mol of Fe, how many moles of O 2 do we need, and how many moles of Fe. O are produced? If we start with 4. 0 mol of Fe, how many moles of O 2 do we need, and how many moles of Fe. O are produced? 4. 0 mol Fe = 2. 0 mol O 2 4. 0 mol Fe = 4. 0 mol Fe. O

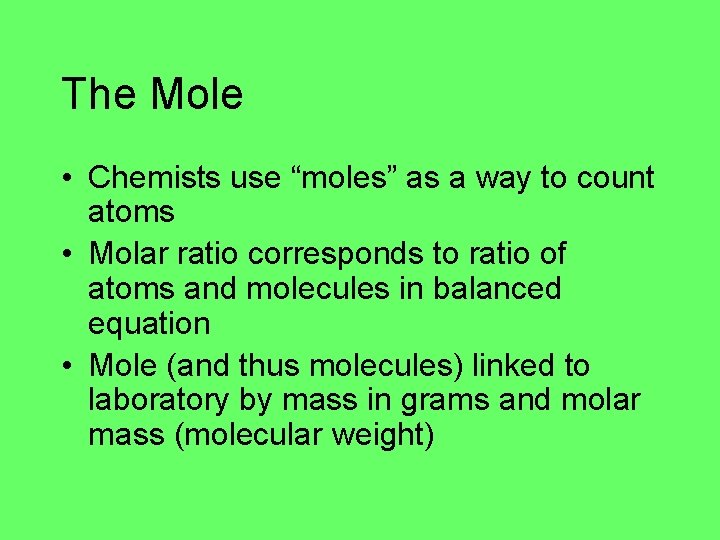
The Mole • Chemists use “moles” as a way to count atoms • Molar ratio corresponds to ratio of atoms and molecules in balanced equation • Mole (and thus molecules) linked to laboratory by mass in grams and molar mass (molecular weight)

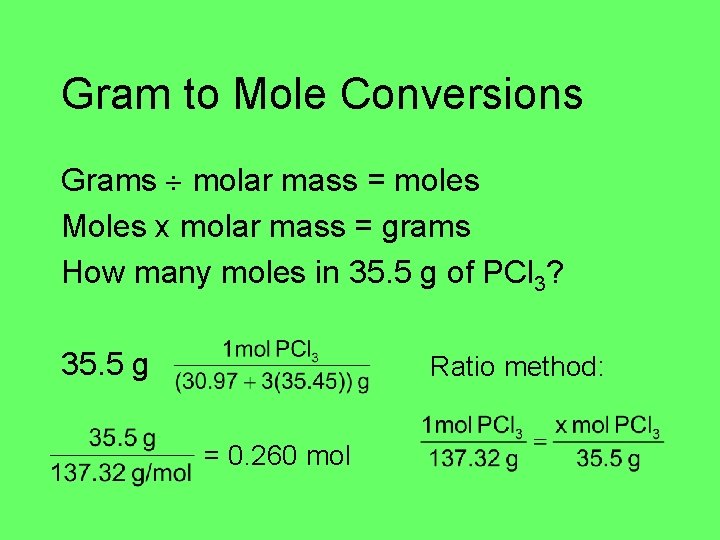
Gram to Mole Conversions Grams molar mass = moles Moles x molar mass = grams How many moles in 35. 5 g of PCl 3? 35. 5 g Ratio method: = 0. 260 mol

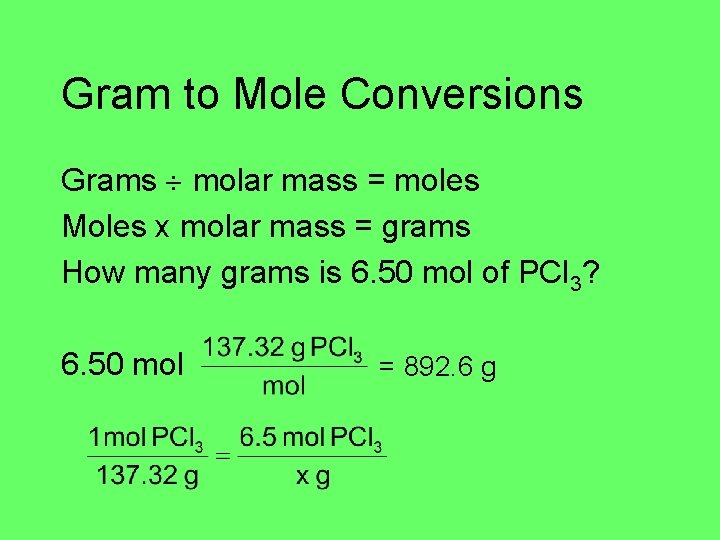
Gram to Mole Conversions Grams molar mass = moles Moles x molar mass = grams How many grams is 6. 50 mol of PCl 3? 6. 50 mol = 892. 6 g

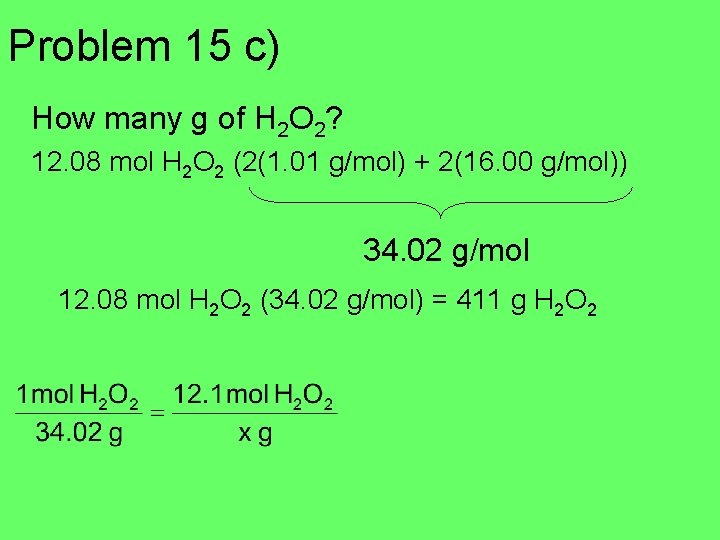
Problem 15 a) Calculate the number of moles in 24. 4 g of H 2. b) If the H 2 reacts with oxygen to make hydrogen peroxide: H 2 + O 2 H 2 O 2 How many moles of H 2 O 2 will form? c) How many g of H 2 O 2?

Problem 15 a) number of moles in 24. 4 g of H 2. 24. 4 = 2. 02 (x) x = 12. 1 mol 24. 4 g = 12. 1 mol H 2 b) H 2 + O 2 H 2 O 2 How many moles of H 2 O 2 will form? 1: 1 ratio 12. 1 mol H 2 O 2

Problem 15 c) How many g of H 2 O 2? 12. 08 mol H 2 O 2 (2(1. 01 g/mol) + 2(16. 00 g/mol)) 34. 02 g/mol 12. 08 mol H 2 O 2 (34. 02 g/mol) = 411 g H 2 O 2